Abstract
AIM: To investigate the relationship between the expression of P-glycoprotein (P-gp) and the degree of vascularization in gallbladder carcinomas.
METHODS: P-gp was stained with streptavidin-peroxidase complex immunohistochemical method in routine paraffin-embedded sections of gallbladder carcinomas. Microvessel counts (MVC) were determined using factor-VIII-related antigens.
RESULTS: The average MVC in 32 cases of gallbladder carcinomas was (34 ± 10)/HP. The value of MVC was closely correlated with Nevin staging and tumor differentiation (P < 0.01 and P < 0.05). The total expression rate of P-gp was 62.5%. The P-gp expression rate in cases of Nevin staging S1-S3 (78.6%) was higher than that of S4-S5 (50.0%) with no statistical significance. The P-gp expression rate was not correlated with tumor differentiation or pathologic types. The value of MVC in P-gp (+) cases was markedly lower than that in P-gp (-) cases (P < 0.01). The positive rate of P-gp was significantly higher in cases of smaller MVC than those of bigger MVC (P < 0.05).
CONCLUSION: MVC may be used as one of the important parameters to reflect the biological behaviors of gallbladder carcinomas. As a major cause of drug resistance, the overexpression of P-gp is closely correlated with the poor vascularization in gallbladder carcinomas.
INTRODUCTION
Because the rate of neovascularization (angiogenesis) frequently fails to keep pace with tumor growth, tumor vasculature is often insufficient in supplying the tumor mass, therefore many solid tumors contain subpopulation of hypoxic cells. Some researches showed that the drug resistance was partially due to the poor tumor vascularization in reducing the influx of cytotoxic agents. Additionally, the hypoxic environment due to poor vascularization could also inhibit tumor cell proliferation, yet noncycling cells would be expected to be less sensitive to many agents. In recent years, some biochemical mechanisms of drug resistance have been identified; one of them is the overexpression of transmembrane transport protein and P-glycoprotein. We therefore linked angiogenesis assessed by microvessel counts with the expression of P-gp in human gallbladder carcinomas. The aim of the present study was to investigate whether MVC could be used as an important parameter to reflect the biological behaviors of gallbladder carcinomas and to illustrate the relationship between P-gp expression and vascularization.
MATERIALS AND METHODS
Clinical materials
Thirty-two cases of gallbladder carcinomas were randomly selected and diagnosed histologically. All the patients were treated surgically in our hospital. No chemotherapy or anti-angiogenesis therapy was used prior to surgery. There were 17 males and 15 females with an average age of 56 years. Histological types included 4 cases of papillary adenocarcinoma (12.5%), 25 cases of tubal adenocarcinoma (78.1%) and 3 cases of mucous adenocarcinoma (9.4%). Twelve cases had well-differentiated gallbladder carcinomas (37.5%), 9 cases moderate-differentiated gallbladder carcinomas (28.1%) and 11 cases poor-differentiated gallbladder carcinomas (34.4%). The Nevin staging (Table 1) was determined based on clinical features: 14 cases at stages S1, S2 and S3, and 18 cases at stages S4 and S5. All available hematoxylin and eosin-stained sections in each case were reviewed.
Table 1.
Nevin staging system for gallbladder cancer[1]
| Stage | Definition |
| 1 | Tumor invades mucosa only |
| 2 | Tumor invades muscularis and mucosa |
| 3 | Tumor invades subserosa, muscularis and mucosa |
| 4 | Tumor invades all layers of gallbladder wall plus cystic lymph node |
| 5 | Tumor extends into liver bed or distant spread |
Immunohistochemical stains
Four micrometer-thick sections from formalin-fixed and paraffin-embedded tissues were placed on poly-L-lysine-coated slides for immunohistochemistry study. The expression of P-gp was assessed by SP immunohistochemical method using a mouse-anti-human P-gp monoclonal antibody (JSB1) and a UltraSensitiveTM S-P kit (kit 9710). Blood vessels were highlighted by staining endothelial cells for factor VIII-related antigens. The deparaffinized sections were boiled in citrate buffer at high temperature and high pressure for antigen retrieval for staining of P-gp, pepsin digestion for factor VIII-related antigen staining, and then incubated with each antibody at 4 °C overnight. Immunohistochemical staining was then performed according to the UltraSensitiveTM S-P kit manual. All reagents were supplied by Maixin-Bio Co, Fuzhou, China. The cells with brown-yellow granules in cytoplasms or on cytomembranes were considered as positive for P-gp expression.
