Abstract
Osteosarcoma is the most common primary malignant bone tumor and affects a significant portion of pediatric oncology patients. Although surgery and adjuvant chemotherapy confer significant survival benefits, many patients go on to develop metastatic disease, particularly to the lungs, secondary to development of drug resistance. Inhibition of protein phosphatase 2A with the small molecule, LB100, has demonstrated potent chemo- and radio-sensitizing properties in numerous pre-clinical tumor models. In this study, we showed that LB100 overcame DNA repair mechanisms in osteosarcoma cells treated with cisplatin, in vitro, and recapitulated these findings in an in vivo xenograft model. Notably, the addition of LB100 to cisplatin prevented development of pulmonary metastases in the majority of treated animals. Our data indicated the mechanism of chemo-sensitization by LB100 involved abrogation of the ATM/ATR-activated DNA damage response, leading to hyperphosphorylation of Chk proteins and persistent cyclin activity. In addition, LB100 exposure suppressed Akt signaling, leading to Mdm2-mediated proteasomal degradation of functional p53. Taken together, LB100 prevented repair of cisplatin-induced DNA damage, resulting in mitotic catastrophe and cell death.
Keywords: cisplatin, chemosensitization, DNA repair, osteosarcoma, protein phosphatase 2A
Introduction
Osteosarcoma is the most common primary malignant bone tumor in the adolescent patient.1 Despite total surgical resection of the primary lesion, approximately 80% of patients go on to develop metastases, particularly to the lungs.2 The addition of systemic chemotherapy has improved 5-year survivals to 70%,3 but long-term survival beyond 10 y remains a challenge, as metastatic disease burden worsens.1,4 Understanding the mechanisms involved in chemotherapeutic resistance, leading to metastases may uncover new therapeutic targets.
It is well known that the drug resistance and metastasis of tumor cells are mediated by multiple defense mechanisms, such as DNA repair, apoptosis and cell cycle arrest.5 Playing a dynamic role in this process is protein phosphatase 2A (PP2A), a serine/threonine phosphatase involved in mitotic progression and cellular responses to DNA damage.6 The traditional Chinese medicine, cantharidin, is a selective inhibitor of PP2A and has been shown to force cancer cells prematurely into mitosis and subsequently induce apoptotic cell death.7,8 Unfortunately, the severe toxicities of cantharidin have precluded its therapeutic use in human patients. However, development of synthetic PP2A inhibitors has gained attention as a strategy of overcoming tumor cell senescence and sensitizing cancer cells to chemotherapies. Recently, a norcantharidin-derived small molecule PP2A inhibitor, dubbed LB100 (Lixte Biotechnology Holdings, Inc.), was approved by the FDA for study in a phase I clinical trial, after having demonstrated marked chemo- and radio-sensitization of tumor cells at non-toxic doses in several tumor models.9-15 When administered together with standard-of-care chemotherapy and radiation regimens, LB100 holds promising potential as a novel means of overcoming treatment resistant cancers. As such, in the present study, we evaluated the efficacy of LB100 to enhance the therapeutic effects of chemotherapy against osteosarcoma. We performed in vitro and in vivo studies in various osteosarcoma cell lines and investigated the cytotoxic effects of LB100 on various signaling pathways involved in cell cycle modulation.
Results
LB100 sensitizes osteosarcoma cells to the cytotoxic effects of cisplatin, in vitro
First, we investigated the effects of LB100 on PP2A enzymatic activity. LB100 exhibited concentration-dependent decreases in PP2A activity in cell lysate from the 143B osteosarcoma cell line (Fig. 1A). The 50% inhibitory concentration (IC50) of LB100 in 143B cells was 10.58 μM (Fig. 1B). In order to characterize the effects of LB100 and cisplatin in osteosarcoma cells, additional experiments were performed in 3 osteosarcoma cell lines with differing p53 mutational status: 143B p53mut, U2OS p53wt, and MG63 p53null. After exposure to 5 μM LB100 for one hour, cells were treated with a cytotoxic dose of cisplatin (4 μg/ml). Cell viability was significantly diminished with combination treatment compared to LB100 or cisplatin alone (Fig. 1C). To demonstrate apoptotic cell death, we performed western blotting of 143B cells, 24 hours after treatment. There was high expression of cleaved caspase-3 and caspase-9 in cells exposed to combination treatment, a finding attenuated in cells treated with only cisplatin and completely abolished in those treated with LB100 alone (Fig. 1D). These findings indicated that the addition of LB100 to cisplatin increased apoptotic cell death of 143B cells, in vitro.
Figure 1.
