Abstract
AIM: To study the expression and serum level of HBxAg, Fas and FasL in tissues of HCC patients, and to assess the relationship between HBxAg and Fas/FasL system.
METHODS: Tissues from 50 patients with HCC were tested for the expression of HBxAg, Fas and FasL by S-P immunohistochemistry. Serum levels of sFas/sFasL and HBsAg/HBeAg were measured by ELISA assay. HBV X gene was detected by PCR in serum and confirmed by automatic sequencing. Fifty cases of liver cirrhosis and 30 normal controls were involved in serum analysis.
RESULTS: The expression of HBxAg, Fas and FasL in carcinoma tissues was 96%, 84% and 98%, respectively. Staining of HBxAg, Fas and FasL was observed predominately in cytoplasms, no significant difference was found in intensity between HBxAg, Fas and FasL (P > 0.05). HBxAg, Fas and FasL might express in the same area of carcinoma tissues and this co-expression could be found in most patients with HCC. The mean levels of sFas in serum from HCC, cirrhosis and normal controls were 762.29 ± 391.56 μg·L-1, 835.36 ± 407.33 μg·L-1 and 238.27 ± 135.29 μg·L-1. The mean levels of sFasL in serum from HCC, cirrhosis and normal controls were 156.36 ± 9.61 μg·L-1, 173.63 ± 18.74 μg·L-1 and 121.96 ± 7.83 μg·L-1. Statistical analysis showed that both sFas and sFasL in HCC and cirrhosis patients were significantly higher than those in normal controls (P < 0.01). Serum HBV X gene was found in 32% of HCC patients and 46% of cirrhotic patients. There was no significant relationship between serum level of sFas/sFasL and serum X gene detection (P > 0.05). Eight percent of HCC patients with negative HBsAg and HBeAg in serum might have X gene in serum and HBxAg expression in carcinoma tissues.
CONCLUSION: Our data suggest that HBxAg and Fas/FasL system plays an important role in the development of human HCC. Expression of HBxAg can leads to expression of Fas/ FasL system which and reverse apoptosis of hepatocellular carcinoma induced by FasL.
INTRODUCTION
Chronic infection with hepatitis B virus (HBV) is a major risk factor for the development of hepatocellular carcinoma (HCC). The pathogenesis of HBV-induced malignant transformation is incompletely understood[1-3]. HBV X gene encodes a 154 amino acid protein called HBV X protein (HBxAg)[4]. This protein is suspected as an oncogenic molecule responsible for hepatocarcinogenesis because of its multifunctional activities affecting gene transcription, intracellular signal transmission, cell proliferation and apoptotic cell death[5-7]. Of these activities, the best known is its promiscuous transactivation activity[8], which is subjected to complex mechanisms such as protein-protein interaction, regulation of phosphorylation, mRNA stablization and alteration of nucleocytoplasmic translocation[9-11]. Several transgenic mice experiments indicate that mice harboring HBV X gene either develop liver cancer or have accelerated development of neoplasms when they are exposed to other carcinogens[12,13]. HBxAg inhibits the function of tumor suppressor protein P53, which is thought to be an early event in hepatocyte transformation before the later accumulation of inactivating P53 point mutations[14]. HBxAg inhibits apoptosis but also exerts pro-apoptotic effects[15,16]. In addition, HBxAg activates cell signaling cascades involving mitogen-activated protein kinase (MAPK) and Janus family tyrosine kinase (JAK) signal transducer and activators of transcription (STAT) pathways[17,18].
