Abstract
Objective
To assess Spanish and Portuguese patients’ and physicians’ preferences regarding type 2 diabetes mellitus (T2DM) treatments and the monthly willingness to pay (WTP) to gain benefits or avoid side effects.
Methods
An observational, multicenter, exploratory study focused on routine clinical practice in Spain and Portugal. Physicians were recruited from multiple hospitals and outpatient clinics, while patients were recruited from eleven centers operating in the public health care system in different autonomous communities in Spain and Portugal. Preferences were measured via a discrete choice experiment by rating multiple T2DM medication attributes. Data were analyzed using the conditional logit model.
Results
Three-hundred and thirty (n=330) patients (49.7% female; mean age 62.4 [SD: 10.3] years, mean T2DM duration 13.9 [8.2] years, mean body mass index 32.5 [6.8] kg/m2, 41.8% received oral + injected medication, 40.3% received oral, and 17.6% injected treatments) and 221 physicians from Spain and Portugal (62% female; mean age 41.9 [SD: 10.5] years, 33.5% endocrinologists, 66.5% primary-care doctors) participated. Patients valued avoiding a gain in bodyweight of 3 kg/6 months (WTP: €68.14 [95% confidence interval: 54.55–85.08]) the most, followed by avoiding one hypoglycemic event/month (WTP: €54.80 [23.29–82.26]). Physicians valued avoiding one hypoglycemia/week (WTP: €287.18 [95% confidence interval: 160.31–1,387.21]) the most, followed by avoiding a 3 kg/6 months gain in bodyweight and decreasing cardiovascular risk (WTP: €166.87 [88.63–843.09] and €154.30 [98.13–434.19], respectively). Physicians and patients were willing to pay €125.92 (73.30–622.75) and €24.28 (18.41–30.31), respectively, to avoid a 1% increase in glycated hemoglobin, and €143.30 (73.39–543.62) and €42.74 (23.89–61.77) to avoid nausea.
Conclusion
Both patients and physicians in Spain and Portugal are willing to pay for the health benefits associated with improved diabetes treatment, the most important being to avoid hypoglycemia and gaining weight. Decreased cardiovascular risk and weight reduction became the third most valued attributes for physicians and patients, respectively.
Keywords: diabetes, discrete choice model, preferences, willingness to pay, hypoglycemia, weight, cardiovascular risk, HbA1c
Introduction
Diabetes mellitus is a complex chronic illness requiring continuous medical care with multifactorial risk reduction strategies beyond glycemic control.1 It is one of the most common metabolic disorders in the world and its prevalence in adults has been increasing for decades. The prevalence of type 2 diabetes mellitus (T2DM) in Spain has been projected between 12.5%2 and 13.8%,3 while in Portugal the prevalence has been estimated to be 7.2%.4 According to the Global estimates of diabetes prevalence for 2013, these numbers are expected to rise to 14.4% and 15.8%, respectively, by 2035.5
The increasing prevalence of T2DM has stimulated the development of many new approaches to treat hyperglycemia safely. The treatment aims in T2DM are to reduce and maintain glucose concentrations as close to normal for as long as possible and thereby prevent the appearance of complications.6 However, despite the benefits of therapy, studies have indicated that recommended glycemic goals are achieved by less than 50% of patients, which may be associated with decreased adherence to medications.7
Medication adherence in T2DM is determined by different factors including patient-centered concerns and beliefs about diabetes as a disease. Moreover, insufficient understanding of the benefits of medication and its side effects, difficulty following lifestyle recommendations as part of the health care plan, medication and health care cost, confusion about how to take medications, and psychological issues associated with chronic disease management can all have an influence on medication adherence.8
Including patients’ preferences into routine clinical practice has been related with an increment in medication adherence, improvements in treatment outcomes, and reduced health care cost.9 Several publications that study patients’ preferences for diabetes medications have been identified in the literature. Conversely, information about physicians’ preferences regarding T2DM has only been identified in two publications,10,11 thus showing that physicians’ preferences can be considered as rational, reasonable, and of great value.12 However, physician and patients disagree about how much risk is tolerable to achieve improvements in efficacy, given that patients are less tolerant in trading risk than physicians are, since the latter also placed great value on effectiveness/efficacy. On the other hand, patients placed greater value on the importance of health-related quality of life (HRQoL) and social attributes than physicians’ believed.13
Although it might seams that this topic has been widely studied, it has not been addressed in patients and physicians in Spain and Portugal, and considering that a patient-centered communication style that incorporates patient preferences into physicians’ judgments is recognized as a strategy for improving diabetes care,14 the information provided by the current study can help overcome the challenge of understanding patients’ behavior and vision of their own health, which can lead to a better diabetes control.
