Abstract
AIM: To explore the feasibility of performing minimally invasive surgery (MIS) on subsets of submucosal gastric cancers that are unlikely to have regional lymph node metastasis.
METHODS: A total of 105 patients underwent radical gastrectomy with lymph node dissection for submucosal gastric cancer at our hospital from January 1995 to December 1995. Besides investigating many clinicopathological features such as tumor size, gross appearance, and differentiation, we measured the depth of invasion into submucosa minutely and analyzed the clinicopathologic features of these patients regarding lymph node metastasis.
RESULTS: The rate of lymph node metastasis in cases where the depth of invasion was < 500 μm, 500-2000 μm, or > 2000 μm was 9% (2/23), 19% (7/36), and 33% (15/46), respectively (P < 0.05). In univariate analysis, no significant correlation was found between lymph node metastasis and clinicopathological characteristics such as age, sex, tumor location, gross appearance, tumor differentiation, Lauren’s classification, and lymphatic invasion. In multivariate analysis, tumor size (> 4 cm vs ≤ 2 cm, odds ratio = 4.80, P = 0.04) and depth of invasion (> 2000 μm vs ≤ 500 μm, odds ratio = 6.81, P = 0.02) were significantly correlated with lymph node metastasis. Combining the depth and size in cases where the depth of invasion was less than 500 μm, we found that lymph node metastasis occurred where the tumor size was greater than 4 cm. In cases where the tumor size was less than 2 cm, lymph node metastasis was found only where the depth of tumor invasion was more than 2000 μm.
CONCLUSION: MIS can be applied to submucosal gastric cancer that is less than 2 cm in size and 500 μm in depth.
INTRODUCTION
Minimally invasive surgeries (MIS) such as endoscopic mucosal resection or laparoscopic surgery are performed as a treatment of early gastric cancer. This limited surgery should be conducted on strict indication in early gastric cancer which has a limited invasion in mucosa. Such a surgery can be attained without lymph node dissection. However, if the case is diagnosed as submucosal gastric cancer after MIS, the patient needs additional gastrectomy and lymph node dissection owing to the limitations of current diagnostic methods in assessing the depth of invasion and lymph node status.
Since the incidence of lymph node metastasis in submucosal gastric cancer is reported as less as about 20%, it can be expected that the chance of further operations will be reduced if we choose patients who have low risk of lymph node metastasis. Thus, we analyzed in our study the clinicopathological factors related to lymph node metastasis in submucosal gastric cancers retrospectively, in order to find out the subsets of submucosal gastric cancers to which MIS can be applied, and to establish the suitable treatment policy of submucosal gastric cancers.
MATERIALS AND METHODS
This study enrolled 105 submucosal gastric cancer cases that were pathologically proven after gastrectomy by lymph node dissection at Seoul National University Hospital from January 1995 to December 1995. The relationship between clinicopathological factors (sex, age, tumor location, gross appearance, tumor size, depth of invasion, tumor differentiation, Lauren’s classification, and lymphatic invasion) and lymph node metastasis was investigated. Submucosal layer was divided into 3 layers (upper third = SM1, middle third = SM2, and lower third = SM3) and submucosal depth of invasion was directly measured from muscularis mucosae by a micrometer (Figure 1). χ2 test and logistic regression analysis were used to evaluate risk factors for lymph node metastasis and P < 0.05 was considered statistically significant.
Figure 1.