The signaling pathway mediated by "death factors" including TNFR1, Fas and TRAILR1 and their cognate ligands is an important mechanism to regulate apoptosis[19]. Fas is the first identified member of "death receptors" and the crosslinking of Fas by its ligand FasL binding leads to conformational changes of Fas, which result in formation of DISC (death induced signaling complex) followed by activation of Caspase-8 and finally induce apoptosis by cleaving their substrates. The Fas/FasL system is likely to play an important role in the regulation of apoptosis including apoptosis of tumor cells[20]. Up-regulation of Fas in the liver has been demonstrated in active viral hepatitis[21]. Human HCC cell lines have been shown to be resistant to Fas-mediated apoptosis[22], but very limited data are available on FasL expression in HCC tissue and its relationship with HBxAg. We investigated the immunohistochemical expression of HBxAg, Fas and FasL in specimens of HCC. The serum level of sFas, sFasL and HBV X gene was also determined.
MATERIALS AND METHODS
Tissue and blood samples
Tumor samples were randomly collected from 50 patients undergoing hepatic resection at the Affiliated Union Hospital of Fujian Medical University from 1999-2001. Formalin-fixed and paraffin embedded tissues from these samples were used for immunohistochemical analysis. The diagnosis of each tumor was confirmed by pathologists. Blood samples of the tumor patients were taken from the cubital vein on day 3 of hospital admission before the tumor resection. Serum was separated within 30 minutes in a refrigerated centrifuge at 4 °C, centrifuged at 1000 g for 5 minutes and stored at –70 °C until analysis of sFas/sFasL and HBsAg/HBeAg. Anti- HCV analysis was also performed in order to exclude HCV-positive HCC. The sera of 50 patients with hepatic cirrhosis and 30 normal controls from the blood donators were enrolled in this study. Diagnosis of cirrhotic patients was made on the basis of clinical history, clinical examinations, laboratory findings, gastroscopy and ultrasonography. All the patients had decompensated cirrhosis complicated with ascites, variceal bleeding, or hepatic encephalopathy without infection. All the cirrhotic patients had no HCV infection and alcoholic cirrhosis.
Immunohistochemical analysis
Immunohistochemical staining of HBxAg, Fas and FasL was performed by S-P method. Paraffin embedded sections of 4 μm thickness were cut from the resected tumor samples and transferred onto glass slides. The slides were dewaxed and re-hydrated through a graded descending alcohol series (100%, 90%, and 70%). The slides’ endogenous peroxidase activity was blocked by covering the sections with freshly prepared 0.5% H2O2 in methanol, the slides were incubated with horse serum (Vector Laboratories) to block non-specific binding of antibodies. Then, the slides were incubated with a 1:100 dilution of mouse anti-HBx monoclonal antibody, a 1:100 dilution of rabbit polyclonal anti-Fas and anti-FasL antibodies (Maixin-Bio) respectively, washed and incubated with secondary antibody. The slides were subsequently incubated with a freshly prepared 0.1% v/v diaminobenzidine/TBS solution and counter-stained with haematoxylin. Stained slides were differentiated in acid alcohol prior to blueing in Scott’s solution (Sigma), followed by a wash in running tap water. Finally, the sections were dehydrated through a graded ascending (70%, 90%, and 100%) alcohol series and mixed xylenes on the resulting slides, the sites of immunoperoxidase activity were stained brown and nuclei were blue. As a negative control for HBxAg immunohistochemistry, we used nonimmune mouse serum instead of HBxAg antibody. As negative controls of Fas and FasL, we used nonimmune sera of rabbits. The reaction for immunohistochemistry was evaluated as strong (3+), moderate (2+), weak (+) or negative (-).
DNA extraction and PCR amplification
DNA was extracted from the serum by using phenol-chloroform extracting method. In brief, the serum was mixed with phenol-chloroform-iso-pentanol (volume fraction 25:24:1). After centrifugation at 10000 g for 10 minutes at 4 °C. The supernatant was stored in 0.1 M sodium citrate and 100% ethanol for 30 mintues at -20 °C. The resulting pellet was dissolved in sterilized water. The sequences of oligonucleotide primers optimized to X region were 5’-ACGGAATTCATGGCTGCTAGGCTGTG-3’, 3’-ATCCTGCAGAGGTGAAAAAGTTGCAT-5’, respectively. PCR was carried in a final volume of 50 μl containing 5 μl of DNA solution, 20 pmol of each primer, 50 mM of each dNTP, PCR buffer (10 mmol·L-1 Tris-HCl, 50 mmol·L-1 KCl, 1.5 mmol·L-1 MgCl2, 0.001% gelatin), and 2.5 units of Taq DNA polymerase. Each PCR was as follows: 35 cycles of at 93 °C for 30 seconds, at 55 °C for 1 minute and at 72 °C for 1 minute. The resulting PCR products were separated in a 1% agarose gel. Assessment of the positive results of PCR was essentially performed on ethidium bromide-stained gel. To confirm the results, PCR products of a positive sample were analyzed by automatic sequencing (Shanghai Shenggong).
