Bone is a site prone to the development of different tumors and a preferred site for hematogenous metastasis from solid tumors such as breast, prostate or lung cancer. Studying the bone microenvironment and its malignancies is challenging as it hosts the interplay of numerous cellular and extracellular components, and its location and structure render it difficult to access and investigate. Over the last decade, major research efforts have focused on developing alternative models to study physiological and pathological processes within the bone microenvironment. Bioengineered tissues generated subcutaneously in immunodeficient mice have proven advantageous in terms of the ease of surgical procedure, accessibility for live imaging and monitoring, and design flexibility, which allows interrogating the contribution of different tissue components to tumor development or metastatic invasion. We discuss here key characteristics for an “ideal” engineered bone model to study human bone malignancies.
A proof-of-concept for the use of engineered tissues as metastatic sites was first shown by Moreau et al. who observed metastases in implanted tissue constructs after SUM1315 breast cancer cells were injected in the mammary fat pad of NOD/SCID mice.1 It is now well known that microenvironmental cues are critical in attracting and determining the fate of malignant cells in the skeleton. However, the histological analysis showed that the engineered microenvironments conducive to the development of metastases in this study consisted of calcified fibrous tissue and did not recapitulate bone. More recently, Lee et al. employed polyacrylamide hydrogels seeded with human bone marrow stromal cells (hBMSCs) to engineer hematopoietic tissue in NSG mice.2 Hematopoietic cells and tumor cells highjack the same pathways to gain foothold within the bone microenvironment; therefore recapitulating a hematopoietic compartment provides a suitable model to mimic metastasis to bone. The model was applied to study metastasis of prostate cancer cells,2,3 yet its relevance is questionable as extramedullary bone marrow without a supporting bone matrix mimics rather a disease state than a physiological condition. When applied to osteotropic tumors such as prostate cancer, the absence of a bone matrix prevents the use of the model to analyze tumor-induced osteoclastic/osteoblastic effects. In brief, replicating closely the morphology and structure of the marrow-containing bone organ is essential to develop a clinically relevant bone model to study tumor growth, metastasis and tumor-induced bone remodeling.
Cell-free approaches have been utilized by some research groups to generate host-derived ectopic bone tissues;4 however, these do not exploit a key advantage of scaffold-cell based constructs, which is the opportunity to transplant human cells into immunodeficient hosts and therefore use the mouse organism as a bioreactor to recreate human tissue substitutes. In fact, the limited ability so far to translate results obtained from traditional xenograft models into the clinic has been attributed to a major extent to the lack of a species-specific microenvironment to study tumor development. We and others have described the development of large volume bone implants with a physiologically relevant morphology and which include a high number of human stromal cells.5,6 Different research groups utilize hBMSCs to engineer ectopic humanized bone tissues. However, most of them do not demonstrate the retention of the human cells after transplantation, nor do they characterize the degree of humanization of the newly formed tissue.1,2 Bersani et al. have shown that in hBMSC-seeded scaffolds the human cells were fully replaced by endogenous mouse stromal cells within 4 weeks after subcutaneous implantation in NSG mice,3 highlighting the importance of a validation of the humanization of the engineered tissue. Another key aspect which is largely missing in most studies is evidence not only of the presence of human cells in the engineered tissues but also of the incorporation of human-derived extracellular matrix (ECM).6 In fact the bone niche regulates the behavior of normal and malignant cells via various chemo-attractive and adhesive pathways, which include cell-cell as well as cell-ECM interactions. In our recently published paper we have shown that the β1 integrin cell-ECM adhesion molecules expressed by breast cancer cells play a significant role in the development of metastases within a humanized bone microenvironment.7 Therefore, humanization of the bone cells and ECM is critical to study specific human tumor-bone interactions.
In essence, engineered humanized microenvironments for the study of primary bone tumors and bone metastases should (1) reproduce closely the bone organ physiology and structure, and (2) achieve a high level of humanization for both the cellular and extracellular components. These platforms may further be applied to other research areas, in particular as models for human hematopoiesis and leukemia research. In fact, the ability to generate a marrow-rich bone organ provides the opportunity to engraft human hematopoietic cells within the humanized bone microenvironment and to study local factors that influence human stem cell behavior during normal and malignant hematopoiesis. We and others are also developing advanced humanized models where the blood vessels, including the endothelial cells and surrounding pericytes, within the bone marrow are humanized to allow even more physiological studies of hematopoietic and cancer cell trafficking and interaction with a human vascular niche (unpublished work).
Figure 1.
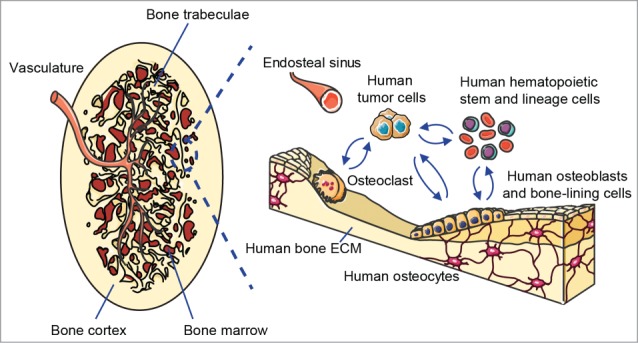
Schematic of the humanized bone “organ” as a model of human bone malignancies. Left panel shows the clinically relevant morphology of the bioengineered ectopic bone “organ,” while the right panel indicates the different humanized cellular and extracellular components that are comprised within the model and interact with human bone tumor or metastatic cells.
References
- 1.Moreau JE, et al.. Cancer Research 2007; 67(21):10304-8; PMID:17974972; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2483 [DOI] [PubMed] [Google Scholar]
- 2.Lee J, et al.. Proc Natl Acad Sci U S A 2012; 109(48):19638-43; PMID:23150542; http://dx.doi.org/ 10.1073/pnas.1208384109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bersani F, et al.. Cancer Res 2014; 74(24):7229-38; PMID:25339351; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seib FP, et al.. Biomaterials 2015; 51(0):313-19; PMID:25771021; http://dx.doi.org/ 10.1016/j.biomaterials.2015.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scotti C, et al.. Proc Natl Acad Sci U S A 2013; 110(10):3997-4002; PMID:23401508; http://dx.doi.org/ 10.1073/pnas.1220108110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibaudeau L, et al.. Dis Model Mech 2014; 7(2):299-309; PMID:24713276; http://dx.doi.org/ 10.1242/dmm.014076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thibaudeau L, et al.. Oncotarget 2015; 6(1):332-44; PMID:25426561 [DOI] [PMC free article] [PubMed] [Google Scholar]