Discrete choice experiments (DCE) allow the analysis of preferences for complex multiattribute goods, such as medications.15 This technique is used in market research to assess how consumers value the characteristics that comprise a specific product, described in terms of underlying attributes.16 DCE are distinct from other conjoint methods because preferences are elicited by asking the respondent to choose one alternative from those presented, simulating real market situations.17 When out-of-the-pocket cost is included as an attribute in the DCE, the results can be used to calculate the willingness to pay (WTP) for the attributes being measured.18
This study aims to assess the preferences of Spanish and Portuguese patients with T2DM and their physicians with regard to diabetes treatments, including their side effects and methods of administration. Additional objectives are to determine the T2DM patients’ and physicians’ WTP to achieve certain therapeutic benefits and avoid side effects, as well as their WTP for alternative ways of treatment administration and changes in the frequency of self-monitoring blood glucose levels. Achieving these aims will in turn make it possible to identify the factors that may explain the patients’ and physicians’ preferences and to recognize potential differences between T2DM patients and physicians.
Methods
Design
This is an observational, descriptive, multicenter, exploratory study in the context of routine clinical practice in Spain and Portugal, not linked to any specific antidiabetic drug or treatment.
Discrete choice experiment
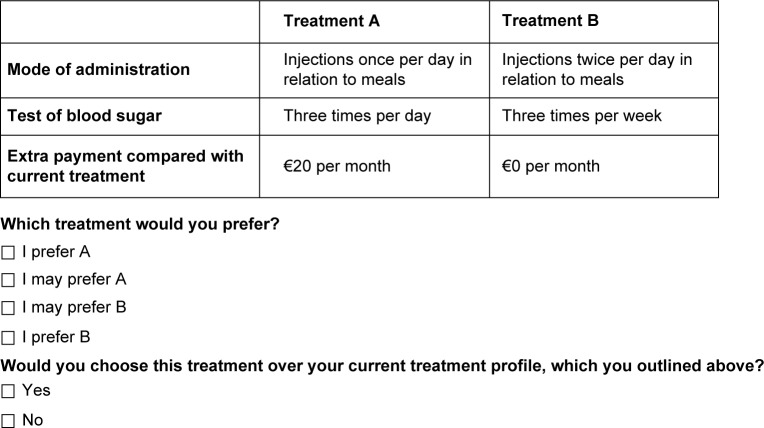
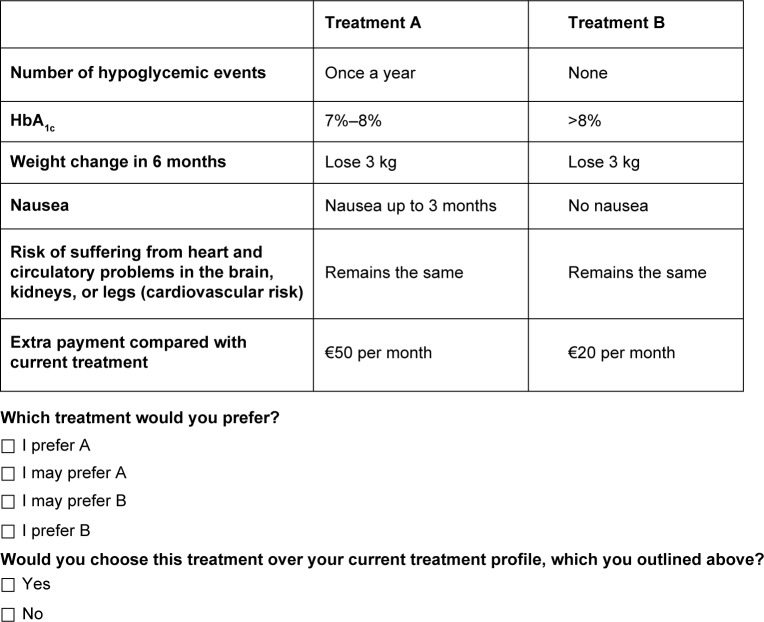
This technique is an attribute-based measure of benefit, based on the assumption that health care interventions can be described by their attributes and that an individual’s evaluation depends upon the levels of these attributes. Respondents are asked to choose between two alternatives. Resulting choices reveal an underlying utility function.19 Examples of alternatives presented to responders are described in Figures 1 and 2.