Enzyme-linked immunosorbent assay
Serum level of sFas, sFasL, HBsAg and HBeAg was measured using a sandwich enzyme-linked immunosorbent assay (ELISA). Commercially available ELISA kits of sFas, sFasL, HBsAg and HBeAg (MBL) were used. ELISA was performed according to the manufacturer’s instructions. Briefly, diluted serum samples were added in duplicate to 96-well plates coated with antibody and incubated at 37 °C for 2 hours. After each well was washed five times with washing buffer (saline containing 0.05% Tween20), peroxidase-labeled secondary antibody was added to each well and the plate was incubated at 37 °C for 1 hour. After each well was washed in a similar manner, the plate was incubated with tetramethylbenzine at room temperature for 20 minutes. The reaction was stopped by adding 1 N sulfuric acid. Optical density was measured at 450 nm using a spectrophotometric microtiter plate reader. The concentration of sample was determined from a standard curve.
Statistical analysis
The expression of HBxAg, Fas and FasL was analyzed using Redit analysis. The sFas and sFasL levels were expressed as mean ± SD, and analyzed using t-test. P value less than 0.05 was regarded as significant.
RESULTS
Expression of HBxAg and Fas/FasL system
HBxAg, Fas and FasL were detected in the majority of specimens from HCC patients. The results are summarized in Table 1. In most specimens HBxAg, Fas and FasL were detectable in a large number of carcinoma cells(Figure 1, Figure 2 and Figure 3). No significant differences were found in the expression degrees of HBxAg whether the HCC patients were sero-positive in HBsAg/HBeAg or not (P > 0.05). Staining of HBxAg, Fas and FasL was observed predominantly in cytoplasms, but no significant difference was found in intensity between HBxAg and Fas/FasL system (P > 0.05). HBxAg could also express in membrane of carcinoma cells. Surprisingly, we found that HBxAg, Fas and FasL might express in the same area of HCC tissues and this co-expression could be found in most patients with HCC.
Table 1.
Expression of HBxAg, Fas and FasL in HCC (n = 50)
| Expression | HBxAg (%) | Fas(%) | FasL(%) |
| - | 2(4) | 8(16) | 1(2) |
| + | 5(10) | 9(18) | 4(8) |
| ++ | 11(22) | 9(18) | 12(24) |
| +++ | 32(64) | 24(48) | 33(66) |
Figure 1.
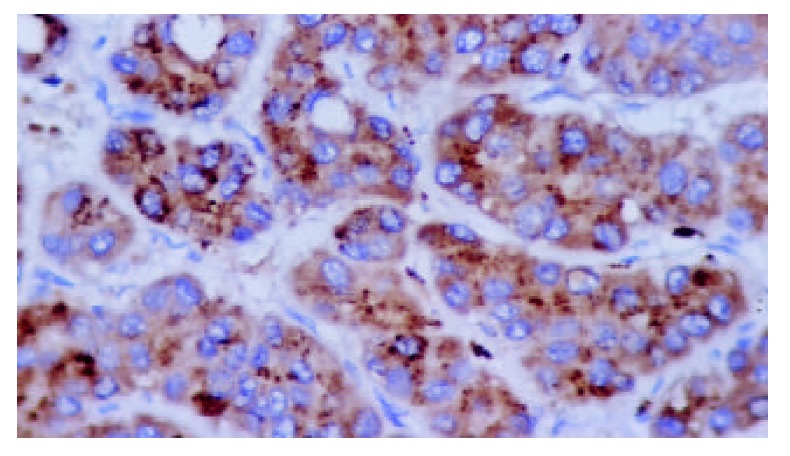
Positive expression of HBxAg in plasma of hepato-cellular carcinoma (S-P stain, ×400).
