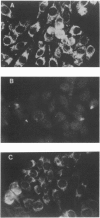
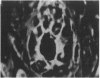
Abstract
mRNA from a regional lymph node of a patient with primary biliary cirrhosis (PBC) was used to construct a combinatorial immunoglobulin library in the lambda phage vector system. Six human monoclonal IgG Fab clones (LC1-LC6) specific for the major autoantigen of PBC--dihydrolipoamide acetyltransferase, the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2)--were isolated, appearing at a frequency of 0.01% in the combinatorial immunoglobulin library. These Fab clones recognize human PDC-E2 with high affinity (Ka = 10(-7)-10(-9) M-1). Using both immunoblotting and ELISA, LC1-LC6 showed little cross-reactivity to any of the other autoantigens commonly recognized by PBC sera or to other antigens commonly recognized by PBC sera or to other antigens such as histone, calf thymus DNA, and bovine serum albumin. The Fab monoclonal antibodies show a typical anti-mitochondrial staining pattern in HEp-2 cells but react strongly with the luminal aspect of biliary epithelial cells of patients with PBC. Our results demonstrate that a recombinant combinatorial immunoglobulin library can be used to isolate high-affinity Fabs against a specific autoantigen. Such reagents will facilitate the analysis of immunoglobulin gene structure, idiotype, and antigen-antibody interactions in autoimmune disease.
Full text
PDF

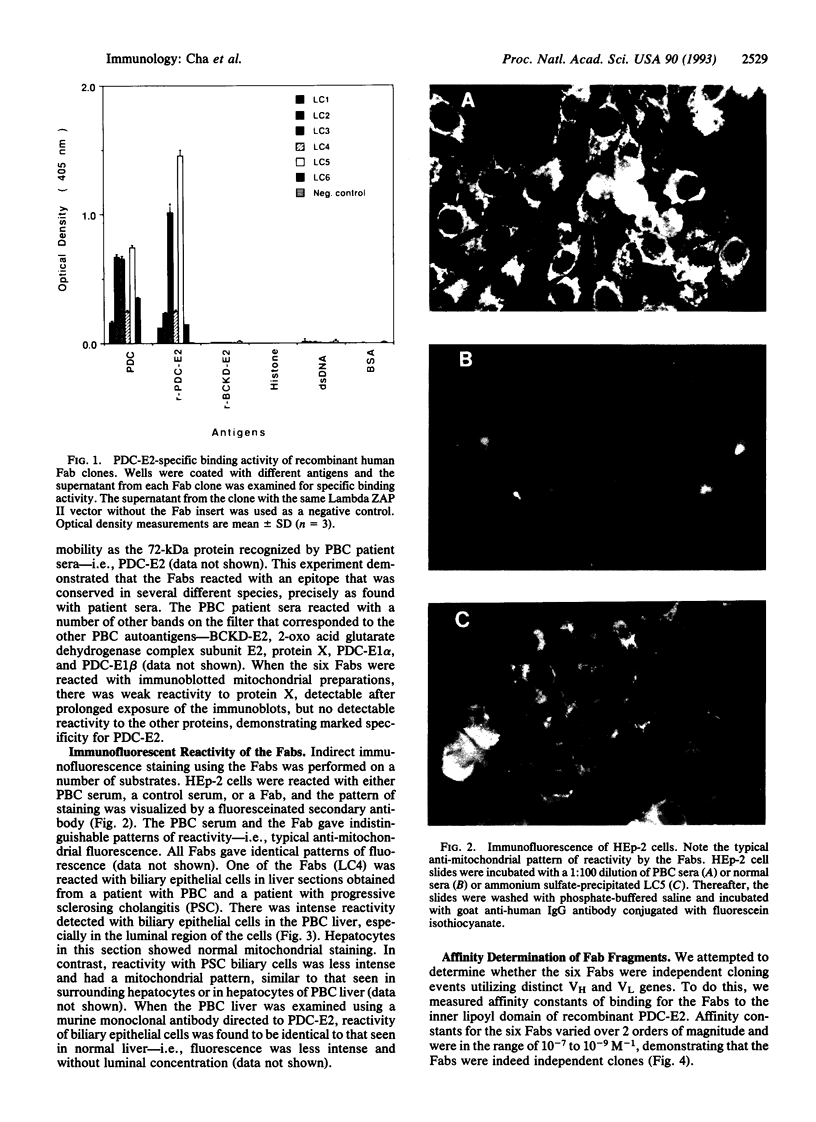
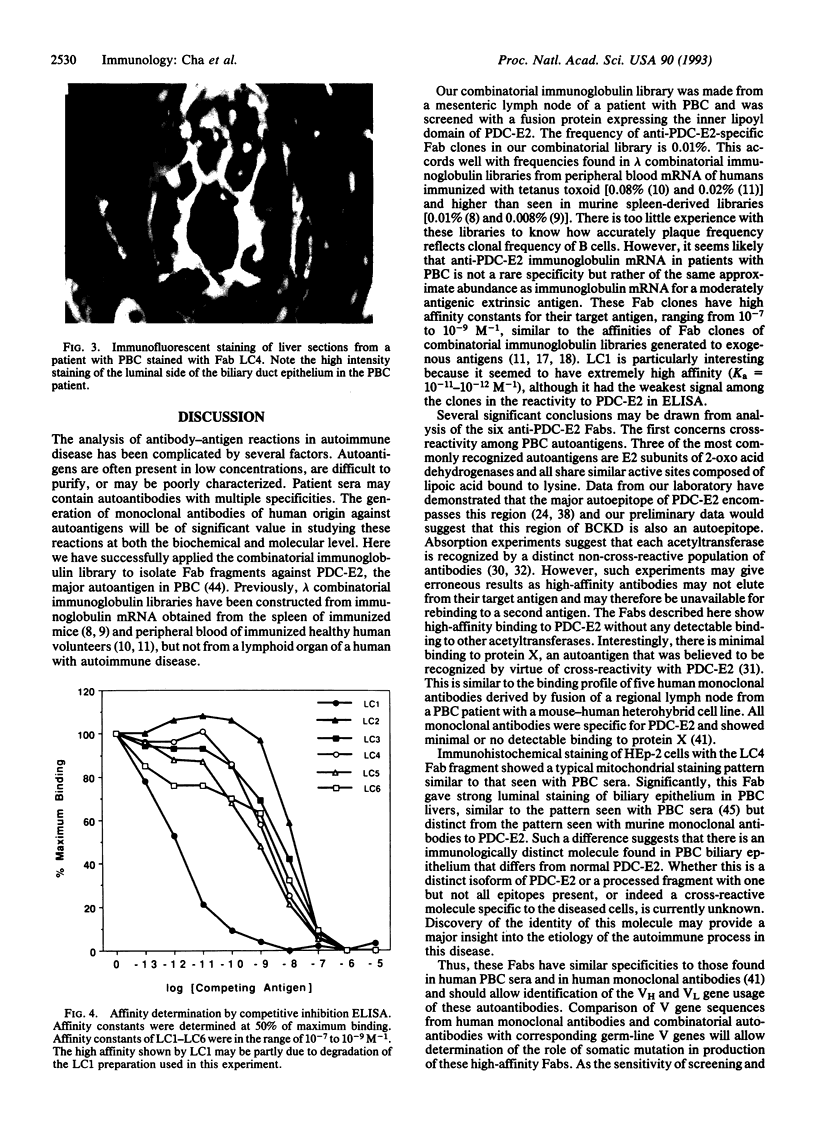

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer M. A., Bazan H. A., Kim J., Morrow S. L., Shaikh T. H., Arcot S. S., Deininger P. L. Large-scale subcloning of bacteriophage lambda ZAP clones. Biotechniques. 1992 Mar;12(3):370–371. [PubMed] [Google Scholar]
- Brüggemann M., Winter G., Waldmann H., Neuberger M. S. The immunogenicity of chimeric antibodies. J Exp Med. 1989 Dec 1;170(6):2153–2157. doi: 10.1084/jem.170.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Koprowski H. Influenza virus hemagglutinin-specific antibodies isolated from a combinatorial expression library are closely related to the immune response of the donor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6450–6454. doi: 10.1073/pnas.87.16.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiswell D. J., McCafferty J. Phage antibodies: will new 'coliclonal' antibodies replace monoclonal antibodies? Trends Biotechnol. 1992 Mar;10(3):80–84. doi: 10.1016/0167-7799(92)90178-x. [DOI] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., McNeilage L. J., Surh C. D., Van de Water J., Spithill T. W., Whittingham S., Gershwin M. E. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7317–7321. doi: 10.1073/pnas.85.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchosal M. A., Eming S. A., Fischer P., Leturcq D., Barbas C. F., 3rd, McConahey P. J., Caothien R. H., Thornton G. B., Dixon F. J., Burton D. R. Immunization of hu-PBL-SCID mice and the rescue of human monoclonal Fab fragments through combinatorial libraries. Nature. 1992 Jan 16;355(6357):258–262. doi: 10.1038/355258a0. [DOI] [PubMed] [Google Scholar]
- Fregeau D. R., Davis P. A., Danner D. J., Ansari A., Coppel R. L., Dickson E. R., Gershwin M. E. Antimitochondrial antibodies of primary biliary cirrhosis recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha-ketoacid dehydrogenase complex. J Immunol. 1989 Jun 1;142(11):3815–3820. [PubMed] [Google Scholar]
- Fregeau D. R., Roche T. E., Davis P. A., Coppel R., Gershwin M. E. Primary biliary cirrhosis. Inhibition of pyruvate dehydrogenase complex activity by autoantibodies specific for E1 alpha, a non-lipoic acid containing mitochondrial enzyme. J Immunol. 1990 Mar 1;144(5):1671–1676. [PubMed] [Google Scholar]
- Fussey S. P., Guest J. R., James O. F., Bassendine M. F., Yeaman S. J. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8654–8658. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R. Primary biliary cirrhosis: paradigm or paradox for autoimmunity. Gastroenterology. 1991 Mar;100(3):822–833. doi: 10.1016/0016-5085(91)80033-6. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Griffin T. A., Chuang D. T. Genetic reconstruction and characterization of the recombinant transacylase (E2b) component of bovine branched-chain alpha-keto acid dehydrogenase complex. Implication of histidine 391 as an active site residue. J Biol Chem. 1990 Aug 5;265(22):13174–13180. [PubMed] [Google Scholar]
- Griffin T. A., Wynn R. M., Chuang D. T. Expression and assembly of mature apotransacylase (E2b) of bovine branched-chain alpha-keto acid dehydrogenase complex in Escherichia coli. Demonstration of transacylase activity and modification by lipoylation. J Biol Chem. 1990 Jul 15;265(20):12104–12110. [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Kang A. S., Barbas C. F., Janda K. D., Benkovic S. J., Lerner R. A. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M. Primary biliary cirrhosis. Adv Intern Med. 1987;32:359–377. [PubMed] [Google Scholar]