Immunostaining
P-gp Stained slide was examined by two independent observers and scored semi-quantitatively. Staining intensity was assessed in comparison with positive slide of colon cancer, supplied by Maixin-Bio Co, Fuzhou, China. The staining intensity was scored as none (0), weak (1), moderate (2) and strong (3). The slides were classified as negative (0), positive (1), strong positive (2) and strongest positive (3) with corresponding rates of positive cells at < 10%, 10%-20%, 20%-40%, and > 40%, respectively. When the mean score in each group was 3 or more, the slide was considered as positive. Negative controls were stained without primary antibody.
Microvessel counts
MVCs were assessed according to Weidner et al[2]. The hot spots were selected under a microscope (40x), then individual counts were made under 200x field (Olympus BH-2 microscope,0.74 mm2 per field). The average counts in 5 fields were recorded. Any single highlighted endothelial cell or endothelial cell cluster clearly separated from adjacent microvessel, and distinct clusters of brown-staining endothelial cells were counted as separate microvessels. Vessel lumens were not the sole criteria in identifying a microvessel.
Statistical analysis
Statistical analysis was performed using the Chi-square test and t test with SPSS software (Ver.10.0). P < 0.01 or P < 0.05 was considered as significant.
RESULTS
Expression of P-gp and MVC
The microvessels in malignant tissues were heterogeneously distributed. These highly neovascularized areas distributed within the tumor and dominated around the tumor margins (Figure 1). The P-gp was stained in cytoplasms and on the cytomembranes of gallbladder carcinoma cells (Figure 2).
Figure 1.
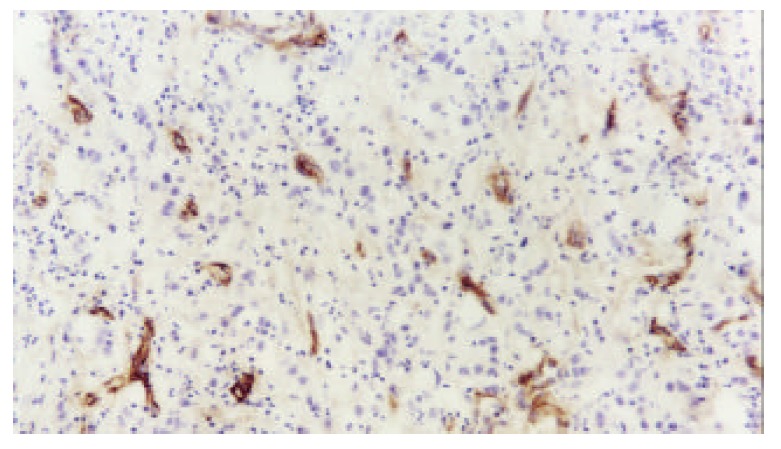
Distribution of microvessels in section of gallbladder carcinoma (S-P ×200).
Figure 2.
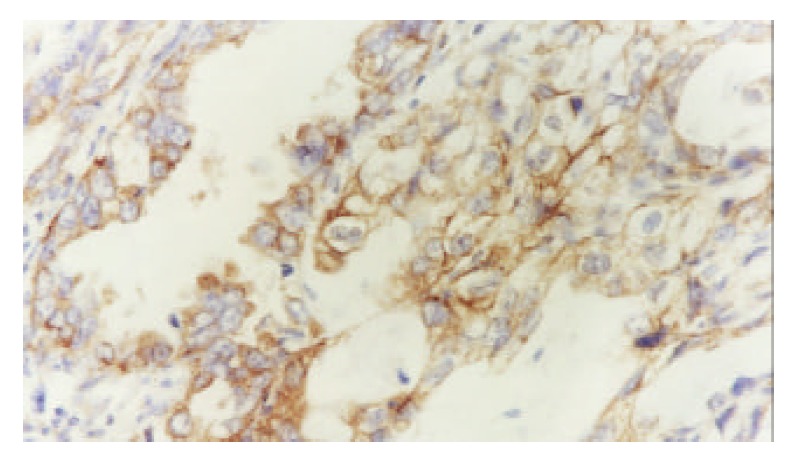
Expressed P-gp in gallbladder carcinoma (S-P ×400).
