Abstract
Purpose
Light flicker has been shown to stimulate retinal neural activity, increase blood flow, and alter inner retinal oxygen metabolism (MO2) and delivery (DO2). The purpose of the study was to determine the change in MO2 relative to DO2 due to light flicker stimulation in humans, as assessed by the inner retinal oxygen extraction fraction (OEF).
Methods
An optical imaging system, based on a modified slit lamp biomicroscope, was developed for simultaneous measurements of retinal vascular diameter (D) and oxygen saturation (SO2). Retinal images were acquired in 20 healthy subjects before and during light flicker stimulation. Arterial and venous D (DA and DV) and SO2 (SO2A and SO2V) were quantified within a circumpapillary region. Oxygen extraction fraction was defined as the ratio of MO2 to DO2 and was calculated as (SO2A − SO2V)/SO2A. Reproducibility of measurements was assessed.
Results
Coefficients of variation and intraclass correlation coefficients of repeated measurements were <5% and ≥0.83, respectively. During light flicker stimulation, DA, DV , and SO2V significantly increased (P ≤ 0.004). Oxygen extraction fraction was 0.37 ± 0.08 before light flicker and significantly decreased to 0.31 ± 0.07 during light flicker (P = 0.001).
Conclusions
Oxygen extraction fraction before and during light flicker stimulation is reported in human subjects for the first time. Oxygen extraction fraction decreased during light flicker stimulation, indicating the change in DO2 exceeded that of MO2. This technology is potentially useful for the detection of changes in OEF response to light flicker in physiological and pathological retinal conditions.
Keywords: retina, oxygen extraction fraction, oxygen saturation, vessel diameter, oxygen metabolism, oxygen delivery, human
Oxygen extraction fraction before and during light flicker stimulation is reported in human subjects for the first time. Oxygen extraction fraction decreased during light flicker stimulation, indicating the change in DO2 exceeded that of MO2. This technology is potentially useful for the detection of changes in OEF response to light flicker in physiological and pathological retinal conditions
The effect of light flicker stimulation on change in oxygen delivery relative to that of oxygen metabolism was determined using the inner retinal oxygen extraction fraction in healthy human subjects.
The retina is one of the most metabolically active tissues in the human body, requiring a constant supply of oxygen to meet its energy demand.1,2 Diffuse light flicker stimulation has been shown to stimulate neural activity3 which dilates retinal vessels,4 increases blood flow,5 and alters the vascular oxygen saturation (SO2) of hemoglobin,6 implying an increase in oxygen delivery from the retinal circulation. This process is known as functional hyperemia.7 Moreover, light flicker stimulation has been reported to increase retinal glucose8,9 and oxygen metabolism9,10 in animals. However, the change in inner retinal oxygen metabolism (MO2) relative to inner retinal oxygen delivery (DO2) due to light flicker stimulation has not been reported in humans. This change in MO2 relative to DO2 is indicative of the capacity of the retinal circulation to address alterations in tissue metabolic demand due to light flicker stimulation.
Inner retinal oxygen extraction fraction (OEF) quantifies the ratio of MO2 to DO2, although it does not provide an absolute measurement of either quantity. In this study, MO2 is defined as the rate that oxygen is extracted from the retinal circulation for energy metabolism in units of volume of oxygen per unit time, and DO2 is defined as the rate that oxygen becomes available from the retinal circulation in the same units. According to Fick's principle,11 MO2 is the product of retinal blood flow (BF) and the arteriovenous oxygen content difference, and DO2 is the product of BF and the arterial oxygen content. Since the dissolved oxygen content of blood is minimal,12 SO2 can be used to estimate oxygen content. Furthermore, since BF is a determinant of both MO2 and DO2, the ratio defined by OEF is independent of BF. Thus, as shown in Equation 1, OEF can be calculated by measurement of arterial and venous SO2 (SO2A and SO2V , respectively),13 without determining either MO2 or DO2:
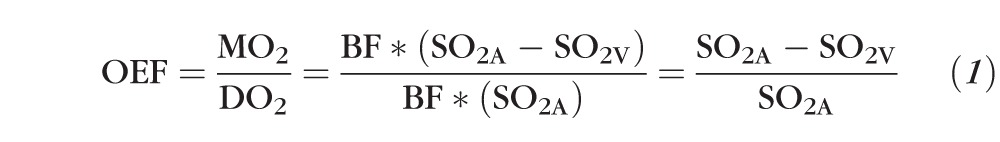 |
In the current study, we report an optical imaging system developed for simultaneous measurement of retinal vascular diameter (D) and SO2 before and during light flicker stimulation in human subjects. We tested the hypothesis that OEF remains unchanged during light flicker stimulation, indicating the change in DO2 matches that of MO2 in healthy human subjects.
