Abstract
AIM: To evaluate the clinic features of eosinophilic gastroenteritis and examine the diagnosis, treatment, long-term outcome of this disease.
METHODS: Charts with a diagnosis of eosinophilic gastroenteritis from 1984 to 2002 at Mackay Memorial Hospital were reviewed retrospectively. There were 15 patients diagnosed with eosinophilic gastroenteritis. The diagnosis was established in 13 by histologic evaluation of endoscopic biopsy or operative specimen and in 2 by radiologic imaging and the presence of eosinophilic ascites.
RESULTS: All the patients had gastrointestinal symptoms and 12 (80%) had hypereosinophilia (absolute eosinophil count 1008 to 31360/cm3). The most common symptoms were abdominal pain and diarrhea. Five of the 15 patients had a history of allergy. Seven patients had involvement of the mucosa, 2 of muscularis, and 6 of subserosa. One with a history of seafood allergy was successfully treated with an elimination diet. Another patient improved spontaneously after fasting for several days. The remaining 13 patients were treated with oral prednisolone, 10 to 40 mg/day initially, which was then tapered. The symptoms in all the patients subsided within two weeks. Eleven of the 15 patients were followed up for more than 12 months (12 to 104 months, mean 48.7), of whom 5 had relapses after discontinuing steroids (13 episodes). Two of these patients required long-term maintenance oral prednisolone (5 to 10 mg/day).
CONCLUSION: Eosinophilic gastroenteritis is a rare condition of unclear etiology characterized by relapses and remissions. Short courses of corticosteroids are the mainstay of treatment, although some patients with relapsing disease require long-term low-dose steroids.
INTRODUCTION
Eosinophilic gastroenteritis is a rare disease characterized by eosinophilic infiltration into one or more layers of the gastrointestinal (GI) tract. It affects adults as well as children. The pathogenesis is poorly understood. Up to 40% of cases were reported to have an underlying allergic basis[1]. It might involve any area of the gastrointestinal tract from the esophagus to the rectum[2,3], although the stomach and proximal small bowel were most commonly affected. Klein et al[4] suggested a classification, based on the histology of the lesion: mucosal, muscularis, and subserosal disease. Clinical features depend on which layer and site are involved. Mucosal involvement leads to protein-losing enteropathy, fecal blood loss, and mal-absorption, muscularis disease often causes gastric outlet or small bowel obstruction, and subserosal involvement manifests as eosinophilic ascites. The disease often waxes and wanes in severity. Only a few studies have described the long-term outcome[5] and none have evaluated risk factors for relapse. In this study, we described the clinical manifestations of 15 patients with eosinophilic gastroenteritis.
MATERIALS AND METHODS
Charts with a diagnosis of eosinophilic gastroenteritis from 1984 to 2002 at Mackay Memorial Hospital were reviewed retrospectively. The diagnostic criteria included 1) the presence of gastrointestinal symptoms, 2) an eosinophilic infiltrate on a biopsy or operative specimen from the GI tract or else a high eosinophil count in ascitic fluid, 3) absence of parasite infestation, 4) no eosinophilic disease outside the GI tract, and 5) exclusion of intestinal lymphoma, Crohn’s disease or other tumors. As peripheral blood hypereosinophilia was not a universal finding in eosinophilic gastroenteritis, hypereosinophilia was not required for the diagnosis. Data collected from the charts included age, sex, presenting symptoms, allergy history (drug or food allergy, atopy, asthma, hay fever, etc), absolute eosinophil count, endoscopic, sonographic and radiological findings, operative records, histology of biopsies or operative specimens, response to medication, length of follow-up, and number of relapses.
An eosinophilic infiltrate was defined as at least 20 eosinophils per high power field. Klein’s criteria were followed: 1) mucosal disease was defined as infiltration of the mucosa without involvement of the muscularis or serosa, 2) muscular disease was defined as complete or incomplete intestinal obstruction and eosinophilic infiltration of the muscularis without eosinophilic ascites, and 3) subserosal disease was defined as eosinophilic infiltration of the GI tract with eosinophilic ascites.
RESULTS
Fifteen patients (6 men, 9 women), with a mean age of 38.4 ± 14.2 (3 to 58) years, were diagnosed as eosinophilic gastroenteritis according to the above criteria. The patients are listed in Table 1 according to Klein’s classification. Hypereosinophilia (1008 to 31360/cm3) in peripheral blood was noted in 12 (80%) cases. The most common symptoms and signs in our series are shown in Table 2.
