Abstract
AIM: To investigate the incidence of CD117-positive immunohistochemical staining in previously diagnosed GI tract stromal tumors (GIST) and to analyze the tumors’ clinical manifestations and prognostic factors.
METHODS: We retrospectively reviewed 91 cases with a previous diagnosis of GI stromal tumor, leiomyoma, or leiomyosarcoma. Tissue samples were assessed with CD117, CD34, SMA and S100 immunohistochemical staining. Clinical and pathological characteristics were analyzed for prognostic factors.
RESULTS: CD117 was positive in 81 (89%) of 91 tissue samples. There were 59 cases (72.8%) positive for CD34, 13 (16%) positive for SMA, and 12 (14.8%) positive for S100. There was no gender difference in patients with CD117-positive GIST. Their mean age was 65 years. There were 44 (54%) tumors located in the stomach and 29 (36%) in the small intestine. The most frequent presenting symptoms were abdominal pain and GI bleeding. The mean tumor size was 7.5 ± 5.7 cm. There were 35 cases (43.2%) with tumors > 5 cm. The tumor size correlated significantly with tumor mitotic count and resectability. Tumor size, mitotic count, and resectability correlated significantly with tumor recurrence and survival. There was recurrent disease in 39% of our patients, and their mean survival after recurrence was 16.6 months. Most recurrences were at the primary site or metastatic to the liver. Twenty-six percent of our patients died of their disease.
CONCLUSION: Traditional histologic criteria are not specific enough to diagnose GIST. This diagnosis must be confirmed with CD117 immunohistochemical staining. Prognosis is dependent on tumor size, mitotic count, and resectability.
INTRODUCTION
Leiomyoma of the gastrointestinal (GI) tract has a typical pathologic picture with parallel spindle cells arranged in a fascicular pattern[1,2]. However, a number of tumors have atypical findings, with epithelioid cells or pleomorphic cells instead of spindle cells or even characteristics of neurons that stain for neuron-specific enolase. Therefore, pathologists used the umbrella term “stromal tumor” to refer to such atypical mesenchymal tumors. It has been thought that these tumors might originate from mesenchymal or stromal cells within the muscle layer of the GI tract[3-5]. Molecular biochemical studies have revealed expression of kit protein (CD117) in the primitive mesenchymal cells. CD117 can be demonstrated by immunohistochemical staining using anti-kit antibodies[6]. It has been found that most mesenchymal or stromal cell tumors of the GI tract are positive for CD117[7]. Leiomyoma or leiomyosarcoma of the GI tract with typical pathologic features also stain positive for CD117. There is therefore now a consensus that these tumors originate from a common cell and that the term gastrointestinal stromal tumors (GIST) be reserved for CD117-positive neoplasms[8].
These tumors arise from primitive cells with dual characteristics of muscle and neural cells, similar to interstitial cell of Cajal[9]. Cytogenetic analysis shows that most of these tumors have a mutation of the c-kit gene. Oncogenic mutations enable the kit protein, a transmembrane tyrosine kinase receptor, to phosphorylate various substrate proteins, leading to activation of signal transduction cascades which regulate cell proliferation, apoptosis, chemotaxis, and adhesion[9]. Additional chromosomal derangements in 22q or 24q may promote GIST development[10,11]. C-kit gene mutation and kit protein over-expression appear to be essential for the pathogenesis of GIST. Imatinib is a tyrosine kinase inhibitor that has resulted in remarkable myxoid degeneration and fibrosis of GIST in clinical trials[12]. Since specifically targeted therapy is thus becoming available, it is important to know if tumors with histology consistent with GIST in fact express the kit protein[13]. We retrospectively reviewed cases in our hospital in the past 13 years with a tissue diagnosis of leiomyoma, leiomyosarcoma, or GIST to assess their immunohistochemical staining patterns, clinical manifestations, and prognostic factors.
MATERIALS AND METHODS
Patients
Using our hospital database, we collected records with a pathologic diagnosis of leiomyoma or leiomyosarcoma or stromal cell tumor of the GI tract from 1988 until 2001. There were 91 such cases. All the patients had undergone surgical resection of their tumor. We recorded the patients’ age, gender, clinical manifestations, tumor site, maximal tumor diameter, duration of surgery, resectability of the tumor, the presence and date of local recurrence or distance metastasis, and the outcome, including date of death. The data of patients who were still alive in January 2002 were censored.