Methods
Subjects
The research study was approved by an Institutional Review Board at the University of Illinois at Chicago. Prior to enrollment, the research study was explained to the subjects and informed consents were obtained according to the tenets of the Declaration of Helsinki. Subjects' pupils were dilated using 1% tropicamide and 2.5% phenylephrine. Subjects were seated in front of a modified slit lamp biomicroscope with their heads resting on a chin and forehead support and a light-emitting diode was presented to the fellow eye as a fixation target. While the room lights were turned off, retinal imaging was performed in the study eye before and during light flicker stimulation. Since subjects were exposed to the instrument's retinal illumination light throughout imaging, they were continuously light adapted. Twenty healthy subjects (age: 53 ± 17 years; 11 male, 9 female) participated in the study. In four subjects, three sets of repeated images were acquired to determine the reproducibility of the measurements.
Instrumentation
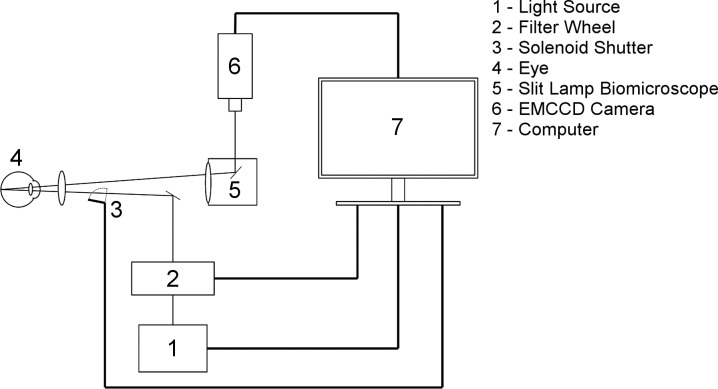
An optical imaging system (Fig. 1) was developed for simultaneous measurement of retinal vascular D and SO2 before and during light flicker stimulation. A slit lamp biomicroscope (Carl Zeiss Microscopy GmbH, Jena, Germany) was modified to incorporate a rapid-switching filter wheel (FW103H, Thorlabs, Newton, NJ, USA) which integrated three bandpass filters with transmission wavelengths of 606 ± 5 nm, 570 ± 5 nm, and 530 ± 5 nm (Edmund Optics, Barrington, NJ, USA). Light at 606 nm and 570 nm corresponded to oxygen-sensitive and oxygen-insensitive imaging wavelengths, respectively,14 whereas the 530-nm wavelength was chosen for light flicker stimulation to elicit a maximal vasodilatory response.15,16 A solenoid with an attached shutter was also integrated into the optical imaging system to provide light flicker at a frequency of 10 Hz17 with a 50% duty cycle. Prior to image acquisition, image alignment was performed in less than 2 minutes using light at 530 nm and 45 μW. Image acquisition before light flicker was achieved within 3 seconds by capturing 9 retinal reflectance images at 606 (230 μW) and 570 nm (180 μW), using an electron multiplying charge coupled device (Rolera EM-C2; QImaging, Surrey, British Columbia). Then light flicker was provided with light at 530 nm and 25 μW for 60 seconds. Image acquisition during light flicker was then performed through continued light flicker and rapid filter wheel adjustment to acquire images at 606 and 570 nm at approximately 50% of the light level before light flicker. A customized computer program was developed using a visual programming language (LabView 2013, National Instruments, Austin, TX, USA) and incorporated an Arduino microprocessor (Arduino Duemilanove; Arduino, Ivrea, Italy) to synchronize filter changes, light flicker stimulation and image acquisition.