Figure 1.
Example of choice question convenience attributes.
Figure 2.
Example of choice question clinical attributes.
Abbreviation: HbA1c, glycated hemoglobin.
Medication attributes and levels selection
In accordance with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) good practices for conjoint analysis in health, the methodology for survey instrument development included: 1) Identification of medication attributes and levels, 2) Medication attributes and levels selection, 3) Experimental design, and 4) Construction of task.20
For the identification of attributes and levels, a comprehensive literature review with the objective of identifying, selecting, and summarizing published studies that assessed the preferences of T2DM patients for the attributes of antidiabetic treatments and/or their WTP for gaining health benefits or avoiding side effects was performed. Key words in English and Spanish joined by Booleans operators “or” and “and” (“diabetes mellitus”, “type 2 diabetes mellitus”, “treatment”, “medication”, “conjoint”, “conjoint analysis”, “conjoint measurement”, “conjoint studies”, “discrete choice experiments”, “DCE”, “discrete choice modeling”, and “preference studies”) were used. The search was carried out in a pool of free publicly-accessible databases that are recommended by the Cochrane Handbook for Systematic Reviews of Interventions21 and in the main national (Spanish) databases (MedLine/PubMed, Cochrane Library, ISI WOK, SCOPUS, and Medicina en Español-MEDES). Articles published until October 1, 2013 were selected.
Seven publications studying the preferences of T2DM patients for the attributes of antidiabetic treatments and/or their WTP for gaining health benefits or avoiding side effects were retrieved.10,22–27 Although the questionnaire used for this study was based on surveys utilized previous studies, due to cultural differences between studied populations and Spanish and Portuguese people, it was deemed important to confirm that the attributes and levels identified in the literature were also important for Spain and Portugal.28,29
For that purpose, an online consult with a group of six diabetes’ experts (three endocrinologists from Spain and Portugal, respectively) with vast experience in the management of T2DM and another consult with nine patients helped define, from the attributes and levels of antidiabetics that were identified, the ones that represented the antidiabetic medication characteristics that were more significant for these patients and clinical practice.
The result of such consults produced eight attributes and 28 levels. Given the amount of attributes levels, a decision was made to divide the attributes into two groups: convenience attributes (mode of administration and blood glucose monitoring) and clinical attributes (number of hypoglycemic events, glycated hemoglobin – HbA1c, bodyweight change in 6 months, nausea, and cardiovascular risk) performing two separate DCE. Furthermore, additional cost per month was included as an attribute in the choice question so as to enable calculation of the WTP (Table 1).
Table 1.
Attributes and levels for the choice questions
| Attributes | Levels |
|---|---|
| Mode of administration | Injections twice a day in relation to meals |
| Injection once a day in relation to meals | |
| Injection once a day irrespective of meals | |
| Oral antidiabetics (OAD) up to three times a day with meals | |
| Blood glucose monitoring | Three times a day |
| Once a day | |
| Three times per week | |
| No need for testing | |
| Number of hypoglycemic events | None |
| Once a week | |
| Once a month | |
| Once a year | |
| HbA1c | <6% |
| 6%–7% | |
| 7%–8% | |
| >8% | |
| Weight change in 6 months | Gain 3 kg |
| Remains the same weight | |
| Loses 3 kg | |
| Loses 6 kg | |
| Nausea | Mild nausea for up to 3 months |
| None | |
| Cardiovascular risk | Decreases |
| Remains the same | |
| Additional payment per month | €100 |
| €50 | |
| €20 | |
| €0 |
Abbreviation: HbA1c, glycated hemoglobin.