Immunohistochemistry
The tumor samples from all the 91 cases were examined for various markers by using immunohistochemistry with commercially available antibodies against CD117 (1:50 dilution), S-100 protein (1:1500), desmin (1:50), and SMA (1:100) (Dako, Carpinteria, CA). Immunoreaction was detected according to the manufacturer’s instructions (Ventana Medical Systems, Tucson, AZ). The risk of aggressive behavior of the tumors was calculated according to the NIH consensus statement of 2001[13]. Briefly, a tumor size < 2 cm and mitotic count < 5/50 high power fields (HPF) was graded as very low risk, a tumor between 2 and 5 cm and a mitotic count < 5/50 HPF as low risk, a tumor < 5 cm and mitotic count between 5/50 HPF and 10/50 HPF or a tumor between 5 and 10 cm and a mitotic count less than 5/50 HPF as intermediate risk, and a tumor > 10 cm or mitotic count > 10/50 HPF or a tumor > 5 cm and mitotic count > 5/50 HPF as high risk.
Statistic analysis
Statistic analysis was performed with SPSS software (SPSS for Window 9.0). Student’s t test was used to compare continuous variables. And a χ2 test was used for dichotomous variables. Survival was calculated from the day of diagnosis until death or the last day of a patient’s visit to the outpatient clinic. The disease-free survival was calculated from the first diagnosis until tumor recurrence or distant metastases were found. Kaplan Meier analysis with a log rank test was used to compare survival and disease-free survival. The Cox proportional hazard method was used to evaluate prognostic factors.
RESULTS
Of the 91 tissue samples tested, 81 (89%) were positive for CD117 staining. There were no significant differences in the distribution of previous pathologic diagnoses between CD117-positive and –negative samples. The highest incidence of CD117 positivity was in samples previously diagnosed as malignant GIST (Table 1). Of the 10 cases negative for CD117, 6 tumors were located in the stomach, 2 in the small bowel, and 1 each in the colon and retroperitoneum. Three of these tumors were positive for CD34, 3 for SMA, and 3 S100 (Table 2). These 10 cases were excluded from further analysis.
Table 1.
Immunohistochemical staining for CD117 in tumors with a previous pathologic diagnosis of leiomyoma, leiomyo-sarcoma or GIST
| Previous pathologic diagnosis | CD117-positive (n) CD117-negative (n) | |
| Leiomyoma | 6 (86%) | 1 (14%) |
| Leiomyosarcoma | 14 (78%) | 4 (22%) |
| Benign GIST | 10 (77%) | 3 (23%) |
| Malignant GIST | 51 (96%) | 2 (4%) |
| Total | 81 (89%) | 10 (11%) |
Table 2.
Previous pathologic diagnoses, tumor site, and other immunohistochemical markers in CD117-negative patients
| Previous pathologic diagnosis | n | Site | CD34 | SMA | S100 |
| Leiomyoma | 1 | Stomach | + | - | - |
| Leiomyosarcoma | 1 | Stomach | - | - | - |
| 1 | Small bowel | - | - | + | |
| 1 | Colon | - | - | - | |
| 1 | Other | - | + | - | |
| Benign GIST | 2 | Stomach | - | - | + |
| 1 | Stomach | + | + | + | |
| Malignant GIST | 1 | Stomach | + | - | - |
| 1 | Small bowel | - | + | - |
Of the 81 patients with CD117-positive tumors, there were 42 males and 39 females. Their ages ranged from 21 to 91 years (mean 65.2 ± 13.6 years), with over half (44/81, 54%) between 60 and 79 years of age (Table 3). Additional immunohistochemical staining showed that the tissue samples were positive for CD34 in 59 cases (73%), for SMA in 13 (16%), and for S100 in 12 cases (15%).
Table 3.