Figure 1.
Schematic diagram of the optical imaging system for simultaneous measurement of retinal vascular diameter and oxygen saturation before and during light flicker stimulation. Thick lines represent physical connections between system components and thin lines represent the optical path.
Image Analysis
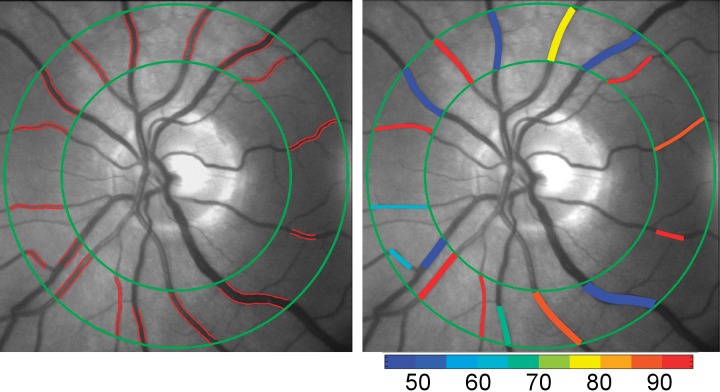
Images which displayed low contrast, reduced focus, large eye movements or blinks were excluded by visual inspection. The remaining images were registered using ImageJ (National Institutes of Health, Bethesda, MD, USA) and then averaged to generate a mean image at each imaging wavelength (image606 and image570). These mean images were manually registered to compensate for any minor eye motion that occurred between filter changes. Our previously reported customized image analysis algorithm18 programmed in a computing environment (MATLAB 2013b; MathWorks, Natick, MA, USA) was modified to segment retinal vessels using a Hessian-based Frangi “vesselness” filter19 within a circumpapillary region of interest which extended between one and two optic disk radii from the optic disk edge (Fig. 2). Vessel centerlines within the region of interest were automatically generated for each segmented vessel using a Euclidian distance transformation. Every seven pixels (∼45 μm) along the vessel centerlines, a perpendicular intensity profile (PIP) was generated from both mean images.
Figure 2.
Example of a retinal image displaying vessel boundaries (red lines), obtained from multiple diameter measurements along blood vessels within a circumpapillary region of interest enclosed by two concentric green circles (left). Mean retinal vascular oxygen saturation measurements overlaid on the retinal image in pseudocolor (right). Color bar indicates oxygen saturation in percent.
Vessel D and boundaries were determined from the full width at half maximum of the PIPs generated from image570, as previously described.20 Gross boundary errors were manually removed by visual inspection and D measurements were averaged along each blood vessel segment. Measurements of D in units of pixels were converted to microns (μm) using a constant calibration factor of 6.63 μm/pixel, which was derived based on previously published optic disk size in healthy human subjects.21
Optical densities (OD) were calculated for each vessel using the PIPs from both mean images. As previously reported,22 optical density was calculated as log(Ioutside/Iinside), where Iinside and Ioutside represent the average pixel intensity inside and outside the vessel along the PIP, respectively. We measured Iinside by averaging the lowest 50% of pixel values within the vessel boundaries to help eliminate specular reflection.23 Based on the vessel diameter, Ioutside was determined by averaging a percentage of background pixel values at locations corresponding to the maximum negative curvatures of PIPs. These locations were determined based on the minima of a second order derivative of the PIPs, as previously described.20
Optical density ratios were calculated as OD606/OD570, where OD606 and OD570 are the optical densities calculated from image606 and image570, respectively.22 Previous studies have described a linear relationship between optical density ratio and vessel size which is due to imaging artifacts24 and physiological factors.25 Since artifactual factors account for most of the linear dependence, a previously described calibration method which did not differentiate between the two factors was employed.23 Adjusted optical density ratio values were converted to SO223,26 by performing a linear regression with previously published retinal arterial and venous SO2 values (92.2% and 57.9%, respectively).27 Measurements of SO2 were averaged along each blood vessel segment.