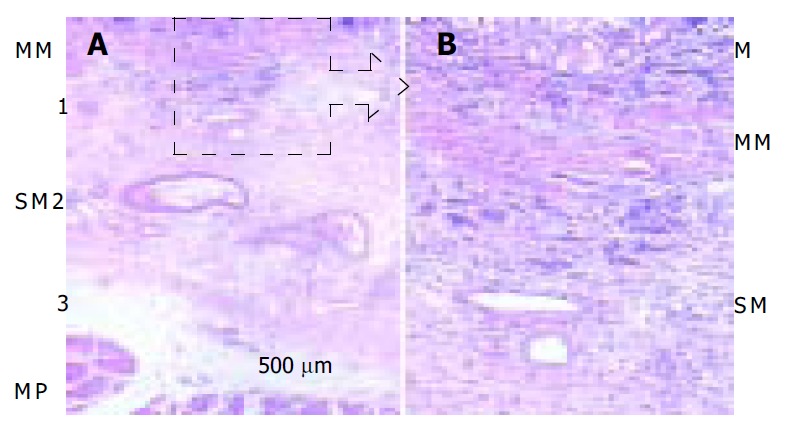
Depth of submucosal invasion of gastric cancer (arrow) in hematoxylin and eosin staining in A (×10) and B (×100). M: mucosa, MM: muscularis mucosae, SM: submucosa, MP: musculrais propria. This case was classified as SM1 or less than 500 μm from musculasis mucosae.
RESULTS
Patient characteristics
A total of 105 patients with submucosal gastric cancer were included in the study. Of them, 74 were males and 31 were females. Their mean age was 54.9 years (18-80 years). The rate of lymph node metastasis was 22.9% (24/105 cases).
Depth of invasion (Table 1)
Table 1.
Depth of submucosal invasion as compared with three SM category measurements
| Depth (μm) | SM1 (%) | SM2 (%) | SM3 (%) |
| ≤ 500 (n = 23) | 19 (68) | 4 (8) | 0 (0) |
| 500-1000 (n = 17) | 8 (28) | 8 (17) | 1 (3) |
| 1000-2000 (n = 19) | 1 (4) | 14 (30) | 4 (13) |
| > 2000 (n = 46) | 0 (0) | 21 (45) | 25 (84) |
| Total | 28 | 47 | 30 |
According to the SM category, SM1, SM2, and SM3 lesions were 28 (27%), 47 (45%), and 30 (28%) respectively. The depth of invasion measured directly from muscularis mucosae was less than 500 μm in 68% of SM1 gastric cancers, more than 1000 μm (1 mm) in 75% of SM2 gastric cancers, and more than 2000 μm in 84% of SM3 gastric cancers.
Relationship between clinicopathological factors and lymph node metastasis (Table 2)
Table 2.
Relationship between clinicopathological factors and lymph node metastasis
| Total | Node-negative | Node-positive (n, %) | P | |
| Sex | ||||
| M | 74 | 57 | 17 (23.0) | 0.965 |
| F | 31 | 24 | 17 (22.6) | |
| Age (yr) | 105 | 54.8 ± 12.5 | 55.4 ± 12.2 | 0.822 |
| Tumor location | ||||
| Upper | 1 | 1 | 0 (0) | 0.614 |
| Middle | 53 | 42 | 11 (20.8) | |
| Lower | 48 | 35 | 13 (27.1) | |
| Entire | 3 | 3 | 0 (0) | |
| Gross appearance | ||||
| Elevated | 23 | 15 | 8 (34.8) | 0.055 |
| Flat | 6 | 3 | 3 (50.0) | |
| Depressed | 76 | 63 | 13 (17.1) | |
| Tumor differentiation | ||||
| Differentiated | 47 | 35 | 12 (25.5) | 0.557 |
| Undifferentiated | 58 | 46 | 12 (20.7) | |
| Lauren’s classification | ||||
| Intestinal | 44 | 35 | 9 (20.5) | 0.572 |
| Diffuse | 53 | 41 | 12 (22.6) | |
| Mixed | 8 | 5 | 3 (37.5) | |
| Depth of invasion | ||||
| SM1 | 28 | 24 | 4 (14.3) | 0.448 |
| SM2 | 47 | 35 | 12 (25.5) | |
| SM3 | 30 | 22 | 8 (26.7) | |
| Lymphatic invasion | ||||
| Absence | 86 | 69 | 17 (19.8) | 0.109 |
| Presence | 19 | 12 | 7 (36.8) | |
In univariate analysis, there was no significant factor related to lymph node metastasis among sex, age, tumor location, gross appearance, tumor differentiation, Lauren’s classification, and lymphatic invasion.