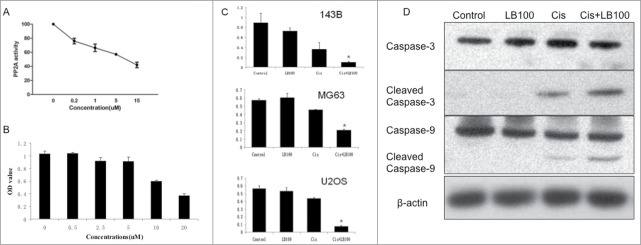
Inhibition of PP2A by LB100 sensitizes osteosarcoma cells to cisplatin cytotoxicity. (A) PP2A activity assay showing inhibition of PP2A in 143B cells in the presence of increasing concentrations of LB100 after 3 hours of exposure. (B) Absorbance values of serially diluted LB100 showing IC50 of 10.58 μM. (C) CCK-8 assay showing increased cytotoxicity in 143B, MG63, and U2OS cells when cells were pre-treated with LB100 and cisplatin compared to either drug alone for 48 hours. * indicates statistical significance (p < 0 .05). (D) Western blots following 24 hours of treatment demonstrating cellular apoptosis via increased expression of cleaved caspase-3 and caspase-9 after combined LB100 and cisplatin treatment.
LB100 abrogates cell cycle arrest and permits DNA damage in a Mad2-dependent manner
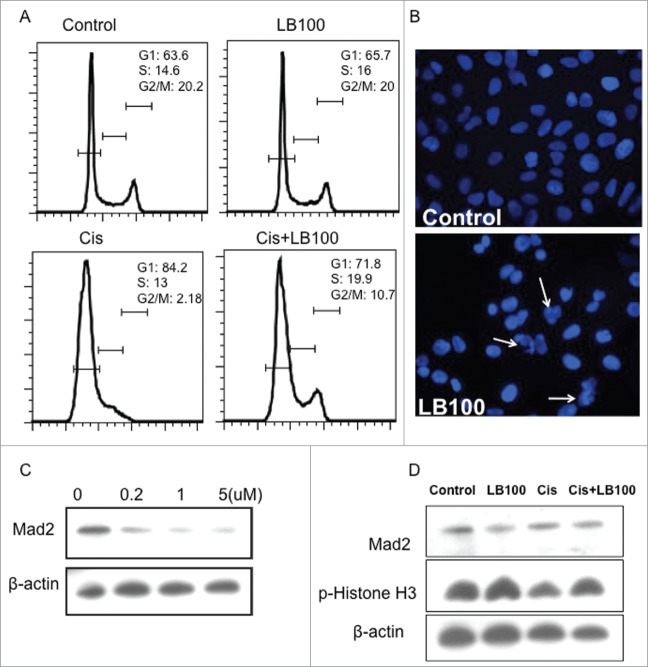
To further investigate the effects of LB100 on cell cycle regulation, we performed cell cycle analysis of cultured 143B cells exposed LB100, cisplatin, or combination treatment (Fig. 2A). Compared to control, 24-hour exposure to 5 μM LB100 did not alter percentages of cells in different phases of the cell cycle. Exposure to 4 μg/ml cisplatin alone diminished the percentage of cells in G2/M and markedly increased those in G1, indicating increased tumor cell senescence. However, combination treatment reversed this effect and prominently increased percentage of cells in S and G2/M phases, compatible with loss of G1 and G2 checkpoints ordinarily induced by acute DNA damage (Fig. 2A). Immunofluorescent staining of nuclei in 143B cells exposed to LB100 for 24 hours demonstrated presence of abnormal mitotic figures, indicative of mitotic catastrophe (Fig. 2B).
Figure 2.
Cell cycle analysis after LB100 treatment of osteosarcoma cells. (A) FACS analysis of cell cycle distribution of 143B cells after treatment with LB100, cisplatin, and combination treatment. (B) Nuclear (DAPI) staining of 143B cells after 24 hour exposure to 5 mM LB100 showing numerous irregular nuclei with clumped chromatin (white arrows). (C) Western blots showing increased abrogation of Mad2 by LB100 in 143B cells in a dose-dependent manner. (D) LB100 alone or in combination with cisplatin reduced Mad2 expression and phosphorylation of Histone H3, indicating cell cycle progression after LB100 treatment.
We further explored the effects of LB100 on expression of mitotic arrest deficiency 2 (Mad2), a critical component of the spindle checkpoint that maintains proper chromosomal segregation and genomic stability. Mad2 overexpression is a hallmark in many cancers, and PP2A inhibition has been suggested as a therapeutic means of targeting Mad2-overexpressing tumor cells.16 Western blotting demonstrated that LB100 exhibited a concentration-dependent decrease in Mad2 expression in 143B cells (Fig. 2C). Furthermore, combination treatment attenuated Mad2 levels and increased expression of phosphorylated-Histone H3 compared to cisplatin exposure alone, indicative of continued cell cycle progression into mitosis (Fig. 2D).