Figure 2.
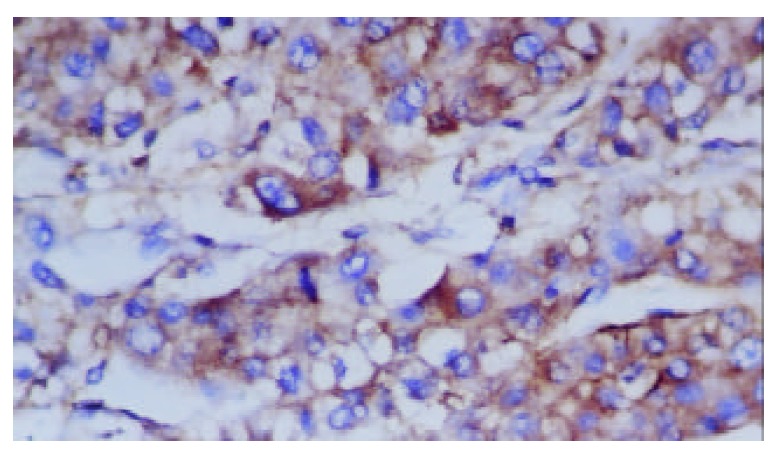
Positive expression of Fas in plasma of hepatocellu-lar carcinoma (S-P stain, ×400).
Figure 3.
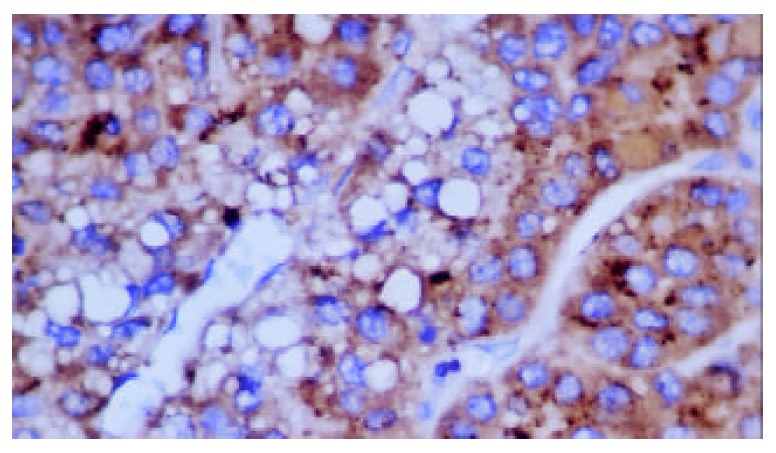
Positive expression of FasL in plasma of hepatocellu-lar carcinoma (S-P stain, ×400).
Serum level of sFas and sFasL
Concentrations of sFas and sFasL in HCC, cirrhotic patients and normal controls are shown in Table 2. The serum levels of sFas and sFasL in both HCC and cirrhotic patients were significantly higher than those of normal controls (P < 0.01), but there was no significant difference between cirrhosis and HCC patients in serum level of sFas and sFasL (P > 0.05). In addition, we did not find a correlation between sFas/sFasL concentrations and serum HBV X gene detection (P > 0.05).
Table 2.
Serum levels of sFas and sFasL in patients with HCC and Cirrhosis (μg·L-1)
| Group | n | sFas | sFasL |
| HCC | 50 | 762.29 ± 391.56a | 158.36 ± 9.67a |
| Cirrhosis | 50 | 835.63 ± 407.33a | 173.63 ± 18.74a |
| Control | 30 | 238.27 ± 135.29 | 121.96 ± 7.83 |
P < 0.01, vs control.