Lymph node status in term of depth of invasion (Table 3)
Table 3.
Lymph node status according to depth of invasion
| Depth (μm) |
Lymph node status (%) |
|||
| pN0 | pN+ | pN1 | pN2 | |
| ≤ 500 (n = 23) | 21 (91) | 2 (9)a | 2 | 0 |
| 500-1000 (n = 17) | 14 (82) | 3 (18) | 3 | 0 |
| 1000-2000 (n = 19) | 15 (79) | 4 (21) | 3 | 1 |
| > 2000 (n = 46) | 31 (67) | 15 (33)a | 13 | 2 |
P < 0.05.
The depth of invasion was significantly associated with lymph node metastasis. Especially, when the depth of invasion was more than 1000 μm, three cases out of 65 (4.6%) were revealed to have more than 7 lymph node metastases (N2 stage).
Lymph node status in term of tumor size (Table 4)
Table 4.
Lymph node status according to tumor size
| Size (cm) |
Lymph node status (%) |
|||
| pN0 | pN+ | pN1 | pN2 | |
| ≤ 2 (n = 27) | 24 (89) | 3 (11) | 3 | 0 |
| 2-3 (n = 30) | 22 (73) | 8 (27) | 7 | 1 |
| 3-4 (n = 20) | 15 (75) | 5 (25) | 5 | 0 |
| > 4 (n = 28) | 20 (71) | 8 (29) | 6 | 2 |
P = 0.403.
Though there was no statistical significance in univariate analysis, the rate of lymph node metastasis was increased as the tumor size increased (≤ 2 cm, 11%; 2-4 cm, 26%; > 4 cm, 29%).
Logistic regression analysis of factors associated with lymph node metastasis in submucosal gastric cancer (Table 5)
Table 5.
Logistic regression analysis for factors associated with lymph node metastasis in submucosal gastric cancer
| Variables | Odds ratio | 95%CI | P-value |
| Depth of invasion (μm) | |||
| ≤ 500 | 1 | ||
| 500-1000 | 2.02 | 0.30-13.86 | 0.473 |
| 1000-2000 | 3.14 | 0.49-20.24 | 0.228 |
| > 2000 | 6.81 | 1.36-34.17 | 0.020 |
| Tumor size (cm) | |||
| ≤ 2 | 1 | ||
| 2-3 | 4.35 | 0.95-19.96 | 0.059 |
| 3-4 | 4.05 | 0.78-20.97 | 0.096 |
| > 4 | 4.80 | 1.05-22.06 | 0.044 |
CI: Confidence interval.
In multivariate analysis, the tumor size (> 4 cm in diameter vs ≤ 2 cm, odds ratio = 4.80, P = 0.04) and depth of invasion (> 2000 μm of depth vs ≤ 500 μm, odds ratio = 6.81, P = 0.02) were significantly associated with lymph node metastasis.
Lymph node status in term of size and depth in submucosal gastric cancer (Table 6)
Table 6.
Lymph node status assessed by co-factor of size and depth in submucosal gastric cancer
| Depth (μm) |
Size (cm) |
|||
| ≤ 2 | 2-3 | 3-4 | > 4 | |
| ≤ 500 (n = 23) | 0a/4b | 0/6 | 0/6 | 2/7 |
| 500-1000 (n = 17) | 0/1 | 1/6 | 1/4 | 1/6 |
| 1000-2000 (n = 19) | 0/5 | 2/8 | 1/2 | 1/4 |
| > 2000 (n = 46) | 3/17 | 5/10 | 3/8 | 4/11 |
Number of cases with lymph node metastasis;
Number of total cases.