Experimental design and survey instrument
Following the recommendations of the ISPOR task force report,30 the R statistical software package31 was used to generate the DCE design that complied with the desirable properties of orthogonality and balance. The orthogonal design ensured that the resulting parameter estimates were uncorrelated and could be determined independently of the other attributes, while a balanced design ensured that the attribute levels occurred with equal frequency within each attribute, yielding equally robust results for all levels.23 Two fractional factorial analysis (orthogonal matrix), one for each group of attributes, produced two blocks of 16 sets of choices (scenarios) each containing multiple choices linked together in each question. Resulting scenarios were checked for dominated alternatives. Dominated alternatives were treated according to ISPOR guidelines.30
Considering the length of the sociodemographic and clinical variables questions included in the survey and the fact that respondents may present fatigue after 17 choice sets,32 following the standard practice, the sets of choices were distributed among four versions of questionnaires of eight multiple choice questions apiece (four convenience attribute scenarios and four clinical attribute scenarios). Physicians and patients were randomly assigned to one version of the survey. Examples of choice sets for each group of attributes are presented in Figures 1 and 2.
The final survey instruments for physicians included sociodemographic variables and the DCE for convenience and clinical attributes, while the surveys used for patients included three sections: clinical variables, including current diabetes treatment, to be completed by the physician, and sociodemographic and clinical variables to be completed by the patients, and the DCE for convenience and clinical attributes. The questionnaire did not contain any information regarding any specific diabetes medication.
Study sample and data collection procedures
A sample size of 195 physicians and 267 patients was estimated using the maximum variability criterion, with a confidence level of 95% and assuming that the variability in response preferences would be higher within the patients group. A precision of 7% for the group of physicians and of 6% for the group of patients was considered in order to obtain a larger sample of patients in comparison with the physicians. Other parameters used were the size of the Spanish and Portuguese adult population (between 20 and 79 years)33,34 and the T2DM prevalence of 12.5%2 and 7.2%,4 respectively, for patients and the number of primary-care physicians and family medicine doctors operating in the public health care system in Spain35 and Portugal,36 respectively. Although new practical guides for sample size requirements for DCE have recently been published,37 this publication also states that for DCE designs, sample sizes over 100 are able to provide a basis for modeling preference data.
Sample size was distributed proportionally throughout Spain and Portugal. Physicians were recruited from diverse hospitals and outpatient clinics located throughout both territories, while patients were recruited from eleven centers (hospitals and outpatient clinics) operating in the national health care system in different autonomous communities in Spain and Portugal.
Physicians were selected if they were primary-care doctors, family medicine doctors, or endocrinologists with at least 3 years’ experience in managing T2DM patients and were working in the national health care system in either of the countries. Inclusion criteria for patients comprised: being over 18 years old, diagnosed with T2DM for at least 5 years, and receiving treatment during the year prior to inclusion.
Physicians were invited to participate by answering an online survey. Physicians were asked which of the hypothetical treatments they would like to prescribe to their patients considering the attributes levels presented in each of the choice task. In other words, physicians were asked to choose which treatment they were willing to prescribe for a given cost if it would guarantee the associated benefits.
Patients were recruited from medical consults. During face-to-face interviews, patients who agreed to participate and signed the informed consent, were asked to complete a printed study survey. To make sure participants understood the DCE exercise, an internal consistency check was included at the beginning of the DCE. This included a dominance test, where one option was clearly superior to the other. Respondents that failed the test were excluded from the analysis.38
Statistical analyses
Once the collection of data was completed, a database containing the DCE results for each group of participants (physicians and patients) was created. The database was validated and checked for consistency and errors before the descriptive analyses were performed. These descriptive analyses included relative and absolute frequency calculations for qualitative variables, and central tendency and dispersion measures for quantitative ones, for each group of participants.