LB100 abrogates cisplatin-induced cell cycle arrest by inhibiting cell cycle checkpoints
Following DNA damage by chemotherapeutic agents, cells activate the kinases ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and rad3 related (ATR), which subsequently phosphorylate checkpoint kinase 1 (Chk1) and Chk2.17 Notably, the functional integrity of Chks is maintained by continuous dephosphorylation by PP2A.18 Activated Chk1 and Chk2 subsequently induce cell cycle arrest by ubiquitinating the phosphatase cdc25A, which normally functions to suppress cyclin E/cdc2 complex formation.19 Among its many functions, this classical complex promotes the G1/S transition via phosphorylation of retinoblastoma (Rb), which frees E2F to activate additional mitotic kinases.20 Compared to control and cisplatin treatment alone, both LB100 and combination treatment induced hyper-phosphorylation (i.e. deactivation) of Chk1 and Chk2. Furthermore, LB100 alone and combination treatment reduced expression of phosphorylated cdc2 with no change in total cdc2, suggesting that the diminished levels of phosphorylated cdc2 was related to dysfunction of Chk1 and Chk2 by LB100 (Fig. 3A). Likewise, LB100 and combination treatment increased expression of phosphorylated Rb, compared to cisplatin exposure alone, suggesting continued cell cycle progression. Additionally, we explored the effect of LB100 on free 14–3–3 protein levels. The 14–3–3 proteins exhibit a diverse array of regulatory functions, among which include inhibitory binding of cdc25 in the face of cell cycle checkpoint activation in response to DNA damage.21 After LB100 treatment, both alone and combined with cisplatin, there was increased expression of 14–3–3 proteins compared to cisplatin alone or control, further suggesting unrestrained mitotic progression and absent cell cycle checkpoint activation (Fig. 3A).
Figure 3.
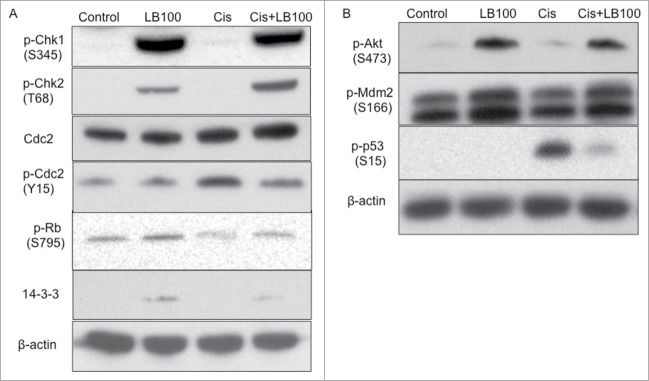
Potential mechanisms of cytotoxic cell cycle alterations after combined LB100 and cisplatin exposure. (A) Western blots of 143B cells treated with LB100, cisplatin, and combined treatment for 24 hours showing suppression of cell cycle checkpoints with addition of LB100 to cisplatin treatment via hyper-phosphorylation of Chk1, Chk2, and Rb, diminished phosphorylation of cdc2, as well as increased levels of free 14–3–3 protein. (B) Additional protein gel blots demonstrating attenuation of the Akt-Mdm2-p53 signaling cascade with addition of LB100 to cisplatin treatment.
Next, we further explored the effects of LB100 treatment on upstream signaling molecules involved in p53 activation. In response to DNA damage, PP2A suppresses protein kinase B (Akt1) signaling, which normally functions to phosphorylatively activate Mdm2, which then ubiquitinates p53 for proteasomal degradation.22 Control cells had low basal expressions of phosphorylated Akt, phosphorylated Mdm2, and phosphorylated p53 (Fig. 3B). Comparatively, treatment with cisplatin alone strongly increased phosphorylated p53 expression, indicative of the cellular response to DNA damage. LB100 treatment increased expression of phosphorylated Akt and Mdm2, consistent with PP2A inhibition, and when administered together with cisplatin, resulted in markedly reduced phosphorylated p53 levels, compared to cisplatin alone. Taken together, these results demonstrated that PP2A inhibition by LB100 activated the Akt-Mdm2-p53 pathway, diminishing levels of functional p53 in the context of cisplatin-induced DNA damage.
LB100 sensitizes osteosarcoma cells to cisplatin and reduces the incidence of pulmonary metastasis in vivo
We then investigated whether LB100 could enhance the efficacy of cisplatin treatment in an in vivo mouse model of osteosarcoma that exhibited a high rate of pulmonary metastases. There were no significant differences in average body weights among the control and LB100-treatment groups (data not shown). LB100 alone did not reduce tumor growth but when administered with cisplatin at the same doses and schedules, it significantly decreased growth of all xenografts compared with cisplatin alone (Fig. 4A, B). Strikingly, only 2/5 mice receiving both LB00 and cisplatin developed pulmonary metastases, in comparison to much higher rates in the other treatment arms: 5/5 in the control group, 5/5 in the LB100 group, and 4/5 in the cisplatin group (Fig. 4C). Diagnoses of metastatic nodules were confirmed histologically on routine H&E stains (Fig. 4D).