Clinicopathologic characteristics of MVC and P-gp expressions
The average MVC in 32 cases of gallbladder carcinoma was (34 ± 10)/HP. The number of MVC was markedly higher in cases of Nevin stages S4-S5 than in those of Nevin stages S1-S3 (t = 2.833, P = 0.008). MVC in moderately or poorly differentiated group was higher than that in well-differentiated group (t = 2.581, P = 0.015). The differences of MVC among the different pathologic types were not statistically significant (P = 0.313, 0.822, 0.168) (Table 2).
Table 2.
Characteristics of MVC in gallbladder carcinoma
| Characteristics | n | MVC |
| Pathologic types | ||
| Papillary adenocacinoma | 4 | 27 ± 8a |
| Tubal adenocarcinoma | 25 | 35 ± 10a |
| Mucous adenocarcinoma | 3 | 32 ± 10a |
| Tumor differentiation | ||
| Well | 12 | 28 ± 9b |
| Moderate-poor | 20 | 37 ± 9b |
| Nevin staging | ||
| S1, S2, S3 | 14 | 28 ± 10c |
| S4, S5 | 18 | 38 ± 8c |
P > 0.05,
P < 0.05,
P < 0.01.
The P-gp expression rate was 62.5% in these 32 cases. The positive rate of P-gp was higher in cases of Nevin stages S1-S3 (78.6%) than in those of Nevin stages S4-S5 (50.0%) with no statistical significance ( χ2 = 2.743, P > 0.05). The expression rate of P-gp was not correlated with tumor differentiation or pathologic types (P > 0.05) (Table 3).
Table 3.
Characteristics of P-gp expression in gallbladder carcinoma
| Characteristics | n | + | % |
| Pathologic types | |||
| Papillary adenocacinoma | 4 | 3 | 75.0a |
| Tubal adenocarcinoma | 25 | 15 | 60.0a |
| Mucous adenocarcinoma | 3 | 2 | 66.7a |
| Tumor differentiation | |||
| Well | 12 | 9 | 75.0b |
| Moderate-poor | 20 | 11 | 55.0b |
| Nevin staging | |||
| S1, S2, S3 | 14 | 11 | 78.6c |
| S4,S5 | 18 | 9 | 50.0c |
P > 0.05,
P > 0.05,
P > 0.05.
Relationship between expression of P-gp and MVC
The value of MVC in P-gp (+) cases was 30 ± 9/HP which was significantly lower than that in P-gp (-) cases (40 ± 8/HP) (t = 2.987, P = 0.006). The P-gp expression rate was significantly higher in cases with less median MVC (33.6/HP) than in those with MVC over median MVC (81.3% vs 43.8%, χ2 = 4.800, P < 0.05).
DISCUSSION
In 1971, Folkman proposed that tumor growth be dependent on angiogenesis, and then considerable evidences showed that tumor growth was angiogenesis dependent, and the neovascularization was closely associated with the growth, invasion, metastasis, staging and prognosis of tumors[2-15]. Our study indicated that MVC was correlated to Nevin staging and tumor differentiation. The case at later stage and with poorer differentiation had higher level of MVC in gallbladder carcinomas. MVC might be one of the most important parameters in reflecting the biologic behaviors of gallbladder carcinomas.
Though tumor growth depends on the angiogenesis, its rate often fails to keep pace with tumor growth, as tumor vasculature is inadequate for the tumor mass. Therefore, many solid tumors have subpopulations of hypoxic cells. Studies showed that the hypoxic tumor cells were relatively resistant to certain cytotoxic drugs[16]. In the past, authors proposed that drug resistance be partly caused by poor tumor vascularization in reducing the influx of cytotoxic agents. Additionally, the hypoxic environment due to poor vascularization inhibited proliferation of tumor cells, yet noncycling cells would be expected to be less sensitive to many agents. In recent years, the identified biochemical mechanism of drug resistance was the overexpression of the transmembrane transport protein, P-glycoprotein (P-gp). P-gp is an ATP-binding-cassette transporter that is ubiquitously expressed, and often has high concentrations on plasma membrane of cancer cells, where it causes multidrug resistance by pumping lipophilic drugs out of the cell. The expression of P-gp influenced the efficacy of postoperative chemotherapy[17-24]. In our study, P-gp expression rate was 62.5% which was similar to the result of another report on hepatocellular carcinoma[25]. Our result showed that overexpression of P-gp in gallbladder carcinoma tissue might be an important cause of drug resistance.