Lymph node status was analyzed in terms of tumor size and depth of invasion in submucosal gastric cancer. In cases where the depth of invasion was less than 500 μm, lymph node metastasis was found only when the tumor size was greater than 4 cm. In cases where the tumor size was less than 2 cm, lymph node metastasis was found only when the depth of invasion was more than 2000 μm.
DISCUSSION
Gastric cancer is the most prevalent cancer in Korea, which accounted for 20.3% of the whole cancer cases[1]. According to the report of Korea Gastric Cancer Association, the ratio of early gastric cancer was increased from 28.6% in 1995 to 32.8% in 1999[2], while this tendency was more obvious in Japan, where the ratio of early gastric cancer in the last 20 years was increased from 18% to 57%[3].
Lymph node metastasis was the most important prognostic factor in gastric cancer[4,5]. Radical gastrectomy with D2 lymph node dissection, therefore, has been recognized as the standard surgical operation for early gastric cancer. But, as the five year survival rate of early gastric cancer patients has reached 93%-98% recently, there arises more interest in preservation of body function and maintenance of quality of life rather than in radical treatment in order to reduce complications or sequelae, such as Dumping syndrome, reflux gastroesophagitis, nutritional deficit, and weight loss. Therefore, interest in MIS such as endoscopic mucosal resection and laparoscopic surgery or function-preserving surgery has been increased.
MIS with no or limited lymph node dissection should be applied to tumors that are unlikely to metastasize into lymph nodes. Endoscopic ultrasonography (EUS) has been introduced recently to diagnose the depth of invasion and lymph node status. An overall accuracy of EUS was reported over 80%[6,7]. Yet, as the accuracy of EUS is not high enough to predict accurate lymph node status, it is important to know the prognostic factors related to lymph node metastasis. There have been already many reports on the prognostic factors associated with lymph node metastasis in early gastric cancer[8-16], such as sex[8], tumor size[3,9,10,12], depth of invasion[11], gross appearance of tumor[8], tumor differentiation[13] and lymphatic invasion[14,15]. If we combine these prognostic factors successfully in the diagnosis of certain subtypes that have no lymph node metastasis, then they may become an indication of MIS. For example, the current indications of endoscopic mucosal resection of early gastric cancer are as follows, namely differentiated adenocarcinoma, mucosal cancer < 10 mm in diameter in IIb and IIc lesions without ulcer or ulcer scar, and mucosal cancer < 20 mm in diameter in IIa lesion[17,18]. The reason why endoscopic mucosal resection can be applied to them is that these lesions are considered to have no lymph node metastasis.
When the depth of invasion reached mucosa in early gastric cancer, the rate of lymph node metastasis was reported as 1%-3%, and submucosa as 11%-20%[19]. At present if endoscopic mucosal resection for early gastric cancer can detect the invasion of submucosal layer, additional gastrectomy and lymph node dissection are regarded necessary. However, as the incidence of lymph node metastasis was about 20% in submucosal gastric cancer, lymph node dissection could be omitted if we chose cases with good selection of indications. In our study on submucosal gastric cancer, we found that sex, age, tumor location, tumor differentiation, Lauren’s classification, gross appearance of tumor, were not associated with lymph node metastasis, but tumor size and depth of invasion were associated with lymph node metastasis. A recent study reported that there was a significant difference in the rate of lymph node metastasis according to submucosal depth of invasion, and the possibility of limited surgery for superficial submucosal gastric cancer was therefore suggested[20]. We divided submucosal layer minutely into SM1, SM2, and