Age distribution of patients with CD117-positive GIST
| Age range | No. of cases (n) | Percentage (%) |
| < 30 | 1 | 1 |
| 30-39 | 2 | 3 |
| 40-49 | 7 | 9 |
| 50-59 | 15 | 19 |
| 60-69 | 19 | 24 |
| 70-79 | 26 | 32 |
| 80-89 | 10 | 12 |
| > 90 | 1 | 1 |
| Total | 81 | 100 |
The tumors were located in the stomach in 44 cases (54%), the small intestine in 29 (36%), the colon in 5 (6%), and the retroperitoneum or mesentery in 3 (4%). The most frequent presenting symptoms were GI tract bleeding and abdominal pain in 27 cases (33%) each, abdominal fullness and discomfort in 14 (17%). Four patients had anemia on presentation, 7 had a palpable abdominal mass, and 1 presented with vaginal bleeding due to a rectal GIST involving the uterus. One patient was asymptomatic and was incidentally found to have a 3.5 cm GIST in her stomach on routine physical examination.
On pathology of the resected tumors, the mean size was 7.5 ± 5.7 cm, 10 (12%) were < 2 cm, 25 (31%) between 2 and 5 cm, 27 (33%) between 5 and 10 cm, and 19 (24%) > 10 cm. Mitotic counts were < 5/50 HPF in 38 cases (47%), between 5 and 10/50 HPF in 21 (26%), and > 10/50 HPF in 22 (27%). There was a significant correlation between tumor size and mitotic count (Pearson correlation coefficient = 0.541, P < 0.001, Table 4). There was ulceration of the tumor in 47% (38/81), hemorrhage in 53% (43), tumor necrosis in 54% (44), and tumor perforation in 25% (20). Sixty-two (77%) of the tumors were completely resected. There was a significant correlation between tumor size and resectability (Pearson correlation coefficient = 0.505, P < 0.001, Table 5).
Table 4.
Correlation between tumor size and mitotic count in CD117-positive GIST
| Tumor size |
Mitotic counts/50 HPFa |
||
| < 5 | 5-10 | > 10 | |
| ≤ 2 cm | 10 (100%) | 0 | 0 |
| 2 to 5 cm | 14 (56%) | 8 (32%) | 3 (12%) |
| 5 to 10 cm | 12 (44%) | 8 (30%) | 7 (26%) |
| > 10 cm | 2 (11%) | 5 (26%) | 12 (63%) |
Pearson correlation = 0.541, P < 0.001,
HPF = High power fields.
Table 5.
Correlation between tumor size and resectability in CD117-positive GIST
| Tumor sizes | Complete resection | Incomplete resection |
| ≤ 2 cm | 10 (100%) | 0 |
| 2 to 5 cm | 25 (100%) | 0 |
| 5 to 10 cm | 18 (69%) | 8 (30.8%) |
| > 10 cm | 9 (47%) | 10 (52.6%) |
Pearson correlation = 0.505, P < 0.001.
There were 31 patients (39%) with local recurrence or distant metastasis, which was statistically associated with tumor size (P < 0.001, Figure 1). The 31 included 3 of 25 (12%) whose primary tumor had been between 2 and 5 cm, 13 of 27 (48%) whose tumor had been between 5 and 10 cm, and 15 of 19 (79%) whose tumor had been greater than 10 cm. Recurrence was also significantly associated with mitotic count, occurring in 5 of 38 (13%) with counts < 5/50HPF, 10 of 21 (48%) with counts between 5 and 10/50HPF, 16 of 22 (73%) with counts > 10/50HPF (P < 0.001, Figure 2).
Figure 1.
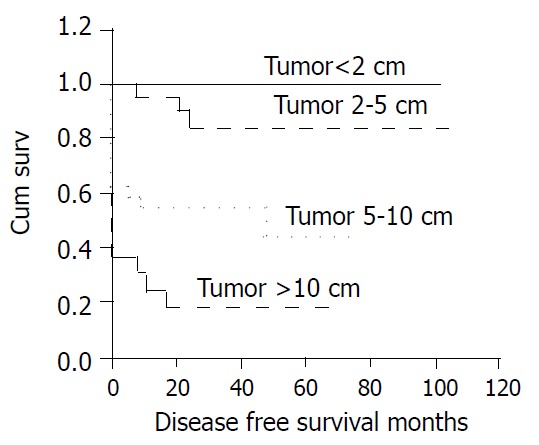
Cumulative disease-free survival for patients with CD117-positive GIST based on tumor size.