Effect of Light Flicker
To study the effect of light flicker stimulation, the fractional change in each metric was assessed as the ratio of the metric value during light flicker to before light flicker, and was denoted by the inclusion of an R to the end of the metric name. As such, the fractional change in OEF (OEFR) was defined in terms of the fractional changes in MO2 and DO2.
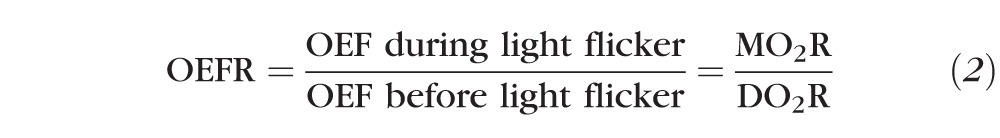 |
The ratio of MO2R to DO2R can result in one of three conditions as reflected by the value of OEFR: 1) if OEFR > 1, then MO2R > DO2R; 2) if OEFR = 1, then MO2R = DO2R; or 3) if OEFR < 1, then MO2R < DO2R. Thus, OEFR provides information about whether the light flicker-induced change in DO2 is lower than, matches, or exceeds that of MO2.
Data Analysis
Reproducibility was determined by calculating the coefficients of variation and intraclass correlation coefficients from three repeated measurements before light flicker in four subjects. Mean arterial D (DA), venous D (Dv), SO2A, and SO2V were calculated from data in at least four vessels and then compiled from all subjects. We calculated OEF from mean SO2A and SO2V according to Equation 1. Mean values of DAR, DVR, SO2AR, SO2VR, and OEFR were calculated from data averaged in all subjects. Paired t-tests were used to evaluate the statistical significance of differences due to vessel type and light flicker. Statistical significance was accepted at P < 0.05.
Results
Mean coefficients of variation for DA, DV , SO2A, SO2V , and OEF were 2.1%, 1.3%, 1.6%, 3.9%, and 4.9%, respectively (n = 4). Intraclass correlation coefficients of DA, DV , SO2A, SO2V , and OEF were 0.85, 0.99, 0.96, 0.93, and 0.83 (n = 4), respectively. As expected, DV was significantly larger than DA and SO2A was significantly greater than SO2V , both before and during light flicker stimulation (P < 0.001; n = 20).
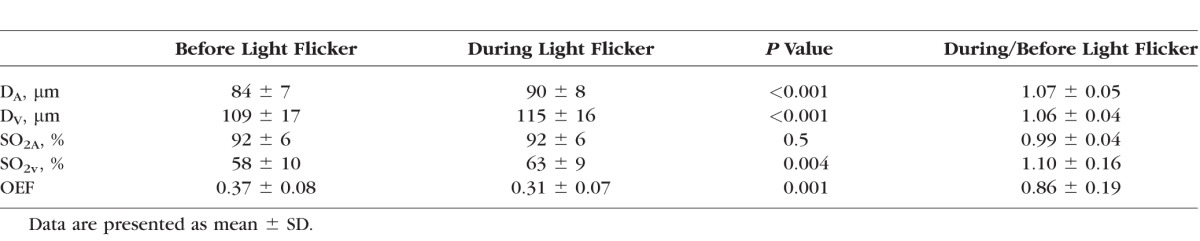
Measurements of DA, DV , SO2A, SO2V , and OEF before and during light flicker are summarized in the Table. Both DA and Dv significantly increased during light flicker (P < 0.001, n = 20). DAR and DVR were 1.07 ± 0.05 (mean ± SD) and 1.06 ± 0.04, indicating average flicker-induced vasodilations of 7% and 6%, respectively. During light flicker, SO2A remained unchanged (P = 0.5, n = 20), while SO2V significantly increased (P = 0.004, n = 20). The value of SO2AR was 0.99 ± 0.04, representing no significant change, whereas SO2VR was 1.10 ± 0.16, indicating an average flicker-induced increase of 10%. Inner retinal OEF was 0.37 ± 0.08 and 0.31 ± 0.07 (P = 0.001, n = 20) before and during light flicker stimulation, respectively. The fractional change in OEF was 0.86 ± 0.19, indicating that OEF decreased on average by 14% during light flicker stimulation.