Figure 4.

Effect of LB100 combined with cisplatin on growth of 143B cells in vivo. (A) Tumor volumes and (B) weights of 143B xenografts are shown after treatment with LB100, cisplatin, and combined treatment. All animals were killed at 34 days, followed by immediate excision of tumors. (C) The number of metastatic lung lesions was significantly fewer in animals receiving combination treatment, compared to the other treatment arms. (D) Histopathology (40x magnification) of pulmonary nodules from excised lungs of 143B cell-bearing mice, confirming metastatic tumor (black arrowheads) in control, LB100-treated, and cisplatin-treated animals but absent in combination-treated animals.
Specimens from the primary tumors of treated animals were histopathologically analyzed after sacrifice. LB100 treatment resulted in decreased cell size and pyknotic nuclei in cells, while cisplatin alone produced cytoplasmic swelling, small pyknotic nuclei and vacuolization interspersed with some necrotic cells. Combination treatment was associated with small pyknotic nuclei and many regions of frank necrosis (Fig. 5A). Proliferating cell nuclear antigen (PCNA) is a classical marker, expressed in the G1 and S phases.23 Utilizing immunohistochemistry, PCNA expression was increased in LB100-treated xenografts, compared to controls, consistent with forced mitotic progression. Comparatively, cisplatin alone modestly diminished PCNA levels while combination treatment further reduced PCNA levels (Fig. 5B). To confirm our previous findings of Mad2 attenuation by LB100 in vitro, we found diminished expression of Mad2 on immunohistochemistry (Fig. 5C) and protein gel blotting (Fig. 5D) in LB100-treated and combination treatment groups, compared to cisplatin-treated samples and controls. As expected, we also found that LB100 increased levels of phosphorylated Chk1 and attenuated levels of phosphorylated p53 when added to cisplatin treatment (Fig. 5D). Taken together, these results confirmed our in vitro findings that LB100 abrogates p53 activation and Chk1-mediated G1/S arrest in response to cisplatin-induced DNA damage and promotes continued mitotic progression in part through attenuation of Mad2.
Figure 5.
Immunohistochemistry of excised 143B xenograft tumors. (A) Histopathologic features (200x magnification) of the primary143B tumors treated with control, cisplatin, or combined treatment. Combination treatment yielded small pyknotic nuclei in most cells and areas of prominent necrosis. Expression of PCNA (B) and Mad2 (C) in 143B xenograft tumors treated with vehicle, LB100, cisplatin, or combined treatment. Combination treatment significantly diminished PCNA and Mad2 expression in tumors compared with cisplatin alone. (D) Western blotting further demonstrating reduced Mad2 expression after LB100 treatment as well as reduced phosphorylated p53 and increased phosphorylated Chk1, in agreement with previous in vitro data.
Discussion
In this study, we have demonstrated that LB100 can sensitize osteosarcoma cells to the cytotoxic effects of cisplatin treatment, in vitro, and validated these findings in a mouse xenograft model, in vivo. Mechanistically, we found that LB100 abrogates activation of cell cycle checkpoints in response to DNA damage, evidenced by persistent hyper-phosphorylation of Chk1 and Chk2. Increased levels of phosphorylated Rb and reduced expression of phosphorylated cdc2 suggested continued mitotic progression in LB100-treated cells. Additionally, LB100 suppressed the normal activation of Akt signaling in response to DNA damage, resulting in Mdm2-mediated proteasomal degradation of p53. Taken together, these molecular changes led to increased apoptosis from mitotic catastrophe, stemming from forced mitotic progression in the setting of cisplatin-induced DNA damage. Furthermore, we-recapitulated the chemosensitizing properties of LB100 in a xenograft mouse model, in vivo. Notably, the majority of mice treated with both LB100 and cisplatin failed to develop pulmonary metastases at time of pre-determined animal sacrifice, in comparison to nearly 100% penetrance in other treatment arms. As such, the chemo-sensitizing properties of LB100 may extend to control of both local tumor growth and distant metastatic spread in osteosarcoma.
