Abstract
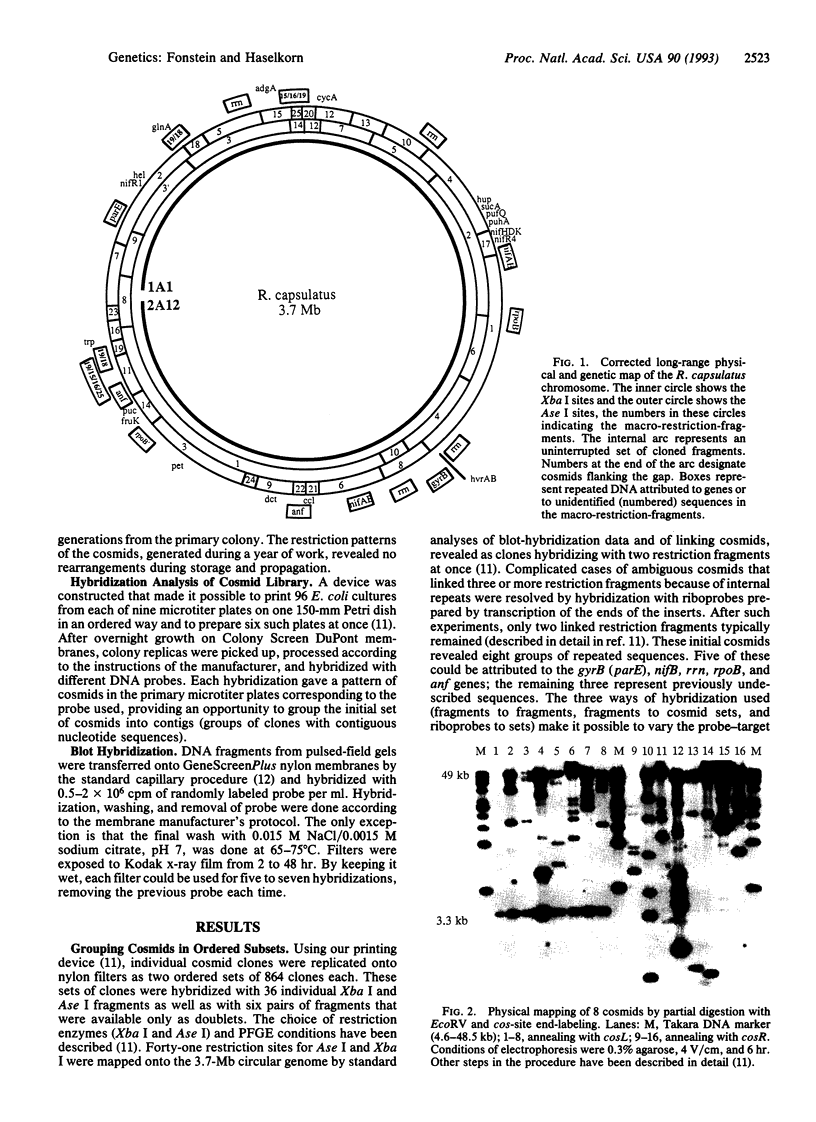
A combination of cosmid genome walking and pulsed-field gel electrophoresis was used to construct a high-resolution physical and genetic map of the 3.8-megabase (Mb) genome of Rhodobacter capsulatus SB1003. The mapping was done by hybridization of pulsed-field gel blots and by grouping and further mapping of the cosmids and bacteriophages from genomic libraries. Cosmid clones formed two uninterrupted and ordered groups, one corresponding to the chromosome of R. capsulatus, the other to its 134-kb plasmid. Cos site end-labeling and partial EcoRV digestion of cosmids were used to construct a high-resolution EcoRV map of the genome. Overlapping of the cosmids was confirmed by the resemblance of the cosmid restriction maps and by direct end-to-end hybridization with SP6- and T7-specific transcripts. Twenty-three previously cloned genes and eight groups of repeated sequences, revealed in this work, were located in the ordered gene library and mapped with an accuracy of 1-10 kb. Blots of a minimal set of 192 cosmids, covering the chromosome and the plasmid with the known map position of each cosmid, give to R. capsulatus the same advantages that the Kohara phage panel gives to E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlebois R. L., Schalkwyk L. C., Hofman J. D., Doolittle W. F. Detailed physical map and set of overlapping clones covering the genome of the archaebacterium Haloferax volcanii DS2. J Mol Biol. 1991 Dec 5;222(3):509–524. doi: 10.1016/0022-2836(91)90493-p. [DOI] [PubMed] [Google Scholar]
- Cohen A., Lam W. L., Charlebois R. L., Doolittle W. F., Schalkwyk L. C. Localizing genes on the map of the genome of Haloferax volcanii, one of the Archaea. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1602–1606. doi: 10.1073/pnas.89.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Kaplan S. Genetic techniques in rhodospirillaceae. Methods Enzymol. 1991;204:459–485. doi: 10.1016/0076-6879(91)04024-i. [DOI] [PubMed] [Google Scholar]