Recent studies showed that hypoxia-induced resistance to doxorubicin and methotrexate was attributed to an amplification of the P-gp gene and the dihydrofolate reductase gene[26-29]. Recently, it has also been shown that poor vascularization in lung carcinomas correlated with an up-regulation of drug-resistance enzymes, such as glutathione S-transferase-Ï, metallothionein and thymidylate synthase[30]. In another study on rectal cancer, poor angiogenesis was also linked to an expression of glutathione S-transferase and metallothionein[31]. Moreover, lung tumors with low vessel density and low VEGF expression have been found to be more frequently resistant to doxorubicin in vitro than tumors with high vessel counts and high expression of VEGF[32]. In our study, the value of MVC was markedly lower in P-gp (+) cases than in P-gp (-) cases. The positive rate of P-gp was significantly higher in cases with small MVC than in cases with big MVC.
In conclusion, the finding that poor vascularization links to overexpression of the most important multidrug resistance enzyme—P-gp provides us an additional insight into drug resistance in gallbladder carcinomas.
Footnotes
Edited by Ren SY and Wang XL
References
- 1.Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37:141–148. doi: 10.1002/1097-0142(197601)37:1<141::aid-cncr2820370121>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 3.Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992;340:145–146. doi: 10.1016/0140-6736(92)93217-b. [DOI] [PubMed] [Google Scholar]
- 4.Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591–595. doi: 10.3748/wjg.v8.i4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XP, Song SB, Li G, Wang DJ, Zhao HL, Wei LX. Correlations of microvessel quantitation in colorectal tumors and clinicopathology. Shijie Huaren Xiaohua Zazhi. 1999;7:37–39. [Google Scholar]
- 6.Gao GL, Yang Y, Yang SF, Ren CW. Relationship between pro-liferation of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282–284. [Google Scholar]
- 7.Jia L, Chen TX, Sun JW, Na ZM, Zhang HH. Relationship be-tween microvessel density and proliferating cell nuclear antigen and prognosis in colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:74–76. [Google Scholar]
- 8.Liu H, Wu JS, Li LH, Yao X. The expression of platelet-derived growth factor and angiogenesis in human coloreactal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:661–664. [Google Scholar]
- 9.Giatromanolaki A, Sivridis E, Koukourakis MI, Polychronidis A, Simopoulos C. Prognostic role of angiogenesis in operable carcinoma of the gallbladder. Am J Clin Oncol. 2002;25:38–41. doi: 10.1097/00000421-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. doi: 10.1097/00006676-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 12.Niemöller K, Jakob C, Heider U, Zavrski I, Eucker J, Kaufmann O, Possinger K, Sezer O. Bone marrow angiogenesis and its correlation with other disease characteristics in multiple myeloma in stage I versus stage II-III. J Cancer Res Clin Oncol. 2003;129:234–238. doi: 10.1007/s00432-003-0432-z. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266–274. doi: 10.1159/000069316. [DOI] [PubMed] [Google Scholar]
- 14.Onogawa S, Tanaka S, Oka S, Morihara M, Kitadai Y, Sumii M, Yoshihara M, Shimamoto F, Haruma K, Chayama K. Clinical significance of angiogenesis in rectal carcinoid tumors. Oncol Rep. 2002;9:489–494. [PubMed] [Google Scholar]
- 15.Joo YE, Sohn YH, Joo SY, Lee WS, Min SW, Park CH, Rew JS, Choi SK, Park CS, Kim YJ, et al. The role of vascular endothelial growth factor (VEGF) and p53 status for angiogenesis in gastric cancer. Korean J Intern Med. 2002;17:211–219. doi: 10.3904/kjim.2002.17.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 17.Chung HC, Rha SY, Kim JH, Roh JK, Min JS, Lee KS, Kim BS, Lee KB. P-glycoprotein: the intermediate end point of drug response to induction chemotherapy in locally advanced breast cancer. Breast Cancer Res Treat. 1997;42:65–72. doi: 10.1023/a:1005739525196. [DOI] [PubMed] [Google Scholar]