Determination of X gene and HBsAg/HBeAg
The positive rates of HBV X gene, HBsAg and HBeAg in HCC and cirrhotic patients are shown in Table 3. Serum HBV X gene was found in 32% of HCC patients and 46% of cirrhotic patients. Eight percent of HCC patients with negative HBsAg and HBeAg in serum might have X gene in serum and HBxAg expression in carcinoma tissues.
Table 3.
Positive rates of HBV X gene, HBsAg and HBeAg in HCC and cirrhotic patients (%)
| Group | HBV X gene | HBsAg | HBeAg |
| HCC | 32 | 86 | 34 |
| Cirrhosis | 46 | 82 | 38 |
The PCR products of X gene positive samples were analyzed by automatic sequencing and the resulting sequence was proved by Genebank.
DISCUSSION
HBV is one of the several agents causing infectious hepatitis, such as acute and chronic viral hepatitis. A strong association was found between chronic HBV infection and the development of HCC in our study. Multiple factors including damage caused by inflammatory cytokines, integration of viral DNA into host cell genomes, host genomic instability, activation of cellular oncogenes, and induction of cell survival pathways have been implicated as causes leading to HCC. However, HBV X gene and HBxAg play a major role in viral infection and carcinogenesis. X gene was the most frequently integrated protein of HBV DNA found in hepatocyte chromosomes during the development of HCC[23]. HBxAg was expressed in these integrated fragments, although no other viral proteins were present in most tumor cells. Recent studies using cDNA microarray analysis revealed that HBxAg expression in HepG2 cells could up- or down-regulate the expression of 39 genes[24]. These genes have a variety of cellular functions including oncogenesis, cell cycle regulation and cell adhesion. Our results demonstrated high levels of HBxAg expression in cirrhotic and HCC cells. Intracellular localization showed that HBxAg was predominant in cytoplasms. Serum levels of sFas and sFasL in both HCC and cirrhotic patients were significantly higher than those in controls. This could be explained by previous observations that X gene was greatly correlated with the development of cirrhosis and integrated into host chromosomes during chronic infection. It seems likely that HBxAg contributes to the initiation of tumor formation in the liver during the process of chronic active hepatitis and cirrhosis. However, whether this process is induced by Fas/FasL system remains to be elucidated.
With PCR detection, we found that some HBsAg and HBeAg negative HCC patients were X gene positive. These results were consistent with a previous report that circulating HBV X gene was detectable in a high proportion of Japanese HCC patients without HBsAg[25]. It could be explained as follows: First, a low level of viremia might exist and hardly result in expression[26]. Second, variant HBV clones might predominantly emerge, the variant HBV might have lost its common "A" determinant of HBsAg and result in loss of HBsAg[27]. Third, disrupted form of HBV was integrated into cellular chromosomes, resulting in failure to produce viral protein translated from deleted DNA[23]. Our results suggested that negative HBsAg and HBeAg HCC patients were required to examine HBV DNA in serum or in liver tissues in order to better understand HBV involvement in the etiology.