The efficacy of adjuvant cisplatin after surgery in prolonging progression-free survival in osteosarcoma was initially demonstrated in 2 large-scale prospective studies.24,25 Despite the passing of over 20 y since these reports, cisplatin continues to be a first-line agent for adjuvant chemotherapy after maximal limb-sparing surgery.26 Doxorubicin is an alternative first-line treatment for osteosarcoma and exerts its cytotoxicity through DNA intercalation.26 While our study only validated chemosensitization by LB100 for the single-agent, cisplatin, previous pre-clinical studies have shown enhancement of doxorubicin treatment after LB100 exposure in tumor models of glioblastoma,27 sarcoma,11 hepatocellular carcinoma,15 and pancreatic cancer.12 Furthermore, Lu et al. demonstrated that abrogation by LB100 of xenograft tumor growth, in vivo, was equally as efficacious for the alkylating agent, temozolomide, as for doxorubicin, suggesting the effects of LB100 were independent of the mechanism of action of adjuvant chemotherapy.9 Notably, LB100 has also been shown to sensitize tumors both in vitro and in vivo to clinically relevant dosing schedules of radiation.13,14 For osteosarcoma, adjuvant radiation has not been shown to improve overall survival after surgery and chemotherapy and therefore is not routinely offered to patients.28 In addition, prophylactic radiation of the lung fields to prevent pulmonary metastases has been studied in the past, but results from randomized studies have been too inconsistent to support its widespread practice.29 Future studies of LB100 in osteosarcoma and other tumors with propensity to spread to the lungs may investigate whether LB100 exposure prior to prophylactic lung field radiation may reduce the incidence of pulmonary metastases in tumor models, in vivo.
In our study, we validated previous studies, demonstrating the downregulatory effects of LB100 on the ATM/ATR-mediated DNA damage response pathway involving Chk protein activation and phosphorylated cdc activity.10,12 Likewise, we found LB100 induced functional p53 degradation by Mdm2 via suppression of the Akt signaling pathway. Although our in vitro and in vivo studies primarily utilized the p53-mutated osteosarcoma 143B cell line, we did show dose-dependent inhibition of p53-wild type and p53-null cell lines in U2OS and MG63 cells, respectively. Our data echoed previous in vitro dose inhibition studies, demonstrating similar effects of LB100 on cultured tumor cells, irrespective of p53 mutation status.9,10,15 Furthermore, Lu et al. demonstrated in both p53 wild type and p53 mutated glioblastoma cells that LB100 induced similar changes in Akt pathway-mediated suppression of activated p53 and induced mitotic catastrophe, suggesting the mechanism of cell death by LB100 exposure may be independent of p53 mutation status.9
Since the adoption of adjuvant chemotherapy after surgery for the treatment of osteosarcoma, the percentage of long-term survivors in patients under age 40 has risen to 60–70%.30 However, for those patients that present with or go on to develop metastatic disease, the prognosis remains poor, mostly due to development of drug resistance in tumors cells.31 As such, LB100 represents a promising strategy for treatment of osteosarcoma, particularly in those patients who develop resistance to cisplatin or other first-line chemotherapeutic agents. Results from the ongoing phase 1 clinical trial for LB100 are highly anticipated and may prompt future investigation into treatment of pediatric patients who make up a significant portion of those afflicted with osteosarcoma. Furthermore, the chemo-sensitizing properties of LB100 may potentially permit dose reduction of routine chemotherapies and alleviate systemic toxicities in the particularly vulnerable adolescent population.
Materials and Methods
Cell culture and reagents
143B (http://www.atcc.org/products/all/CRL-8303.aspx), MG63 (http://www.atcc.org/Products/All/CRL-1427.aspx), and U2OS (http://www.atcc.org/Products/All/HTB-96.aspx) osteosarcoma cells were purchased from American Type Culture Collection. All cells were cultured in Gibco Minimum Essential Medium (Life Technologies, http://www.lifetechnologies.com/us/en/home/life-science/cell-culture/mammalian-cell-culture/classical-media/mem.html) supplemented with 10% fetal bovine serum, 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate. Cisplatin (Sigma-Aldrich, http://www.sigmaaldrich.com/catalog/product/fluka/1134357?lang=enandregion=US) was dissolved in saline and store at 4°C. LB100 was provided by Lixte Biotechnology Holdings, Inc. (http://www.lixte.com/index.php) and was diluted in sterile PBS and stored at −20°C prior to administration.
PP2A phosphatase activity assay
143B cells were grown to 80% confluence in 6-well plates and treated with LB100 as indicated and prepared as described previously.27 Following treatment for 3 hours, cells were washed twice with cold water and lysed in RIPA buffer supplemented with Complete Protease Inhibitor Cocktail (Roche, http://lifescience.roche.com/shop/en/us/products/complete-3271352–1) for 20 minutes on ice. Cell lysates were sonicated for 10 seconds and then centrifuged at 20,000 × g for 15 minutes. Supernatants were assayed with the PP2A Phosphatase Assay Kit (Millipore, http://www.emdmillipore.com/US/en/product/PP2A-Immunoprecipitation-Phosphatase-Assay-Kit,MM_NF-17–313). Experiments were performed in triplicate, and data are presented as a percent mean of relative PP2A activity compared to control, ± SD.