- 18.Chen CY, Zhu ZH. Relationship between expression of P-glyco-protein and efficacy of chemotherapy in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2003;11:36–38. [Google Scholar]
- 19.Cao L, Peng S, Duchrow M. [Expression of P-glycoprotein in benign and malignant gallbladder neoplasms] Zhonghua Zhongliu Zazhi. 1999;21:119–121. [PubMed] [Google Scholar]
- 20.Cao L, Duchrow M, Windhövel U, Kujath P, Bruch HP, Broll R. Expression of MDR1 mRNA and encoding P-glycoprotein in archival formalin-fixed paraffin-embedded gall bladder cancer tissues. Eur J Cancer. 1998;34:1612–1617. doi: 10.1016/s0959-8049(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 21.Monden N, Abe S, Hishikawa Y, Yoshimura H, Kinugasa S, Dhar DK, Tachibana M, Nagasue N. The role of P-glycoprotein in human gastric cancer xenografts in response to chemotherapy. Int J Surg Investig. 1999;1:3–10. [PubMed] [Google Scholar]
- 22.Yokoyama Y, Sato S, Fukushi Y, Sakamoto T, Futagami M, Saito Y. Significance of multi-drug-resistant proteins in predicting chemotherapy response and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 1999;25:387–394. doi: 10.1111/j.1447-0756.1999.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 23.Baekelandt MM, Holm R, Nesland JM, Tropé CG, Kristensen GB. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Res. 2000;20:1061–1067. [PubMed] [Google Scholar]
- 24.Warnakulasuriya S, Jia C, Johnson N, Houghton J. p53 and P-glycoprotein expression are significant prognostic markers in advanced head and neck cancer treated with chemo/radiotherapy. J Pathol. 2000;191:33–38. doi: 10.1002/(SICI)1096-9896(200005)191:1<33::AID-PATH585>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Kong XB, Yang ZK, Liang LJ, Huang JF, Lin HL. Overexpression of P-glycoprotein in hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2000;6:134–135. doi: 10.3748/wjg.v6.i1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice GC, Hoy C, Schimke RT. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1986;83:5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice GC, Ling V, Schimke RT. Frequencies of independent and simultaneous selection of Chinese hamster cells for methotrexate and doxorubicin (adriamycin) resistance. Proc Natl Acad Sci USA. 1987;84:9261–9264. doi: 10.1073/pnas.84.24.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra R, Jones AM, Kirk J, Adams GE, Stratford IJ. The effect of hypoxia on acquired drug resistance and response to epidermal growth factor in Chinese hamster lung fibroblasts and human breast-cancer cells in vitro. Int J Cancer. 1993;54:650–655. doi: 10.1002/ijc.2910540421. [DOI] [PubMed] [Google Scholar]
- 29.Luk CK, Veinot-Drebot L, Tjan E, Tannock IF. Effect of transient hypoxia on sensitivity to doxorubicin in human and murine cell lines. J Natl Cancer Inst. 1990;82:684–692. doi: 10.1093/jnci/82.8.684. [DOI] [PubMed] [Google Scholar]
- 30.Koomägi R, Mattern J, Volm M. Up-regulation of resistance-related proteins in human lung tumors with poor vascularization. Carcinogenesis. 1995;16:2129–2133. doi: 10.1093/carcin/16.9.2129. [DOI] [PubMed] [Google Scholar]
- 31.Mattern J, Kallinowski F, Herfarth C, Volm M. Association of resistance-related protein expression with poor vascularization and low levels of oxygen in human rectal cancer. Int J Cancer. 1996;67:20–23. doi: 10.1002/(SICI)1097-0215(19960703)67:1<20::AID-IJC5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Volm M, Koomägi R, Mattern J. Interrelationships between microvessel density, expression of VEGF and resistance to doxorubicin of non-small lung cell carcinoma. Anticancer Res. 1996;16:213–217. [PubMed] [Google Scholar]