Table 1.
General characteristics of patients with eosinophilic gastroenteritis according to histologic classification
| Group/Patient | Sex | Age | WBC/cm3 | Eos count | % of Eos | |
| I | 1 | M | 43 | 13800 | 1380 | 13 |
| 2 | F | 65 | 12720 | 2540 | 18 | |
| 3 | F | 38 | 11220 | 4704 | 41 | |
| 4 | F | 57 | 33010 | 19475 | 59 | |
| 5 | F | 34 | 7900 | 2291 | 27 | |
| 6 | M | 45 | 10400 | 3340 | 34 | |
| 7 | F | 64 | 13700 | 3973 | 29 | |
| II | 8 | M | 31 | 14500 | 6960 | 43 |
| 9 | F | 58 | 9900 | 396 | 5 | |
| III | 10 | M | 17 | 24800 | 11016 | 42 |
| 11 | F | 38 | 13400 | 1008 | 12 | |
| 12 | F | 20 | 11720 | 3984 | 36 | |
| 13 | M | 35 | 5650 | 621 | 10 | |
| 14 | M | 53 | 31360 | 14500 | 68 | |
| 15 | F | 3 | 8800 | 264 | 3 | |
Group I: mucosal, group II: muscular and group III: subserosal disease. Eos = eosinophil.
Table 2.
Presenting symptoms and signs of the 15 patients
| Symptoms and signs | n = 15 |
| Abdominal pain | 12 |
| Diarrhea | 11 |
| Bloating /fullness | 10 |
| Nausea/vomiting | 9 |
| Hypoalbuminemia ( < 3.5 g/dL) | 11 |
| Fecal blood loss | 6 |
Endoscopic biopsies were performed in 12 patients from 30 different sites in the gastric antrum, duodenum, and colon. Sixteen specimens were positive, yielding the diagnosis in 12. Another 20-year-old female underwent endoscopic laparotomy for refractory ascites and was diagnosed as subserosal eosinophilic gastroenteritis based on a biopsy. The other two patients had general bowel wall thickening on CT scan and eosinophils in their ascitic fluid. Although eosinophilic ascites might also be seen in parasitic disease and abdominal lymphoma, these entities were excluded by the clinical findings and response to treatment.
The endoscopic findings were nonspecific, with most patients having only hyperemic mucosa, although 2 patients had ulcers in the antrum and duodenum. A barium study of the small intestine showed thickening of the duodenal wall in 1 patient with subserosal disease and ischemic changes in the proximal ileum in 1 patient with muscular disease. CT scan or sonography were performed on all the patients with muscular and subserosal diseases (Figure 1 and Figure 2). Intestinal wall thickening was noted in the 2 with muscular disease and in 3 of 6 with subserosal disease. Ascites was present in all the 6 who had subserosal disease.
Figure 1.
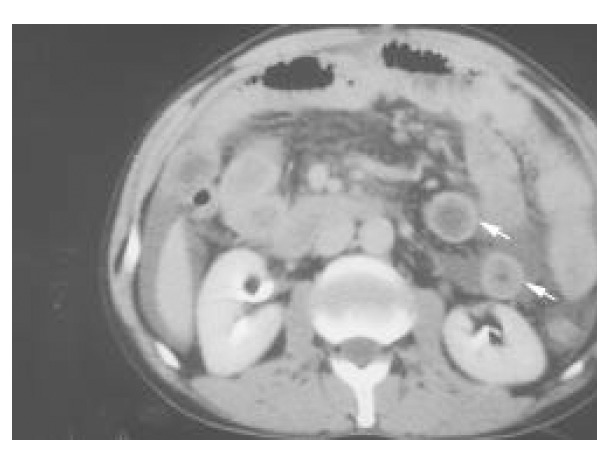
Abdominal computed tomography with intravenous contrast medium showing general thickening in the small bowel wall (white arrows), characteristic of the distribution of eosinophilic gastroenteritis.
Figure 2.