SM3 on microscopic field and measured the depth of invasion by a micrometer directly from muscularis mucosae. Actually, because there was some difference between SM 1, 2, 3 categories and the directly measured depth associated with lymph node metastasis, it was considered more objective to use directly measured depth rather than SM category to describe submucosal tumor invasion. Ishigami et al[21] reported that patients with both slight invasion into submucosa and horizontal expansion that was < 5 mm were often negative in lymph node involvement. Kurihara et al[22] reported that when the pathological report revealed SM1 invasion after laparoscopic or endoscopic surgery, reoperation should be regarded unnecessary because SM1-carcinoma with its diameter less than 2 cm did not usually metastasize to lymph nodes. Moreover, Yasuda et al[23] showed that local resection could be applied when the depth of submucosal invasion was < 300 μm and tumor size was < 1 cm. Yamada et al[24] also showed that local resection might be possible when the depth of submucosal invasion was < 500 μm and tumor size was < 1.5 cm. Gotoda et al[25] proposed to expand the criteria for local treatment of submucosal gastric cancer by showing that none of the 145 differentiated adenocarcinomas, < 30 mm in diameter and without lymphatic or venous permeation, was associated with lymph node metastasis, provided the lesion invaded less than 500 μm into submucosa. In our study, the tumor size and depth of invasion as independent prognostic factors associated with lymph node metastasis were joined together. In cases where the depth of invasion was < 500 μm, lymph node metastasis was not found when the tumor size was < 4 cm. In cases where the tumor size was less than 2 cm, lymph node metastasis was not found when the depth of invasion was less than 2000 μm. Therefore, the results suggest that additional operation is unnecessary when the depth of submucosal invasion is less than 500 μm and the tumor size is smaller than 2 cm after local excision. Moreover, in case that patients cannot endure general anesthesia because of old age or cardiopulmonary disability, in case that gastrectomy itself (with or without lymph node dissection) is considered to be dangerous to patients, or when patients refuse the operation, local excisions such as endoscopic mucosal resection, can be applied to submucosal gastric cancer.
In conclusion, the depth of invasion measured directly by a micrometer is more objective to describe cancer permeation into submucosal layer because it is associated more with lymph node metastasis than with SM category.
Our results suggest that additional operation is not necessary after MIS for submucosal gastric cancer when the depth of invasion is less than 500 μm and the tumor size is smaller than 2 cm.
Footnotes
Edited by Qiu WS and Wang XL Proofread by Chen WW and Xu FM
References
- 1.Korea Central Cancer Registry, Ministry of Health and Welfare Republic of Korea. Annual Report of the Korea Central Cancer Registry 2003 [Google Scholar]
- 2.Nationwide gastric cancer report in Korea. Korea Gastric Can-cer Association. J Korean Gastric Cancer Assoc. 2002;2:105–114. [Google Scholar]
- 3.Maehara Y, Orita H, Okuyama T, Moriguchi S, Tsujitani S, Korenaga D, Sugimachi K. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. doi: 10.1002/bjs.1800790320. [DOI] [PubMed] [Google Scholar]
- 4.Kunisaki C, Shimada H, Takahashi M, Ookubo K, Moriwaki Y, Akiyama H, Nomura M. Prognostic factors in early gastric cancer. Hepatogastroenterology. 2001;48:294–298. [PubMed] [Google Scholar]
- 5.Kim JP, Kim YW, Yang HK, Noh DY. Significant prognostic factors by multivariate analysis of 3926 gastric cancer patients. World J Surg. 1994;18:872–877; discussion 872-877. doi: 10.1007/BF00299091. [DOI] [PubMed] [Google Scholar]
- 6.Tio TL, Schouwink MH, Cikot RJ, Tytgat GN. Preoperative TNM classification of gastric carcinoma by endosonography in comparison with the pathological TNM system: a prospective study of 72 cases. Hepatogastroenterology. 1989;36:51–56. [PubMed] [Google Scholar]