- Krams S. M., Van de Water J., Coppel R. L., Esquivel C., Roberts J., Ansari A., Gershwin M. E. Analysis of hepatic T lymphocyte and immunoglobulin deposits in patients with primary biliary cirrhosis. Hepatology. 1990 Aug;12(2):306–313. doi: 10.1002/hep.1840120219. [DOI] [PubMed] [Google Scholar]
- Leung P. S., Iwayama T., Coppel R. L., Gershwin M. E. Site-directed mutagenesis of lysine within the immunodominant autoepitope of PDC-E2. Hepatology. 1990 Dec;12(6):1321–1328. doi: 10.1002/hep.1840120612. [DOI] [PubMed] [Google Scholar]
- Leung P. S., Krams S., Munoz S., Surh C. P., Ansari A., Kenny T., Robbins D. L., Fung J., Starzl T. E., Maddrey W. Characterization and epitope mapping of human monoclonal antibodies to PDC-E2, the immunodominant autoantigen of primary biliary cirrhosis. J Autoimmun. 1992 Dec;5(6):703–718. doi: 10.1016/0896-8411(92)90187-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay I. R., Gershwin M. E. Molecular basis of mitochondrial autoreactivity in primary biliary cirrhosis. Immunol Today. 1989 Sep;10(9):315–318. doi: 10.1016/0167-5699(89)90088-1. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Mullinax R. L., Gross E. A., Amberg J. R., Hay B. N., Hogrefe H. H., Kubitz M. M., Greener A., Alting-Mees M., Ardourel D., Short J. M. Identification of human antibody fragment clones specific for tetanus toxoid in a bacteriophage lambda immunoexpression library. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8095–8099. doi: 10.1073/pnas.87.20.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Mitchell E. B., Jouhal S. S., Flanagan J. G., Rabbitts T. H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985 Mar 21;314(6008):268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- Persson M. A., Caothien R. H., Burton D. R. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portolano S., Chazenbalk G. D., Seto P., Hutchison J. S., Rapoport B., McLachlan S. M. Recognition by recombinant autoimmune thyroid disease-derived Fab fragments of a dominant conformational epitope on human thyroid peroxidase. J Clin Invest. 1992 Sep;90(3):720–726. doi: 10.1172/JCI115943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S., Stanley C. M., Steward M. W. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J Immunol Methods. 1988 Feb 10;106(2):245–249. doi: 10.1016/0022-1759(88)90204-9. [DOI] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Wunderlich D., Teramoto Y. A. Generation of human monoclonal antibodies reactive with human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6841–6845. doi: 10.1073/pnas.77.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C. D., Coppel R., Gershwin M. E. Structural requirement for autoreactivity on human pyruvate dehydrogenase-E2, the major autoantigen of primary biliary cirrhosis. Implication for a conformational autoepitope. J Immunol. 1990 May 1;144(9):3367–3374. [PubMed] [Google Scholar]