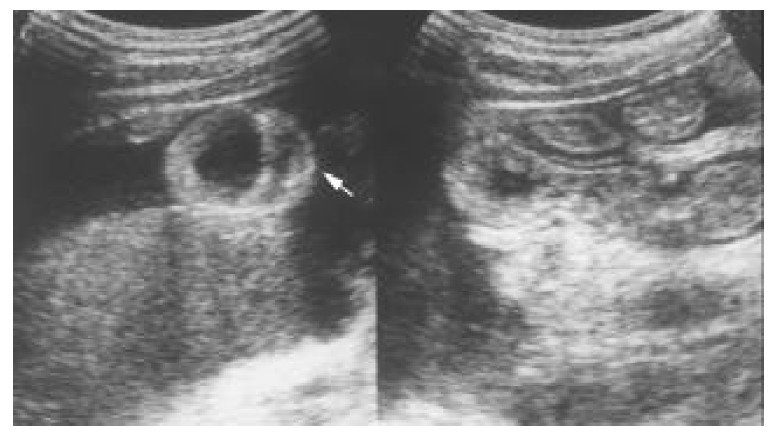
Transverse sonography of the proximal small bowel in the right subcostal area showing general thickening of the wall (white arrow) and ascites.
A history of allergy was noted in 5 of the 15 (34%) patients (1 mucosal, 1 muscular, and 3 subserosal). One patient with mild mucosal disease and allergy to shellfish was successfully treated with an elimination diet. The mucosal disease in another patient (who had no history of allergy) remitted spontaneously after a fast of several days. Symptoms in both of these patients resolved within one week, and neither patient had a relapse over more than three years of follow up. Thirteen patients were treated with prednisolone, 10 to 40 mg/day initially, which was then gradually tapered over 4 to 6 weeks. Of these 13, 11 had relief of symptoms within one week, while 2 patients with subserosal disease improved within two weeks. The average hospital stay was 14.8 days (5 to 27 days).
Eleven of our patients have been followed up for more than one year (12 to 102 months). Of them, 5 (1 with mucosal, 1 with muscular and 3 with subserosal disease) had a total of 13 relapses after discontinuing steroids. A 58-year-old female with muscular disease had a relapse after discontinuing steroids and had an ileal perforation associated with ischemia. She underwent ileal resection. In general, an additional short course of steroids resulted in resolution, although 2 patients maintained on low-dose prednisolone (5 to 10 mg/day) to prevent relapses. Patients with and without relapse are compared in Table 3.
Table 3.
Characteristics of patients with and without relapse
| No. | Sex | Age | Klein classification Relapsing (number of episodes) | Followed up > 12 m | Allergy |
| 4 | F | 57 | Mucosal (3) | + | + |
| 9 | F | 58 | Muscular (4) | + | + |
| 10 | M | 17 | Subserosal (1) | + | |
| 12 | F | 20 | Subserosal (3) | + | |
| 15 | F | 3 | Subserosal (4) | + | |
| Non-relapsing | |||||
| 1 | M | 43 | Mucosal | + | |
| 2 | F | 65 | Mucosal | + | |
| 3 | F | 38 | Mucosal | + | + |
| 5 | F | 34 | Mucosal | ||
| 6 | M | 45 | Muscular | + | |
| 7 | F | 64 | Muscular | ||
| 8 | M | 31 | Muscular | + | |
| 11 | F | 38 | Subserosal | + | + |
| 13 | M | 35 | Subserosal | ||
| 14 | M | 53 | Subserosal | + |
DISCUSSION
The pathogenesis of eosinophilic gastroenteritis is still unknown, but speculation has focused on the selective release of eosinophil major proteins leading to intestinal epithelial damage. Keshavarzian et al[6] demonstrated that the number of activated degranulated eosinophils in the mucosa correlated with the severity of eosinophilic gastroenteritis. The disease was reportedly more prevalent in patients with seasonal allergies, food sensitivities[7], eczema, allergic rhinitis, and asthma[8]. There have been a few cases related to exposure to medications[9,10] The evidence of elevations in IgE suggested that atopy might be involved in the pathogenesis of the disease[11,12], however a history of allergy may be of little help in establishing the diagnosis. In our study 34% of patients had a history of allergy, a proportion similar to that of other studies[1], but there was no correlation between an allergy history and the histologic type of disease.
Hypereosinophilia in the peripheral blood was absent in at least 20% of the cases[1]. In our series, the results were similar. Therefore, the absence of hypereosinophilia should not exclude consideration of the diagnosis of eosinophilic gastroenteritis in patients with unexplained GI symptoms. Eleven of our patients had hypoalbuminemia (serum albumin < 3.5 g/dl). Such a finding in patients with vague symptoms may be a hint to this disease, a chronic, organic rather than a transient, functional character.