Table.
Effects of Light Flicker Stimulation on DA, DV , SO2A, SO2V , and OEF in 20 Healthy Subjects
Discussion
In the current study, inner retinal OEF was determined before and during light flicker stimulation in healthy human subjects for the first time, using a custom designed optical imaging system. OEF decreased with light flicker stimulation, indicating the change in DO2 exceeded that of MO2, thus our hypothesis was rejected.
Values of retinal vessel D, SO2, and OEF obtained by our optical imaging system were repeatable to within 5%. As expected, DV was significantly larger than DA, and SO2A was significantly greater than SO2V. In response to light flicker stimulation, both DA and DV significantly dilated, in agreement with previous studies.5,6,17,28,29 Furthermore, SO2A did not significantly change with light flicker, while SO2V significantly increased, consistent with the findings of a previous study.6 Therefore, our optical imaging system was able to detect the physiological perturbations caused by light flicker stimulation.
In the current study, mean OEF before light flicker stimulation was 0.37, indicating that 37% of oxygen available from the retinal circulation was extracted for energy metabolism by the inner retinal tissue. This value is consistent with previous studies which measured an OEF of 0.44 in human brain30 and 0.46 in rat inner retina.13 Mean OEF during light flicker was 0.31, resulting in a mean OEFR < 1, which indicates the change in DO2 exceeded that of MO2. The change in DO2 likely occurs due to an increase in BF during functional hyperemia as supported by our finding of vasodilation. Although the exact vasoregulatory mechanisms7,31,32 involved in functional hyperemia remain to be elucidated, they result in unequal alterations in DO2 and MO2. This may be necessary to raise venous capillary PO2 in order to drive oxygen to regions of the retina which are farther away from the blood vessels.
Recently, efforts have been made to directly quantify MO2 in humans using combined measurements of SO2 and BF.33 Although, light flicker has been shown to increase MO2 in animals,9,10 its effect on MO2 in humans has not been reported. MO2R can be expressed as the product of OEFR and DO2R, where DO2R is the product of BFR and SO2AR. Hence, MO2R can be expressed as follows:
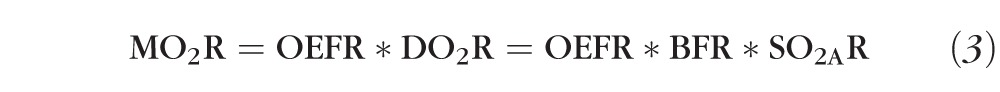 |
Since DO2R was not measured in the current study, MO2R cannot be directly quantified by our measurements of OEFR alone. However, it is possible to use our values of mean OEFR (0.86) and SO2AR (0.99) to calculate the value of BFR at which MO2R = 1, namely, BFR = 1.17. Thus, for MO2 to increase during light flicker, there must be an associated increase in BF of at least 17%. Consequently, an increase in MO2 with light flicker is very likely since Garhofer et al.5 have measured BFR in major retinal arteries to be 1.59. Nevertheless, future studies that simultaneously measure vascular SO2 and BF in the same human subjects are needed to determine MO2 before and during light flicker stimulation.
While OEF provides information about MO2 relative to DO2 based on oxygen saturation measurements in the retinal circulation, it inherently does not account for the potential contribution of oxygen supply from the choroidal circulation. Hence, measurements of OEFR may have been influenced by flicker-induced changes in the relative proportion of the retinal mass supplied by the retinal and choroidal circulations. This relative proportion is determined by oxygen gradients through the retinal depth, which depend on oxygen consumption rates of the inner and outer retina, inner retinal oxygen delivery, and choroidal oxygen delivery. Therefore, without empirical data on intraretinal oxygen tension profiles during light flicker, the effects of dual circulatory beds on OEFR measurements remain to be elucidated.