- Surh C. D., Coppel R., Gershwin M. E. Structural requirement for autoreactivity on human pyruvate dehydrogenase-E2, the major autoantigen of primary biliary cirrhosis. Implication for a conformational autoepitope. J Immunol. 1990 May 1;144(9):3367–3374. [PubMed] [Google Scholar]
- Surh C. D., Danner D. J., Ahmed A., Coppel R. L., Mackay I. R., Dickson E. R., Gershwin M. E. Reactivity of primary biliary cirrhosis sera with a human fetal liver cDNA clone of branched-chain alpha-keto acid dehydrogenase dihydrolipoamide acyltransferase, the 52 kD mitochondrial autoantigen. Hepatology. 1989 Jan;9(1):63–68. doi: 10.1002/hep.1840090110. [DOI] [PubMed] [Google Scholar]
- Surh C. D., Roche T. E., Danner D. J., Ansari A., Coppel R. L., Prindiville T., Dickson E. R., Gershwin M. E. Antimitochondrial autoantibodies in primary biliary cirrhosis recognize cross-reactive epitope(s) on protein X and dihydrolipoamide acetyltransferase of pyruvate dehydrogenase complex. Hepatology. 1989 Aug;10(2):127–133. doi: 10.1002/hep.1840100202. [DOI] [PubMed] [Google Scholar]
- Thompson K. M. Human monoclonal antibodies. Immunol Today. 1988 Apr;9(4):113–117. doi: 10.1016/0167-5699(88)91281-9. [DOI] [PubMed] [Google Scholar]
- Van de Water J., Ansari A. A., Surh C. D., Coppel R., Roche T., Bonkovsky H., Kaplan M., Gershwin M. E. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991 Jan 1;146(1):89–94. [PubMed] [Google Scholar]
- Van de Water J., Fregeau D., Davis P., Ansari A., Danner D., Leung P., Coppel R., Gershwin M. E. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988 Oct 1;141(7):2321–2324. [PubMed] [Google Scholar]
- Van de Water J., Gershwin M. E., Leung P., Ansari A., Coppel R. L. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988 Jun 1;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S. Antibody engineering: the use of Escherichia coli as an expression host. FASEB J. 1992 Apr;6(7):2422–2427. doi: 10.1096/fasebj.6.7.1563594. [DOI] [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Fussey S. P., Danner D. J., James O. F., Mutimer D. J., Bassendine M. F. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988 May 14;1(8594):1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]