Radiologically, the hallmark of eosinophilic gastroenteritis on CT is nodular and irregular thickening of the folds in the distal stomach and proximal small bowel[13,14]. However, similar thickening may also be seen in Menetrier’s disease, lymphoma, scirrhous carcinoma, Crohn’s disease, and granulomatous disease. It is thus not a specific sign of eosinophil gastroenteritis. Mesenteric inflammation as well as ascites are not uncommon but are still nonspecific. Sonography is a useful tool for detecting non-mucosal eosinophilic gastroenteritis in patients without peripheral hypereosinophilia. It may reveal generalized thickening of the intestinal wall as well as ascites, which prompted us to do cytological examination of ascitic fluid or endoscopic biopsy. Based on these sonographic abnormalities, we were able to diagnose eosinophilic gastroenteritis in 3 patients who had a normal serum eosinophil count (396 to 621/cm3). In our series, all the 6 patients with subserosal disease had ascites on sonography, and 5 patients (3 with subserosal and 2 with muscular disease) had a thickened wall. Sonography could also evaluate the response to treatment by measuring the thickness of the affected layer[15].
The endoscopic appearance in eosinophilic gastroenteritis is nonspecific, including erythematous, friable, nodular, and occasional ulcerative changes. In our study, 10 patients had only nonspecific gastritis or colitis, while 2 had shallow gastric or duodenal ulcers. These were most likely peptic ulcers, because no eosinophilic infiltrate was found on biopsy.
Definitive diagnosis requires histological evidence of eosinophilic infiltration. Eosinophilic infiltrates are usually patchy in distribution and may be present in otherwise normal, non-inflamed bowel wall. Therefore, multiple biopsies may be required to avoid missing the diagnosis. In our study, the definitive diagnosis was established by endoscopic biopsy in 12 patients. Several different examinations, such as gastroduodenoscopy and colonoscopy, and multiple deep biopsies may be necessary to establish the diagnosis. Even then it may be difficult to evaluate accurately the degree and extent of disease in most patients, given the patchy distribution of the infiltrates. A new technique using Tc-99m HMPAO-labeled WBC SPECT may be useful in assessing the extent of disease and response to treatment. Lee et al[16] have proposed a grading system using this technology.
Eosinophilic gastroenteritis may present with symptoms suggesting an acute abdomen. There have been reports of the disease mimicking acute appendicitis, an obstructing cecal mass, pancreatitis, giant refractory duodenal ulcer, and intussusception[17-25]. If such patients have peripheral hypereosinophilia, the correct diagnosis of eosinophilic gastroenteritis might be suspected, avoiding an unnecessary operation. Surgery in eosinophilic gastroenteritis should be reserved for obstruction or perforation. One 53-year-old male with subserosal disease in our series underwent surgery twice, once for a refractory ulcer that had perforated, and again for an acute abdomen without definitive etiology, before the diagnosis of eosinophilic gastroenteritis. It is possible that the acute symptoms at the second time were a manifestation of eosinophilic gastroenteritis.
Once the diagnosis has been made, it is useful to look for specific food allergies, as an elimination diet may be successful if a limited number of food allergies are identified. One of our patients with mucosal disease had improvement after elimination of seafood. Katz et al[26] reported that an elimination diet might fail to prevent recurrence, but our patient has remained well for more than 3 years. In general, patients responded quickly and well to steroids[27,28], as was true in our series. The appropriate duration of steroid treatment has been unknown, but short courses followed by a repeat course for a relapse have been described[29]. Patients with refractory relapsing disease are usually placed on long-term low-dose steroids or immunosuppressive therapy. Some authors have described the use of sodium cromoglycate (a stabilizer of mast cell membranes)[30] or montelukast (a selective, competitive leukotriene receptor antagonist)[31] as steroid-sparing agents.
We were able to follow 11 of our patients for more than one year and found that 5 of them had relapses. Three of the 5 relapse patients were all younger than 20 years old in our series. Our numbers are too small to draw firm conclusions, but this observation does raise the possibility that young age may be a risk factor for relapse. Larger series or a meta-analysis would be needed to investigate this possibility.