There were several limitations in the current study. First, as with all optical imaging techniques, image quality may affect measurements. However, the reproducibility of measurements was sufficient for detection of changes due to light flicker. Second, light levels before and during light flicker were not matched. A reduction in the mean light level during the 1-minute duration of light flicker may have induced a small change in the photoreceptor oxygen consumption. However, this change is primarily addressed by the choroidal circulation. Therefore, the reduction in mean light levels between image alignment and light flicker is expected to minimally influence measurements of OEFR which predominately reflects the retinal circulation and inner retinal neural activity. Third, the effects of pigmentation on measurements of SO223 and optical properties on measurements of D were not accounted for. However, since flicker-induced changes were evaluated within subjects and the statistical significance of the differences was high, these factors likely contributed minimally to the results. Fourth, absolute measurements of MO2 and DO2 were not possible since BF was not measured, but their ratio was derived based on measurements of OEF. Consequently, the findings based on OEFR measurements cannot address the adequacy of any light flicker-induced compensatory changes in BF in response to tissue need. Finally, the light flicker–induced change in OEF was calculated based on theoretical considerations which assumed the volume of retinal tissue consuming oxygen that was supplied by the retinal vasculature was identical before and during light flicker stimulation. Direct measurements of MO2 and DO2 are needed to substantiate the findings of the current study.
In summary, with the use of an optical imaging system for simultaneous measurement of retinal vascular D and SO2, OEF was determined before and during light flicker stimulation in human subjects. As indicated by the measured decrease in OEF, the light flicker–induced change in DO2 exceeded that of MO2 in healthy human subjects. Future application of this technique is potentially useful for detection of alterations in inner retinal OEF and OEFR due to physiological and pathological conditions.
Acknowledgments
Supported by NIH Grants DK010439 and EY001792, senior scientific investigator award (MS), and an unrestricted departmental grant from Research to Prevent Blindness. The authors thank Andrew Cross, Ruth Zelkha, and Marek Mori for assistance in acquisition.
Disclosure: A.E. Felder, None; J. Wanek, None; N.P. Blair, None; M. Shahidi, None
References
- 1. Trick GL,, Berkowitz BA. Retinal oxygenation response and retinopathy. Pro Retin Eye Res. 2005; 24: 259–274. [DOI] [PubMed] [Google Scholar]
- 2. Kur J,, Newman EA,, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012; 31: 377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsini B,, Riva CE,, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol. 2002; 43: 2309–2316. [PubMed] [Google Scholar]
- 4. Garhofer G,, Bek T,, Boehm AG,, et al. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010; 88: 717–722. [DOI] [PubMed] [Google Scholar]
- 5. Garhofer G,, Zawinka C,, Resch H,, Huemer KH,, Dorner GT,, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vision Res. 2004; 44: 833–838. [DOI] [PubMed] [Google Scholar]
- 6. Hammer M,, Vilser W,, Riemer T,, et al. Retinal venous oxygen saturation increases by flicker light stimulation. Invest Ophthalmol Vis Sci. 2011; 52: 274–277. [DOI] [PubMed] [Google Scholar]
- 7. Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cerebr Blood Flow Metab. 2013; 33: 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bill A,, Sperber GO. Aspects of oxygen and glucose consumption in the retina - effects of high intraocular-pressure and light. Graefes Arch Clin Exp Ophthamol. 1990; 228: 124–127. [DOI] [PubMed] [Google Scholar]
- 9. Wang L,, Bill A. Effects of constant and flickering light on retinal metabolism in rabbits. Acta Ophthalmol Scand. 1997; 75: 227–231. [DOI] [PubMed] [Google Scholar]