Figure 2.
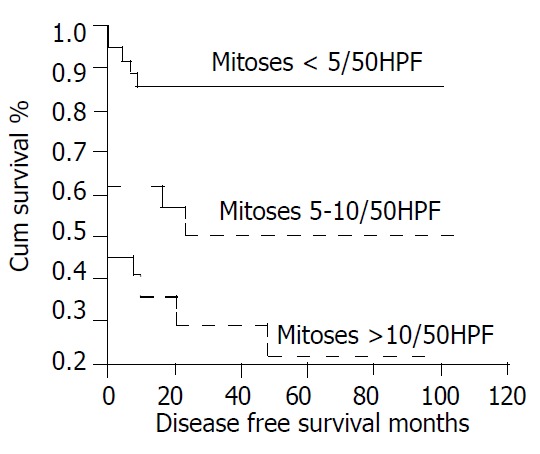
Cumulative disease-free survival for patients with CD117-positive GIST based on mitotic count.
Twenty-one patients (26%) died during follow up. The mean survival time after recurrence was 16.6 ± 14.5 months. Tumor size, mitotic count, and resectability were significantly associated with survival (Figure 3, Figure 4, Figure 5). The 5-year survival was 78% (mean survival 85.5 ± 8.13 months) for patients with tumors between 2 and 5 cm, 57% (mean survival 53.8 ± 6.09 months) for those with tumors between 5 and 10 cm, and 27% (mean survival 30.0 ± 6.41 months) for those with tumors larger than 10 cm (Figure 3, log rank test, P < 0.001). The 5-year survival was 76% (mean survival 80.7 ± 6.77 months) for patients with mitotic counts < 5/50HPF, 73% (mean survival 80.9 ± 10.5 months) for those with counts between 5 and 10/50HPF, and 31% (mean survival time 44.2 ± 9.53 months) for those with counts > 10/50HPF (Figure 4, P < 0.02). Patients whose tumors were completely resected had a 5-year survival of 73% (mean survival 82.0 ± 5.78 months) while those without complete resection had a 5-year survival of 26% (mean survival 27.0 ± 5.23 months) (Figure 5, P < 0.05).
Figure 3.
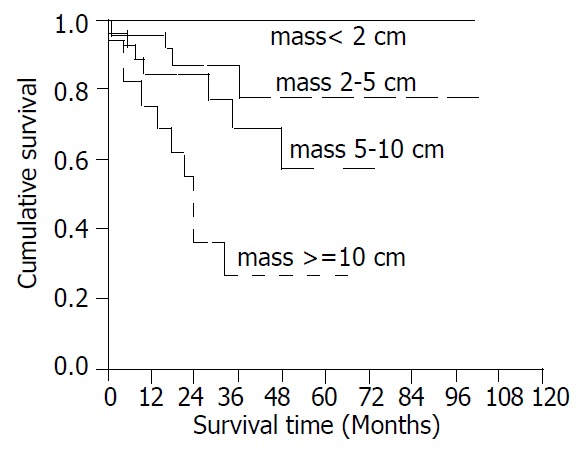
Cumulative survival for patients with CD117-posi-tive GIST based on tumor size.
Figure 4.
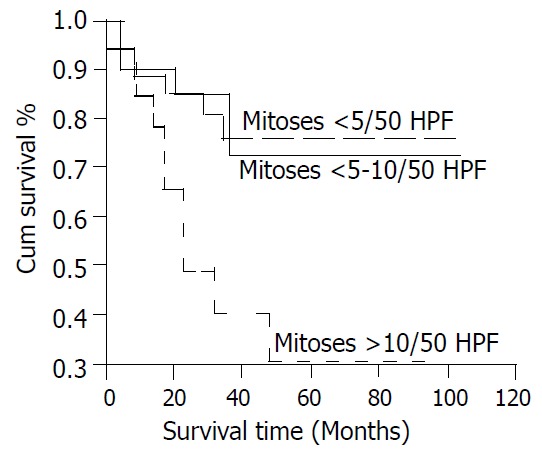
Cumulative survival for patients with CD117-posi-tive GIST based on mitotic count.