Our results indicated that HCC cells could strongly express both Fas and FasL in the same area of carcinoma. What is the target of this ligand There are some hypotheses. First, because HCC cells can co-express Fas and FasL, they may undergo apoptosis induced not only by activated FasL-positive lymphocytes but also by their own FasL in an autocrine or paracrine manner. Such a fratricide mechanism may be involved in chemotherapeutic drug-induced death of HCC cells, as evidenced by the observations that bleomycin upregulated the expression of Fas and FasL in HepG2 cells, and that apoptosis induced by this drug was almost completely inhibited by antibodies that interfere with Fas/FasL interaction[28]. Second, FasL expressed on HCC cells might be important in their infiltration as well as dissemination into the liver[29], as has been demonstrated in the hepatic metastasis of colon cancer cells[30]. The third possibility is the so-called “counterattack” hypothesis. FasL expressed on tumor cells may be engaged with Fas receptors expressed on the surfaces of antitumor immune cells, causing them to undergo apoptosis. Strand et al[31] reported that HepG2 cells, expressing FasL after treatment with cytostatic drugs, could kill Fas-positive Jurkat lymphocytes, providing the first evidence that FasL is functional on HCC. Although the idea that tumor cells counterattack immune cells and escape from antitumor immunity has attracted popular attention, several investigators reported that the presence of surface FasL might be only an inflammatory response. The presence and function of FasL on HCC cells have become the subject of hot debate[32,33]. But why this happened only in HCC Ito et al[34] examined the expression of Fas/FasL system in human HCC tissues and found that FasL expressing HCCs were moderately or poorly differentiated carcinomas. But FasL expression was not a critical factor in determining intrahepatic tumor spread. Fukuzawa et al[35] reported some conflicting results. They examined the expression of Fas and FasL in human HCC tissues, and found that Fas/FasL expression decreased in proportion to the malignancy of tumor cells. According to the concept that tumor cells become resistant to apoptosis during disease progression, their observation implies that FasL expressed on HCC may be involved in a self-regulatory mechanism of apoptosis, rather than being involved in a counterattack against immune cells or infiltration into the liver. Indeed, so far, HCC cells have often been found to be resistant to Fas-mediated apoptosis, despite their expression of Fas receptors[36]. Thus, there is no clinical evidence to directly support the idea that FasL expressed on HCCs was involved in their immune escape or infiltration into the liver. Because the mitochondrial death pathway was predominantly involved in Fas-mediated apoptosis in liver cells[37], overexpression of HBxAg might contribute to FasL-resistance in HCC. We found that Fas, FasL and HBxAg might express in the same area of HCC tissues. The precise mechanism of the co-expression of HBxAg and Fas/FasL system remains to be established. Recently Terradillos et al[38] reported that the proapoptotic activity of HBxAg could overcome or bypass the inhibitory effect of Bcl-2 against Fas cytotoxicity. The inability of Bcl-2 to protect HBxAg-expressing hepatocytes against Fas cytotoxicity might be resulted either from inactivation of Bcl-2 or from execution of a Bcl-2-independent death pathway. These indicate that the dominant function of HBxAg upon Bcl-2-regulated apoptosis might play an important role in carcinogenesis. It is known that HBxAg could activate various cellular transcription factors such as AP-1 and NF-kB[39]. In addition, HBxAg may induce FasL expression through activation of cellular transcription factors. Although there is no confirmative report of transcription factors regulating FasL expression, it has been found that the enhancer region of FasL gene has a putative binding site of NF-kB[40] and the important role of NF-kB has been reported in FasL gene activation. Because HBxAg could activate NF-kB, it is possible that HBxAg might induce FasL expression through NF-kB activation[41,42]. Our results also suggest that the expression of HBxAg can lead to expression of Fas/FasL system, which might not reflex apoptosis of hepatocellular carcinoma induced by FasL. This implies that Fas/FasL expression by itself, cannot be used as a reliable marker of apoptosis in HCC.