Cytotoxicity assay
Cell survival was measured using the CCK-8 kit assay (Dojindo Laboratories, http://www.dojindo.com/store/p/456-Cell-Counting-Kit-8.html). All osteosarcoma cells were seeded at a density of 5,000 cells per well in 96-well plates and incubated at 37°C in humidified 5% CO2. Cells were allowed to attach for 24 hours, and the media was replaced with media containing equal volumes of the appropriate concentrations of LB100 or PBS vehicle. To determine 50% inhibitory concentrations (IC50), serially diluted LB100 was added to amount to the final intended concentrations in 143B cells. Absorbance values were determined at 540 nM on a spectrophotometer (Molecular Devices). All CCK-8 assays were performed in triplicate. In order to determine if LB100 could enhance the cytotoxic effects of cisplatin, cells were pre-treated with either a non-toxic or slightly toxic dose of LB100 for 1 hour prior to the addition of either a low or high dose of cisplatin. Cells were treated with both drugs for 48 hours. Cell viability was analyzed with the CCK-8 assay. Experiments were performed in triplicate and the data are presented as a percent mean ± SD.
Cell cycle analysis
143B cells were incubated in 60 mm sterile dishes for 24 hours and treated with LB100, cisplatin, or LB100 plus cisplatin at the indicated concentrations for 24 hours. For cell-cycle analysis, cells were washed with PBS and fixed overnight in ice-cold 70% methanol and stored at −20°C. The fixed cells were stained with 10 g/ml PI and 1 μg/mL RNase for 30 minutes and analyzed by fluorescent-activated cell sorting (FACS).
Western blotting
Cells were washed with 0.9% saline for 3 times and lysed in RIPA lysis buffer, supplemented with Complete Protease Inhibitor Cocktail (Roche). Proteins were separated by NuPAGE Bis-Tris 4–12% gels (Invitrogen, https://www.lifetechnologies.com/order/catalog/product/NP0321BOX) and transferred to PVDF membranes (Millipore, http://www.emdmillipore.com/US/en/product/Immobilon%C2%AE-Membranes%2C-Sandwiches-and-Blotting-Filter-Paper,MM_NF-C3117?CatalogCategoryID=#overview). Blocking buffer solution was used before immunoblotting with primary antibody. The membranes were detected through an HRP-conjugated species-specific secondary antibody and an ECL kit (Pierce, http://www.piercenet.com/product/pierce-ecl-western-blotting-substrate). Antibodies against the following epitopes were purchased from Cell Signaling Technology: caspase-3 (http://www.cellsignal.com/products/primary-antibodies/9662), cleaved caspase-3 (Asp175) (http://www.cellsignal.com/products/primary-antibodies/9661), caspase-9 (http://www.cellsignal.com/products/primary-antibodies/9502), cleaved caspase-9 (http://www.cellsignal.com/products/primary-antibodies/9505), phosphorylated p53 (Ser15) (http://www.cellsignal.com/products/primary-antibodies/9284), phosphorylated Erk (http://www.cellsignal.com/products/primary-antibodies/9101), phosphorylated Mdm2 (http://www.cellsignal.com/products/primary-antibodies/3521), phosphorylated Akt (http://www.cellsignal.com/products/primary-antibodies/9267), phosphorylated Rb (Ser795) (http://www.cellsignal.com/products/primary-antibodies/9301), Cdc2 (http://www.cellsignal.com/products/primary-antibodies/9116), phosphorylated cdc2 (Tyr15) (http://www.cellsignal.com/products/primary-antibodies/9111), phosphorylated Chk1 (Ser345) (http://www.cellsignal.com/products/primary-antibodies/2341), phosphorylated Chk2 (Thr68) (http://www.cellsignal.com/products/primary-antibodies/2661), phosphorylated histone H3 (Ser10) (http://www.cellsignal.com/products/primary-antibodies/9701), and phosphorylated (Ser) 14–3–3 binding motif (http://www.cellsignal.com/products/primary-antibodies/9601). Additional antibodies were purchased from Santa Cruz Biotechnology: β-actin (http://www.scbt.com/datasheet-47778-β-actin-c4-antibody.html), PCNA (http://www.scbt.com/datasheet-56.html), Gapdh (http://www.scbt.com/datasheet-365062-gapdh-g-9-antibody.html), and Mad2 (http://www.scbt.com/datasheet-6329-mad2-c-19-antibody.html).
In vivo osteosarcoma model and drug treatment
Six-week-old female nude athymic mice (nu/nu) were obtained from the Laboratory Animal Center of the Third Military Medical University (Chongqing, China). Animal protocols were in accordance with the Animal Care and Use Committee Guidelines of the Third Military Medical University. One × 106 143B cells were suspended in 100 µL PBS and injected subcutaneously in the right shoulder. Tumors were allowed to grow 8 d and then were randomly assigned to one of 4 groups (5 animals per group): control group (PBS), cisplatin group, LB100 group, and combination group. LB100 at 1.5 mg/kg, cisplatin at 1.5 mg/kg, and LB100 combined with cisplatin (at aforementioned doses; LB100 was administered 1 hour prior to cisplatin) were given intraperitoneally every other day. Tumor volumes and animal weights were measured every 2 days, as previously described.27 All mice were killed on day 34. Dissected tumors and lungs were weighed and fixed with 4% paraformaldehyde. For protein gel blotting analysis, tumors also were snap-frozen in liquid nitrogen.