We found the incidence of subserosal disease (6/15 = 40%) to be higher than in other studies, for example, 4.5% to 9% in Japan and 13% in the USA[32,1]. Ascites as a manifestation of subserosal disease may be a more worrisome symptom, leading to a more aggressive approach to diagnosis. This in itself would not explain regional differences, but it might be that we under-diagnosed cases of mucosal and muscular disease, attributing vague symptoms to functional GI disease.
The diagnosis of eosinophilic gastroenteritis is problematic because the final diagnosis requires histological confirmation. However, this entity should be considered in the patient with unexplained chronic and relapsing gastrointestinal symptoms.
Footnotes
Edited by Zhu LH
References
- 1.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54–58. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita M, Hajiro K, Morita Y, Takakuwa H, Suzaki T. Eosinophilic gastroenteritis involving the entire digestive tract. Am J Gastroenterol. 1995;90:1868–1870. [PubMed] [Google Scholar]
- 3.Liacouras CA, Markowitz JE. Eosinophilic esophagitis: A subset of eosinophilic gastroenteritis. Curr Gastroenterol Rep. 1999;1:253–258. doi: 10.1007/s11894-999-0043-1. [DOI] [PubMed] [Google Scholar]
- 4.Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore) 1970;49:299–319. doi: 10.1097/00005792-197007000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lee CM, Changchien CS, Chen PC, Lin DY, Sheen IS, Wang CS, Tai DI, Sheen-Chen SM, Chen WJ, Wu CS. Eosinophilic gastroenteritis: 10 years experience. Am J Gastroenterol. 1993;88:70–74. [PubMed] [Google Scholar]
- 6.Keshavarzian A, Saverymuttu SH, Tai PC, Thompson M, Barter S, Spry CJ, Chadwick VS. Activated eosinophils in familial eosinophilic gastroenteritis. Gastroenterology. 1985;88:1041–1049. doi: 10.1016/s0016-5085(85)80026-3. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KJ. Eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr. 2000;30 Suppl:S28–S35. doi: 10.1097/00005176-200001001-00005. [DOI] [PubMed] [Google Scholar]
- 8.von Wattenwyl F, Zimmermann A, Netzer P. Synchronous first manifestation of an idiopathic eosinophilic gastroenteritis and bronchial asthma. Eur J Gastroenterol Hepatol. 2001;13:721–725. doi: 10.1097/00042737-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Medellin MV, Tumpkin C. Allergic reaction to gemfibrozil manifesting as eosinophilic gastroenteritis. South Med J. 2000;93:807–808. [PubMed] [Google Scholar]
- 10.Barak N, Hart J, Sitrin MD. Enalapril-induced eosinophilic gastroenteritis. J Clin Gastroenterol. 2001;33:157–158. doi: 10.1097/00004836-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Cello JP. Eosinophilic gastroenteritis--a complex disease entity. Am J Med. 1979;67:1097–1104. doi: 10.1016/0002-9343(79)90652-1. [DOI] [PubMed] [Google Scholar]
- 12.von Wattenwyl F, Zimmermann A, Netzer P. Synchronous first manifestation of an idiopathic eosinophilic gastroenteritis and bronchial asthma. Eur J Gastroenterol Hepatol. 2001;13:721–725. doi: 10.1097/00042737-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Marco-Doménech SF, Gil-Sánchez S, Jornet-Fayos J, Ambit-Capdevila S, Gonzalez-Añón M. Eosinophilic gastroenteritis: percutaneous biopsy under ultrasound guidance. Abdom Imaging. 1998;23:286–288. doi: 10.1007/s002619900341. [DOI] [PubMed] [Google Scholar]
- 14.Horton KM, Corl FM, Fishman EK. CT of nonneoplastic diseases of the small bowel: spectrum of disease. J Comput Assist Tomogr. 1999;23:417–428. doi: 10.1097/00004728-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Maroy B. Nonmucosal eosinophilic gastroenteritis: sonographic appearance at presentation and during follow-up of response to prednisone therapy. J Clin Ultrasound. 1998;26:483–486. doi: 10.1002/(sici)1097-0096(199811/12)26:9<483::aid-jcu10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee KJ, Hahm KB, Kim YS, Kim JH, Cho SW, Jie H, Park CH, Yim H. The usefulness of Tc-99m HMPAO labeled WBC SPECT in eosinophilic gastroenteritis. Clin Nucl Med. 1997;22:536–541. doi: 10.1097/00003072-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Box JC, Tucker J, Watne AL, Lucas G. Eosinophilic colitis presenting as a left-sided colocolonic intussusception with secondary large bowel obstruction: an uncommon entity with a rare presentation. Am Surg. 1997;63:741–743. [PubMed] [Google Scholar]