Footnotes
Supported by the Science Foundation of Fujian Province, No.99-Z-162
Edited by Wang XL
References
- 1.Koike K, Tsutsumi T, Fujie H, Shintani Y, Kyoji M. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62 Suppl 1:29–37. doi: 10.1159/000048273. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WL, Gu GY, Hu M. Expression and significance of HBV genes and their antigens in human primary intrahepatic cholangiocarcinoma. World J Gastroenterol. 1998;4:392–396. doi: 10.3748/wjg.v4.i5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh CT. Hepatitis B virus X protein: searching for a role in hepatocarcinogenesis. J Gastroenterol Hepatol. 2000;15:339–341. doi: 10.1046/j.1440-1746.2000.02166.x. [DOI] [PubMed] [Google Scholar]
- 5.Lian Z, Liu J, Pan J, Satiroglu Tufan NL, Zhu M, Arbuthnot P, Kew M, Clayton MM, Feitelson MA. A cellular gene up-regulated by hepatitis B virus-encoded X antigen promotes hepatocellular growth and survival. Hepatology. 2001;34:146–157. doi: 10.1053/jhep.2001.25545. [DOI] [PubMed] [Google Scholar]
- 6.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin LL, Su JJ, Li Y, Yang C, Ban KC, Yian RQ. Expression of IGF- II, p53, p21 and HBxAg in precancerous events of hepatocarcinogenesis induced by AFB1 and/or HBV in tree shrews. World J Gastroenterol. 2000;6:138–139. doi: 10.3748/wjg.v6.i1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 9.Diao J, Garces R, Richardson CD. X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001;12:189–205. doi: 10.1016/s1359-6101(00)00034-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang XZ, Jiang XR, Chen XC, Chen ZX, Li D, Lin JY, Tao QM. Seek protein which can interact with hepatitis B virus X protein from human liver cDNA library by yeast two-hybrid system. World J Gastroenterol. 2002;8:95–98. doi: 10.3748/wjg.v8.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong HJ, Hong SH, Lee MY, Kim HD, Lee JW, Cheong J. Direct binding of hepatitis B virus X protein and retinoid X receptor contributes to phosphoenolpyruvate carboxykinase gene transactivation. FEBS Lett. 2000;483:114–118. doi: 10.1016/s0014-5793(00)02091-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 13.Slagle BL, Lee TH, Medina D, Finegold MJ, Butel JS. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol Carcinog. 1996;15:261–269. doi: 10.1002/(SICI)1098-2744(199604)15:4<261::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 15.Pollicino T, Terradillos O, Lecoeur H, Gougeon ML, Buendia MA. Pro-apoptotic effect of the hepatitis B virus X gene. Biomed Pharmacother. 1998;52:363–368. doi: 10.1016/s0753-3322(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 16.Xu ZH, Zhao MJ, Li TP. p73beta inhibits transcriptional activities of enhancer I and X promoter in hepatitis B virus more efficiently than p73alpha. World J Gastroenterol. 2002;8:1094–1097. doi: 10.3748/wjg.v8.i6.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang-Park S, Lee JH, Shin JH, Lee YI. Activation of the IGF-II gene by HBV-X protein requires PKC and p44/p42 map kinase signalings. Biochem Biophys Res Commun. 2001;283:303–307. doi: 10.1006/bbrc.2001.4767. [DOI] [PubMed] [Google Scholar]
- 18.Arbuthnot P, Capovilla A, Kew M. Putative role of hepatitis B virus X protein in hepatocarcinogenesis: effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J Gastroenterol Hepatol. 2000;15:357–368. doi: 10.1046/j.1440-1746.2000.02069.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 20.Takehara T, Hayashi N. Fas and fas ligand in human hepatocellular carcinoma. J Gastroenterol. 2001;36:727–728. doi: 10.1007/s005350170040. [DOI] [PubMed] [Google Scholar]
- 21.Ibuki N, Yamamoto K, Yabushita K, Okano N, Okamoto R, Shimada N, Hakoda T, Mizuno M, Higashi T, Tsuji T. In situ expression of Granzyme B and Fas-ligand in the liver of viral hepatitis. Liver. 2002;22:198–204. doi: 10.1046/j.0106-9543.2002.00tes.x. [DOI] [PubMed] [Google Scholar]
- 22.Natoli G, Ianni A, Costanzo A, De Petrillo G, Ilari I, Chirillo P, Balsano C, Levrero M. Resistance to Fas-mediated apoptosis in human hepatoma cells. Oncogene. 1995;11:1157–1164. [PubMed] [Google Scholar]