Histologic analysis
Fixed tumors were paraffin-embedded, sectioned, and stained routinely with hematoxylin and eosin. Specimens were also stained with PCNA and Mad2 antibodies at 4°C overnight, followed by secondary antibody for 30 minutes at room temperature and DAB chromagen.
Statistical analysis
Mean values were reported as mean ± standard deviation, and t-tests were performed using GraphPad Prism Software (Graphpad Software, Inc.). A p-value of under 0.05 was considered to be of statistical significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Lixte Biotechnology Holdings, Inc. for providing LB100.
funding
This work was supported by the National Natural Science Foundation Program (NSFC81102042) as well as intramural grants from the Xinqiao Hospital, Third Military Medical University (Chongqing, China). This study was also supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (Bethesda, MD, USA).
References
- 1.Mirabello L, Troisi RJ, Savage SA.. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer 2009; 115:1531-43; PMID:19197972; http://dx.doi.org/ 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruland OS, Hoifodt H, Saeter G, Smeland S, Fodstad O.. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res 2005; 11:4666-73; PMID:16000559; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0165 [DOI] [PubMed] [Google Scholar]
- 3.Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC, Egeler RM.. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer 2011; 47:2431-45; PMID:21703851; http://dx.doi.org/ 10.1016/j.ejca.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 4.Janeway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R, Marina N.. Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children's Oncology Group. Cancer 2012; 118:4597-605; PMID:22252521; http://dx.doi.org/ 10.1002/cncr.27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang Z, Lu J, Lonser R, Kovach JS. Enhancement of cancer chemotherapy by simultaneously altering cell cycle progression and DNA-damage defenses through global modification of the serine/threonine phospho-proteome. Cell Cycle 2009; 8:3303-6; PMID:19806030; http://dx.doi.org/ 10.4161/cc.8.20.9689 [DOI] [PubMed] [Google Scholar]
- 6.Eichhorn PJ, Creyghton MP, Bernards R.. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta 2009; 1795:1-15; PMID:18588945 [DOI] [PubMed] [Google Scholar]
- 7.Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, Mao YQ, Kan B, Lei S, Wang GS, et al.. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol 2002; 128:223-30; PMID:11935314; http://dx.doi.org/ 10.1007/s00432-002-0326-5 [DOI] [PubMed] [Google Scholar]
- 8.Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y, Xu Z, Han X.. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci 2010; 101:1226-33; PMID:20331621; http://dx.doi.org/ 10.1111/j.1349-7006.2010.01523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, Zhuang Z.. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci U S A 2009; 106:11697-702; PMID:19564615; http://dx.doi.org/ 10.1073/pnas.0905930106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KE, Wei BR, Madigan JP, Hall MD, Simpson RM, Zhuang Z, Gottesman MM.. The protein phosphatase 2A inhibitor LB100 sensitizes ovarian carcinoma cells to cisplatin-mediated cytotoxicity. Mol Cancer Ther 2015; 14:90-100; PMID:25376608; http://dx.doi.org/ 10.1158/1535-7163.MCT-14-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Peng Y, Wang F, Tan X, Liu N, Fan S, Wang D, Zhang L, Liu D, Wang T, et al.. A synthetic cantharidin analog for the enhancement of doxorubicin suppression of stem cell-derived aggressive sarcoma. Biomaterials 2010; 31:9535-43; PMID:20875681; http://dx.doi.org/ 10.1016/j.biomaterials.2010.08.059 [DOI] [PubMed] [Google Scholar]
- 12.Bai X, Zhi X, Zhang Q, Liang F, Chen W, Liang C, Hu Q, Sun X, Zhuang Z, Liang T.. Inhibition of protein phosphatase 2A sensitizes pancreatic cancer to chemotherapy by increasing drug perfusion via HIF-1alpha-VEGF mediated angiogenesis. Cancer Lett 2014; 355:281-7; PMID:25304380; http://dx.doi.org/ 10.1016/j.canlet.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 13.Lv P, Wang Y, Ma J, Wang Z, Li JL, Hong CS, Zhuang Z, Zeng YX.. Inhibition of protein phosphatase 2A with a small molecule LB100 radiosensitizes nasopharyngeal carcinoma xenografts by inducing mitotic catastrophe and blocking DNA damage repair. Oncotarget 2014; 5:7512-24; PMID:25245035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, et al.. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res 2013; 19:4422-32; PMID:23780887; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai XL, Zhang Q, Ye LY, Hu QD, Fu QH, Zhi X, Su W, Su RG, Ma T, Chen W, et al.. Inhibition of protein phosphatase 2A enhances cytotoxicity and accessibility of chemotherapeutic drugs to hepatocellular carcinomas. Mol Cancer Ther 2014; 13:2062-72; PMID:24867249; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0800 [DOI] [PubMed] [Google Scholar]