- Fonstein M., Zheng S., Haselkorn R. Physical map of the genome of Rhodobacter capsulatus SB 1003. J Bacteriol. 1992 Jun;174(12):4070–4077. doi: 10.1128/jb.174.12.4070-4077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Coulson A. R., Sulston J. E., Little P. F. Lorist2, a cosmid with transcriptional terminators insulating vector genes from interference by promoters within the insert: effect on DNA yield and cloned insert frequency. Gene. 1987;53(2-3):275–281. doi: 10.1016/0378-1119(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Wong W. K., Beatty J. T. Expression of cellulase genes in Rhodobacter capsulatus by use of plasmid expression vectors. J Bacteriol. 1986 Aug;167(2):604–610. doi: 10.1128/jb.167.2.604-610.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipp W., Masepohl B., Pühler A. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol. 1988 Feb;170(2):693–699. doi: 10.1128/jb.170.2.693-699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
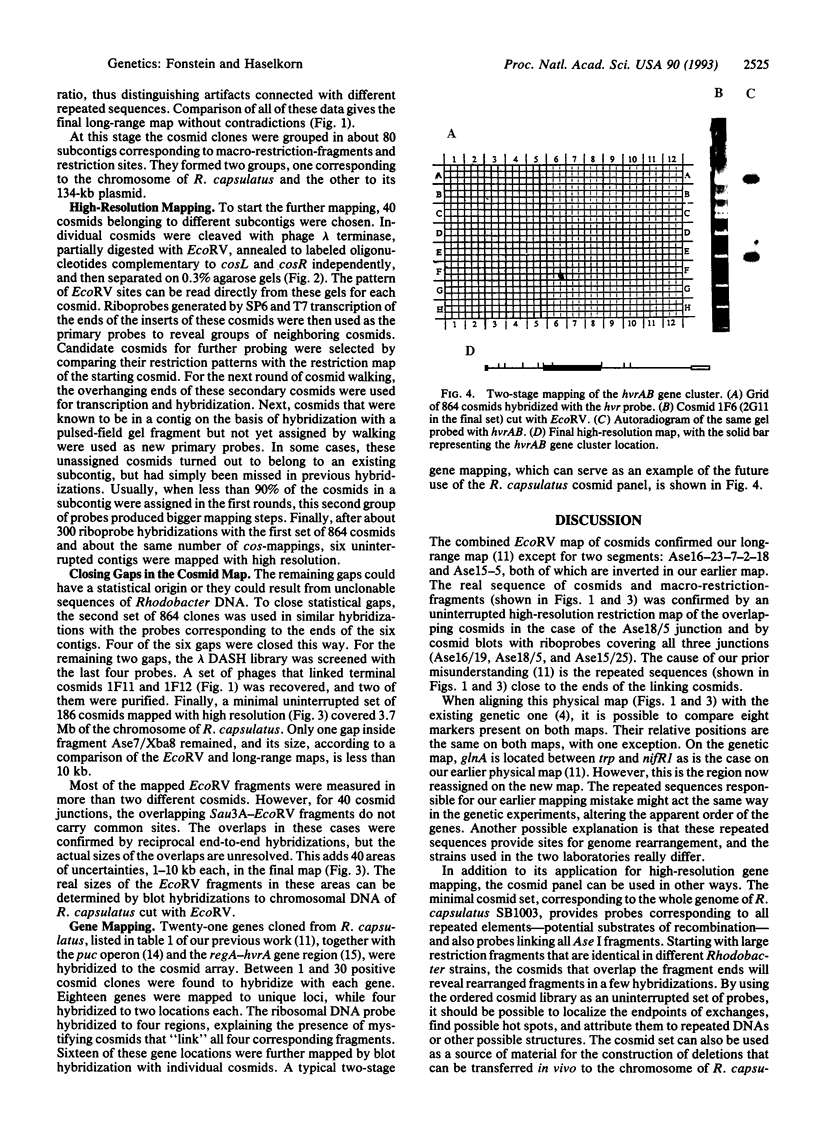
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Komine Y., Inokuchi H. Precise mapping of the rnpB gene encoding the RNA component of RNase P in Escherichia coli K-12. J Bacteriol. 1991 Mar;173(5):1813–1816. doi: 10.1128/jb.173.5.1813-1816.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sganga M. W., Aksamit R. R., Cantoni G. L., Bauer C. E. Mutational and nucleotide sequence analysis of S-adenosyl-L-homocysteine hydrolase from Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6328–6332. doi: 10.1073/pnas.89.14.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sganga M. W., Bauer C. E. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992 Mar 6;68(5):945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Wall J. D., Love J., Quinn S. P. Spontaneous Nif- mutants of Rhodopseudomonas capsulata. J Bacteriol. 1984 Aug;159(2):652–657. doi: 10.1128/jb.159.2.652-657.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]