- 18.Tran D, Salloum L, Tshibaka C, Moser R. Eosinophilic gastroenteritis mimicking acute appendicitis. Am Surg. 2000;66:990–992. [PubMed] [Google Scholar]
- 19.Redondo Cerezo E, Moreno Platero JJ, García Domínguez E, González Aranda Y, Cabello Tapia MJ, Martínez Tirado P, López de Hierro Ruiz M, Gómez García M. Comments to a report: eosinophilic gastroenteritis presenting as an obstructing cecal mass: review literature and our own experience. Am J Gastroenterol. 2000;95:3655–3657. doi: 10.1111/j.1572-0241.2000.03398.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai MJ, Lai NS, Huang YF, Huang YH, Tseng HH. Allergic eosinophilic gastroenteritis in a boy with congenital duodenal obstruction. J Microbiol Immunol Infect. 2000;33:197–201. [PubMed] [Google Scholar]
- 21.Markowitz JE, Russo P, Liacouras CA. Solitary duodenal ulcer: a new presentation of eosinophilic gastroenteritis. Gastrointest Endosc. 2000;52:673–676. doi: 10.1067/mge.2000.110083. [DOI] [PubMed] [Google Scholar]
- 22.Shweiki E, West JC, Klena JW, Kelley SE, Colley AT, Bross RJ, Tyler WB. Eosinophilic gastroenteritis presenting as an obstructing cecal mass--a case report and review of the literature. Am J Gastroenterol. 1999;94:3644–3645. doi: 10.1111/j.1572-0241.1999.01625.x. [DOI] [PubMed] [Google Scholar]
- 23.Kristopaitis T, Neghme C, Yong SL, Chejfec G, Aranha G, Keshavarzian A. Giant antral ulcer: a rare presentation of eosinophilic gastroenteritis--case report and review of the literature. Am J Gastroenterol. 1997;92:1205–1208. [PubMed] [Google Scholar]
- 24.Scolapio JS, DeVault K, Wolfe JT. Eosinophilic gastroenteritis presenting as a giant gastric ulcer. Am J Gastroenterol. 1996;91:804–805. [PubMed] [Google Scholar]
- 25.Siahanidou T, Mandyla H, Dimitriadis D, Van-Vliet C, Anagnostakis D. Eosinophilic gastroenteritis complicated with perforation and intussusception in a neonate. J Pediatr Gastroenterol Nutr. 2001;32:335–337. doi: 10.1097/00005176-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Katz AJ, Twarog FJ, Zeiger RS, Falchuk ZM. Milk-sensitive and eosinophilic gastroenteropathy: similar clinical features with contrasting mechanisms and clinical course. J Allergy Clin Immunol. 1984;74:72–78. doi: 10.1016/0091-6749(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 27.Malaguarnera M, Restuccia N, Pistone G, Panebianco MP, Giugno I, Grasso G, Seminara G. Eosinophilic gastroenteritis. Eur J Gastroenterol Hepatol. 1997;9:533–537. doi: 10.1097/00042737-199705000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26:380–385. doi: 10.1097/00005176-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Naylor AR. Eosinophilic gastroenteritis. Scott Med J. 1990;35:163–165. doi: 10.1177/003693309003500601. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Millán A, Martín-Lorente JL, López-Morante A, Yuguero L, Sáez-Royuela F. Subserosal eosinophilic gastroenteritis treated efficaciously with sodium cromoglycate. Dig Dis Sci. 1997;42:342–344. doi: 10.1023/a:1018818003002. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz DA, Pardi DS, Murray JA. Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig Dis Sci. 2001;46:1787–1790. doi: 10.1023/a:1010682310928. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto T, Shibata T, Matsuura S, Kagesawa M, Ishizawa Y, Tamiya K. Eosinophilic gastroenteritis with ileus and ascites. Intern Med. 1996;35:779–782. doi: 10.2169/internalmedicine.35.779. [DOI] [PubMed] [Google Scholar]