- 16.Bian Y, Kitagawa R, Bansal PK, Fujii Y, Stepanov A, Kitagawa K.. Synthetic genetic array screen identifies PP2A as a therapeutic target in Mad2-overexpressing tumors. Proc Natl Acad Sci U S A 2014; 111:1628-33; PMID:24425774; http://dx.doi.org/ 10.1073/pnas.1315588111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperka T, Wang J, Rudolph KL.. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol 2012; 13:579-90; PMID:22914294; http://dx.doi.org/ 10.1038/nrm3420 [DOI] [PubMed] [Google Scholar]
- 18.Kim MA, Kim HJ, Brown AL, Lee MY, Bae YS, Park JI, Kwak JY, Chung JH, Yun J.. Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Exp Mol Med 2007; 39:205-12; PMID:17464182; http://dx.doi.org/ 10.1038/emm.2007.23 [DOI] [PubMed] [Google Scholar]
- 19.Aleem E, Kiyokawa H, Kaldis P.. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol 2005; 7:831-6; PMID:16007079; http://dx.doi.org/ 10.1038/ncb1284 [DOI] [PubMed] [Google Scholar]
- 20.Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH.. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 1991; 67:293-302; PMID:1655277; http://dx.doi.org/ 10.1016/0092-8674(91)90181-W [DOI] [PubMed] [Google Scholar]
- 21.Margolis SS, Perry JA, Forester CM, Nutt LK, Guo Y, Jardim MJ, Thomenius MJ, Freel CD, Darbandi R, Ahn JH, et al.. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 2006; 127:759-73; PMID:17110335; http://dx.doi.org/ 10.1016/j.cell.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CY, Oliner JD, Zhan Q, Fornace AJ Jr., Vogelstein B, Kastan MB.. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci U S A 1994; 91:2684-8; PMID:8146175; http://dx.doi.org/ 10.1073/pnas.91.7.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews MB, Bernstein RM, Franza BR Jr., Garrels JI.. Identity of the proliferating cell nuclear antigen and cyclin. Nature 1984; 309:374-6; PMID:6145097; http://dx.doi.org/ 10.1038/309374a0 [DOI] [PubMed] [Google Scholar]
- 24.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, et al.. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986; 314:1600-6; PMID:3520317; http://dx.doi.org/ 10.1056/NEJM198606193142502 [DOI] [PubMed] [Google Scholar]
- 25.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, Ayala A, Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the multi-institutional osteosarcoma study. Clin Orthop Relat Res 1991; 270:8-14; PMID:1884563 [PubMed] [Google Scholar]
- 26.Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ, Quintana J, Luchtman-Jones L, Villarroel M, Santana VM.. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children's Research Hospital OS99 trial. Cancer 2011; 117:2770-8; PMID:21656756; http://dx.doi.org/ 10.1002/cncr.25715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Zhuang Z, Song DK, Mehta GU, Ikejiri B, Mushlin H, Park DM, Lonser RR.. The effect of a PP2A inhibitor on the nuclear receptor corepressor pathway in glioma. J Neurosurg 2010; 113:225-33; PMID:20001590; http://dx.doi.org/ 10.3171/2009.11.JNS091272 [DOI] [PubMed] [Google Scholar]
- 28.DeLaney TF, Park L, Goldberg SI, Hug EB, Liebsch NJ, Munzenrider JE, Suit HD.. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005; 61:492-8; PMID:15667972; http://dx.doi.org/ 10.1016/j.ijrobp.2004.05.051 [DOI] [PubMed] [Google Scholar]
- 29.Whelan JS, Burcombe RJ, Janinis J, Baldelli AM, Cassoni AM.. A systematic review of the role of pulmonary irradiation in the management of primary bone tumours. Ann Oncol 2002; 13:23-30; http://dx.doi.org/ 10.1093/annonc/mdf047 [DOI] [PubMed] [Google Scholar]
- 30.Aljubran AH, Griffin A, Pintilie M, Blackstein M.. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol 2009; 20:1136-41; PMID:19153114; http://dx.doi.org/ 10.1093/annonc/mdn731 [DOI] [PubMed] [Google Scholar]
- 31.Asada N, Tsuchiya H, Ueda Y, Tomita K.. Establishment and characterization of an acquired cisplatin-resistant subline in a human osteosarcoma cell line. Anticancer Res 1998; 18:1765-8; PMID:9673402 [PubMed] [Google Scholar]