Figure 5.
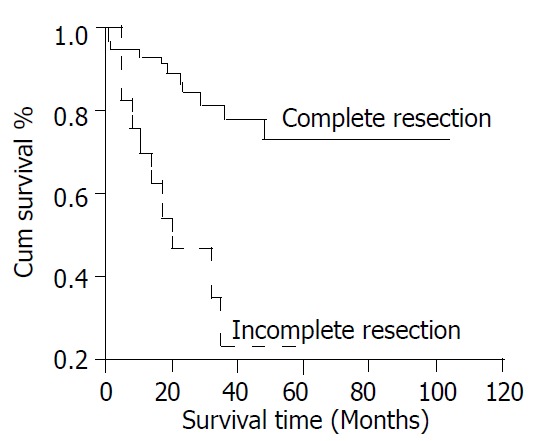
Cumulative survival for patients with CD117-posi-tive GIST based on resectability.
The survival was also significantly correlated with the score for risk of aggressive behaviors (Figure 6, P < 0.0001).
Figure 6.
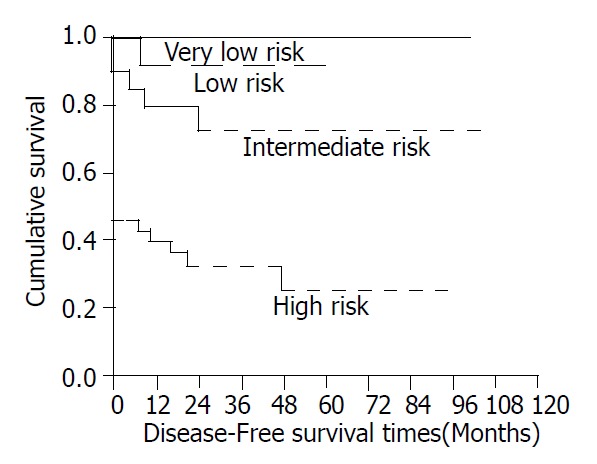
Cumulative disease-free survival for patients with CD117-positive GIST based on risk of aggressive behaviors.
DISCUSSION
Before the development of imatinib, surgical resection was the only treatment for tumors classified pathologically as GIST[14,15]. Tumor size was an independent prognostic factor, with tumors > 10 cm having a disease-specific 5-year survival of only 20% after resection[16]. The tumors are often soft and fragile and prone to rupture or intraperitoneal dissemination during resection. The primary goal of surgery is complete resection of the disease, because rupture at surgery is another poor prognostic factor[17]. A number of other pathologic features have also been correlated with survival, including mitotic index, aneuploidy, cellular morphometry, proliferative index, and percent S-phase fraction[18]. Most patients with malignant stromal tumor or leiomyosarcoma have had either local recurrence or metastasis to the liver[14]. Locally recurrent tumors were usually not amenable to complete resection because of peritoneal implantation[19], nor has chemotherapy been very effective[20]. Solitary liver metastasis could be surgically resected[21], but multiple liver metastases are difficult to manage. Transcatheter arterial embolization has been used, but the partial response rate was low.
Our study confirms the contention that pathology alone is inadequate to confirm GIST by the current diagnostic criterion of CD117 immunohistochemical staining. Of the 91 tissue samples in our series, only 81 (89%) were CD117-positive. Of the 10 negative samples, 2 were positive for SMA but negative for CD34 and S100 and therefore may be leiomyosarcomas. Another 3 were positive for S100 but negative for CD34 and SMA and therefore may be schwannomas. One case was positive and 2 were negative for all the 3 markers (that is, CD34, SMA and S100). The diagnosis in these cases is still unclear. A search for a gene mutation other than in the C-kit gene, for example PDGFR, might be helpful to clarify the diagnosis.
Among the 81cases positive for CD117, 73% were also positive for CD34, 16% for SMA, and 15% for S100. These data are similar to that of previous reports.