- 7.Guo W, Zhang YL, Li GX, Zhou DY, Zhang WD. Comparison of preoperative TN staging of gastric carcinoma by endoscopic ultrasonography with CT examination. China Nati J New Gastroenterol. 1997;3:242–245. doi: 10.3748/wjg.v3.i4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boku T, Nakane Y, Okusa T, Hirozane N, Imabayashi N, Hioki K, Yamamoto M. Strategy for lymphadenectomy of gastric cancer. Surgery. 1989;105:585–592. [PubMed] [Google Scholar]
- 9.Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Analysis of lymph node metastasis in early gastric cancer: rationale of limited surgery. J Surg Oncol. 1997;64:42–47. doi: 10.1002/(sici)1096-9098(199701)64:1<42::aid-jso9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992;79:241–244. doi: 10.1002/bjs.1800790319. [DOI] [PubMed] [Google Scholar]
- 11.Kwak CS, Lee HK, Cho SJ, Yang HK, Lee KU, Choe KJ, Kim JP. Analysis of clinicopathological factors associated with lymph node metastasis in early gastric cancer review of 2.137 cases. J Korean Cancer Assoc. 2000;32:674–681. [Google Scholar]
- 12.Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Indications of limited surgery for gastric cancer with submucosal invasion--analysis of 715 cases with special reference to site of the tumor and level 2 lymph nodes. Hepatogastroenterology. 2003;50:1727–1730. [PubMed] [Google Scholar]
- 13.Iriyama K, Asakawa T, Koike H, Nishiwaki H, Suzuki H. Is extensive lymphadenectomy necessary for surgical treatment of intramucosal carcinoma of the stomach? Arch Surg. 1989;124:309–311. doi: 10.1001/archsurg.1989.01410030059010. [DOI] [PubMed] [Google Scholar]
- 14.Ichikura T, Uefuji K, Tomimatsu S, Okusa Y, Yahara T, Tamakuma S. Surgical strategy for patients with gastric carcinoma with submucosal invasion. A multivariate analysis. Cancer. 1995;76:935–940. doi: 10.1002/1097-0142(19950915)76:6<935::aid-cncr2820760605>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181–185; discussion 186. doi: 10.1002/jso.20018. [DOI] [PubMed] [Google Scholar]
- 16.Kitayama J, Hatano K, Kaisaki S, Suzuki H, Fujii S, Nagawa H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br J Surg. 2004;91:191–198. doi: 10.1002/bjs.4391. [DOI] [PubMed] [Google Scholar]
- 17.Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. doi: 10.1055/s-2007-1008990. [DOI] [PubMed] [Google Scholar]
- 18.Korenaga D, Orita H, Maekawa S, Maruoka A, Sakai K, Ikeda T, Sugimachi K. Pathological appearance of the stomach after endoscopic mucosal resection for early gastric cancer. Br J Surg. 1997;84:1563–1566. [PubMed] [Google Scholar]
- 19.Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333–339. doi: 10.1159/000065829. [DOI] [PubMed] [Google Scholar]
- 20.Amano Y, Ishihara S, Amano K, Hirakawa K, Adachi K, Fukuda R, Watanabe M, Fukumoto S, Fujishiro H, Imaoka T. An assessment of local curability of endoscopic surgery in early gastric cancer without satisfaction of current therapeutic indications. Endoscopy. 1998;30:548–552. doi: 10.1055/s-2007-1001342. [DOI] [PubMed] [Google Scholar]
- 21.Ishigami S, Hokita S, Natsugoe S, Tokushige M, Saihara T, Iwashige H, Aridome K, Aikou T. Carcinomatous infiltration into the submucosa as a predictor of lymph node involvement in early gastric cancer. World J Surg. 1998;22:1056–1059; discussion 1056-1059. doi: 10.1007/s002689900516. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998;85:835–839. doi: 10.1046/j.1365-2168.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999;85:2119–2123. doi: 10.1002/(sici)1097-0142(19990515)85:10<2119::aid-cncr4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Yamada H, Nihei Z, Yamashita T, Shirota Y, Ichikawa W, Sugihara K. Is lymphadenectomy needed for all submucosal gastric cancers? Eur J Surg. 2001;167:199–203. doi: 10.1080/110241501750099393. [DOI] [PubMed] [Google Scholar]
- 25.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]