- 10. Ames A,, Li YY,, Heher EC,, Kimble CR. Energy-metabolism of rabbit retina as related to function - high cost of Na+ transport. J Neurosci. 1992; 12: 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pittman RN. Oxygen transport in normal and pathological situations: defects and compensations. : Pittman RN, Regulation of Tissue Oxygenation. San Rafael: Morgan & Claypool Life Sciences; 2011: 47–50. [PubMed] [Google Scholar]
- 12. Crystal GJ. Principles of cardiovascular physiology. : Estafanous FG,, Barash PG,, Reves JG, Cardiac Anesthesia: Principles and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2001: 37–57. [Google Scholar]
- 13. Teng PY,, Wanek J,, Blair NP,, Shahidi M. Inner retinal oxygen extraction fraction in rat. Invest Ophthamol Vis Sci. 2013; 54: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zijlstra WG,, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991; 37: 1633–1638. [PubMed] [Google Scholar]
- 15. Polak K,, Schmetterer L,, Riva CE. Influence of flicker frequency on flicker-induced changes of retinal vessel diameter. Invest Ophthalmol Vis Sci. 2002; 43: 2721–2726. [PubMed] [Google Scholar]
- 16. Riva CE,, Falsini B,, Logean E. Flicker-evoked responses of human optic nerve head blood flow: luminance versus chromatic modulation. Invest Ophthalmol Vis Sci. 2001; 42: 756–762. [PubMed] [Google Scholar]
- 17. Formaz F,, Riva CE,, Geiser M. Diffuse luminance flicker increases retinal vessel diameter in humans. Curr Eye Res. 1997; 16: 1252–1257. [DOI] [PubMed] [Google Scholar]
- 18. Moss HE,, Treadwell G,, Wanek J,, DeLeon S,, Shahidi M. Retinal vessel diameter assessment in papilledema by semi-automated analysis of SLO images: feasibility and reliability. Invest Ophthalmol Vis Sci. 2014; 55: 2049–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frangi AF,, Niessen WJ,, Vincken KL,, Viergever MA. Multiscale vessel enhancement filtering. Lect Notes Comput Sci. 1998; 1496: 130–137. [Google Scholar]
- 20. Pedersen L,, Grunkin M,, Ersboll B,, et al. Quantitative measurement of changes in retinal vessel diameter in ocular fundus images. Pattern Recogn Lett. 2000; 21: 1215–1223. [Google Scholar]
- 21. Quigley HA,, Brown AE,, Morrison JD,, Drance SM. The size and shape of the optic disc in normal human eyes. Arch Ophthamol. 1990; 108: 51–57. [DOI] [PubMed] [Google Scholar]
- 22. Beach JM,, Schwenzer KJ,, Srinivas S,, Kim D,, Tiedeman JS. Oximetry of retinal vessels by dual-wavelength imaging: calibration and influence of pigmentation. J Appl Physiol. 1999; 86: 748–758. [DOI] [PubMed] [Google Scholar]
- 23. Hammer M,, Vilser W,, Riemer T,, Schweitzer D. Retinal vessel oximetry-calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. J Biomed Opt. 2008; 13: 054015 [DOI] [PubMed] [Google Scholar]
- 24. Hardarson SH. Retinal oximetry. Acta Ophthalmol. 2013; 91 Thesis 2: 1–47. [DOI] [PubMed] [Google Scholar]
- 25. Geirsdottir A,, Palsson O,, Hardarson SH,, Olafsdottir OB,, Kristjansdottir JV,, Stefansson E. Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci. 2012; 53: 5433–5442. [DOI] [PubMed] [Google Scholar]
- 26. Beach J. Pathway to retinal oximetry. Trans Vis Sci Technol. 2014; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kristjansdottir JV,, Hardarson SH,, Halldorsson GH,, Karlsson RA,, Eliasdottir TS,, Stefansson E. Retinal oximetry with a scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2014; 55: 3120–3126. [DOI] [PubMed] [Google Scholar]
- 28. Nagel E,, Vilser W. Flicker observation light induces diameter response in retinal arterioles: a clinical methodological study. Br J Ophthalmol. 2004; 88: 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palkovits S,, Told R,, Boltz A,, et al. Effect of increased oxygen tension on flicker-induced vasodilatation in the human retina. J Cereb Blood FlowMetab. 2014; 34: 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ito H,, Kanno I,, Kato C,, et al. Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with O-15-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging. 2004; 31: 635–643. [DOI] [PubMed] [Google Scholar]
- 31. Roy CS,, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890; 11: 85–158 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Attwell D,, Buchan AM,, Charpak S,, Lauritzen M,, Macvicar BA,, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palkovits S,, Lasta M,, Told R,, et al. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 4707–4713. [DOI] [PubMed] [Google Scholar]