Esophagogastroduodenoscopy is usually performed in Taiwan for any patient complaining of abdominal discomfort. As a result, we can, on occasion, find small submucosal tumors in the stomach. Most of our cases with a tumor less than 5 cm were located in the stomach. This accounted for 43% of our patients with tumors less than 5 cm in size, all of whom had completely resectable lesions. None of our patients with tumors less than 4 cm in size died (data not shown). Our findings confired that complete resection of the tumor is one of the most important factors related to survival[16]. In our analysis, tumor size was significantly correlated with mitotic count and resectability, with larger tumors usually having higher mitotic counts and being more likely to be unresectable. Most of our patients with small intestinal GIST had lesions larger than 5 cm and a poorer outcome than those with gastric tumors. At the present time it is probably unwise to characterize any GIST benign[13]. This diagnosis mandates treatment.
Tumor grade was correlated, as expected, with mortality. No patients with very low grade tumors died, but the mortality increased as tumor grade increased, 14% for low grade tumor, 30% for moderate grade, and 35% for high grade. Thirty-nine percent of our patients had recurrence, with a mean survival of 16.6 months after recurrence. Twenty-six percent of our patients died of their diseases.
We have done a clinical trial using imatinib 800 mg daily for 3 months in 11 patients with recurrent GIST. There was partial response in 3 cases and stable disease in 6 cases (unpublished data).
In conclusion, two recent developments are important with regard to GIST. The first is the ability to diagnose the tumor based on immunohistochemical staining. Our study confirms that traditional histologic criteria alone are not specific enough. The second is the development of imatinib, which specifically targets CD117, providing us a new tool to combat GIST. Early diagnosis and complete resection remain the ideal, as surgical removal provides the best prognosis. However, specific molecular diagnosis and treatment are expanding our ability to manage this disease.
Footnotes
Edited by Zhu LH
References
- 1.Ranchod M, Kempson RL. Smooth muscle tumors of the gastrointestinal tract and retroperitoneum: a pathologic analysis of 100 cases. Cancer. 1977;39:255–262. doi: 10.1002/1097-0142(197701)39:1<255::aid-cncr2820390139>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer. 1978;42:1375–1384. doi: 10.1002/1097-0142(197809)42:3<1375::aid-cncr2820420348>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Casper ES. Gastrointestinal stromal tumors. Curr Treat Options Oncol. 2000;1:267–273. doi: 10.1007/s11864-000-0039-4. [DOI] [PubMed] [Google Scholar]
- 4.Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen's disease with minimal smooth muscle differentiation. Hum Pathol. 1984;15:229–232. doi: 10.1016/s0046-8177(84)80184-7. [DOI] [PubMed] [Google Scholar]
- 5.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 7.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–1572. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J, Sjögren H, Meis-Kindblom JM, Stenman G, Aman P, Kindblom LG. The complexity of KIT gene mutations and chromosome rearrangements and their clinical correlation in gastrointestinal stromal (pacemaker cell) tumors. Am J Pathol. 2002;160:15–22. doi: 10.1016/S0002-9440(10)64343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunawan B, Bergmann F, Höer J, Langer C, Schumpelick V, Becker H, Füzesi L. Biological and clinical significance of cytogenetic abnormalities in low-risk and high-risk gastrointestinal stromal tumors. Hum Pathol. 2002;33:316–321. doi: 10.1053/hupa.2002.32216. [DOI] [PubMed] [Google Scholar]
- 12.Croom KF, Perry CM. Imatinib mesylate: in the treatment of gastrointestinal stromal tumours. Drugs. 2003;63:513–522; discussion 523-524. doi: 10.2165/00003495-200363050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81–89. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 14.Blanke CD, Eisenberg BL, Heinrich MC. Gastrointestinal stromal tumors. Curr Treat Options Oncol. 2001;2:485–491. doi: 10.1007/s11864-001-0070-0. [DOI] [PubMed] [Google Scholar]
- 15.Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer. 1982;49:177–187. doi: 10.1002/1097-0142(19820101)49:1<177::aid-cncr2820490136>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 19.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 20.Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211–3220. doi: 10.1200/JCO.2000.18.18.3211. [DOI] [PubMed] [Google Scholar]
- 21.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547; discussion 540-547;. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