- 23.Paterlini P, Poussin K, Kew M, Franco D, Brechot C. Selective accumulation of the X transcript of hepatitis B virus in patients negative for hepatitis B surface antigen with hepatocellular carcinoma. Hepatology. 1995;21:313–321. [PubMed] [Google Scholar]
- 24.Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000;272:525–530. doi: 10.1006/bbrc.2000.2801. [DOI] [PubMed] [Google Scholar]
- 25.Shiota G, Oyama K, Udagawa A, Tanaka K, Nomi T, Kitamura A, Tsutsumi A, Noguchi N, Takano Y, Yashima K, et al. Occult hepatitis B virus infection in HBs antigen-negative hepatocellular carcinoma in a Japanese population: involvement of HBx and p53. J Med Virol. 2000;62:151–158. doi: 10.1002/1096-9071(200010)62:2<151::aid-jmv5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Niitsuma H, Ishii M, Miura M, Kobayashi K, Toyota T. Low level hepatitis B viremia detected by polymerase chain reaction accompanies the absence of HBe antigenemia and hepatitis in hepatitis B virus carriers. Am J Gastroenterol. 1997;92:119–123. [PubMed] [Google Scholar]
- 27.Carman WF. S gene variation of HBV. Acta Gastroenterol Belg. 2000;63:182–184. [PubMed] [Google Scholar]
- 28.Müller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roskams T, Libbrecht L, Van Damme B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma; can cancer induce suicide in peritumoural cells. J Pathol. 2000;191:150–153. doi: 10.1002/(SICI)1096-9896(200006)191:2<150::AID-PATH612>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci U S A. 1997;94:6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion. Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell J, Houston A, Bennett MW, O'Sullivan GC, Shanahan F. Immune privilege or inflammation Insights into the Fas ligand enigma. Nat Med. 2001;7:271–274. doi: 10.1038/85395. [DOI] [PubMed] [Google Scholar]
- 33.Restifo NP. Countering the 'counterattack' hypothesis. Nat Med. 2001;7:259. doi: 10.1038/85357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito Y, Monden M, Takeda T, Eguchi H, Umeshita K, Nagano H, Nakamori S, Dono K, Sakon M, Nakamura M, et al. The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br J Cancer. 2000;82:1211–1217. doi: 10.1054/bjoc.1999.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuzawa K, Takahashi K, Furuta K, Tagaya T, Ishikawa T, Wada K, Omoto Y, Koji T, Kakumu S. Expression of fas/fas ligand (fasL) and its involvement in infiltrating lymphocytes in hepatocellular carcinoma (HCC) J Gastroenterol. 2001;36:681–688. doi: 10.1007/s005350170031. [DOI] [PubMed] [Google Scholar]
- 36.Shin EC, Shin JS, Park JH, Kim H, Kim SJ. Expression of fas ligand in human hepatoma cell lines: role of hepatitis-B virus X (HBX) in induction of Fas ligand. Int J Cancer. 1999;82:587–591. doi: 10.1002/(sici)1097-0215(19990812)82:4<587::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 38.Terradillos O, de La Coste A, Pollicino T, Neuveut C, Sitterlin D, Lecoeur H, Gougeon ML, Kahn A, Buendia MA. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene. 2002;21:377–386. doi: 10.1038/sj.onc.1205110. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xu Z, Zheng Y, Johnson DL, Ou JH. Regulation of hepatocyte nuclear factor 1 activity by wild-type and mutant hepatitis B virus X proteins. J Virol. 2002;76:5875–5881. doi: 10.1128/JVI.76.12.5875-5881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Wang L, Medan D, Toledo D, Huang C, Chen F, Shi X, Rojanasakul Y. Regulation of Fas (CD95)-induced apoptosis by nuclear factor-kappaB and tumor necrosis factor-alpha in macrophages. Am J Physiol Cell Physiol. 2002;283:C831–C838. doi: 10.1152/ajpcell.00045.2002. [DOI] [PubMed] [Google Scholar]
- 41.Kasibhatla S, Genestier L, Green DR. Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor kappaB. J Biol Chem. 1999;274:987–992. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- 42.Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340–344. doi: 10.3748/wjg.v7.i3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