For the analysis of the DCE data, the conditional logit model was used, which is available through the mlogit statistical package of the R statistical software.39 This model is recommended when the attributes that are included in the hypothetical treatment are the only variables that are to be assessed. The conditional logit model is a mathematical regression model designed to estimate the part-worth (β) of the attribute level (characteristic) that contributes to the utility of each hypothetical treatment presented in the discrete choice. The utility corresponds to the value that individuals assign to a product through the combination of its attributes, so that its value is the maximum for the choice made in a set of options. From these utility values, the probability of each treatment being selected is calculated. The higher the utility value, the higher the probability of a treatment being selected, and fulfilling the rule that the sum of all probabilities adds up to one.40
For the analysis of the convenience and clinical attributes, two models were constructed, one for each group of attributes. The attributes characterizing the treatments were collected and coded as qualitative variables, named as variables with different dummy categories, defined by the levels that were included. Given that individuals typically show a relative disregard for distant gains and losses in comparison with more immediate ones,41,42 and that in routine clinical practice it is recommended to set short-term weight goals in 6-month frames,43 the statistical analysis of the hypoglycemic events and weight attributes was performed using the attributes just as they were asked (categorical variables), thus avoiding bias. However, a linear transformation of certain attributes was considered (transformation of some levels into values). These changes were tested using likelihood tests. The transformed variables were blood glucose monitoring (per month), HbA1c, and additional payment per month.
As the importance of different attributes cannot be compared directly using the parameter estimates due to the likelihood of confounding with the underlying utility scales, the relative impact of attributes is usually examined by converting estimates into a common scale, in this case the marginal rates of substitution in the form of WTP.44
The WTP for the attribute levels was obtained from the quotient between the utility value of each attribute and the utility value calculated for the payment.20 Although the WTP calculations are the same for qualitative and quantitative variables, certain nuances must be considered:
Quantitative variables: when an attribute is expressed as a quantitative variable (ie, HbA1c), the WTP is obtained directly from the utility values of the given attribute and the payment. It is interpreted as the cost that a participant is willing to pay to increase the attribute by one unit (ie, WTP to increase HbA1c by 1%).
Qualitative variables: when an attribute is expressed as a qualitative variable (ie, mode of administration), WTP is obtained from the quotient between the difference in utility values among the categories to be exchanged and the payment. It is interpreted as the cost that a participant is willing to pay in order to change the attribute being assessed (ie, WTP for oral antidiabetics instead of injections in relation to meals).
Since WTP was calculated as a ratio between two variables, confidence intervals (CIs) could not be derived directly from the parameters of the conditional logit estimation, and therefore the bootstrap technique was utilized. The purpose of this methodology is to simulate a large number of samples with replacements (10,000 simulations) of the same size as the original sample used in the study. In these samples, the Krinsky Robb method for obtaining the CIs was used.45
To identify conditioning factors that might explain either physicians’ or patients’ preferences, a multinomial logit model was used, where the preferences will be the dependent variables and the sociodemographic and clinical variables the explanatory ones.39 For the comparison of results among patients and physicians, the Z-test was utilized. This test is used for the comparison of regression coefficients when they have been estimated by maximum likelihood, as in the case of a conditional logit model.46–48
Ethical considerations
This study followed the principles of the Declaration of Helsinki. It was developed to ensure that Good Clinical Practices were observed and in keeping with The Tripartite Harmonized ICH Guideline49 principles. The study protocol was submitted to the Agencia Española del Medicamento y Productos Sanitarios and Comissão Nacional de Protecção de Dados for its classification and to all participant centers’ Clinical Research Ethic Committees for approval. Data were treated confidentially and dissociated in accordance with the Spanish (15/1999 Personal Data Protection Law) and Portuguese regulation (Lei n⍛ 67/98).
Results
Patients’ results
Three-hundred and fifty-five patients were recruited. From those 25 were excluded due to failure in the DCE dominance test. Three-hundred and thirty patients participated in the study; 84% of them were Spaniards and were distributed similarly by sex. Patients had a mean age of 62.4 (SD: 10.3) years. Mean body mass index (32.5±6.8) showed an obese population in which more than half (53%) presented T2DM complications. All sociodemographic and clinical variables are presented in Table 2.
Table 2.
Patients’ and physicians’ sociodemographic and clinical characteristics
| Patients’ sociodemographic characteristic | Spain | Portugal | Total | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 62.29 | 10.24 | 62.93 | 9.20 | 62.39 | 10.27 |
| Time since diagnosis (years) | 14.32 | 8.39 | 12.15 | 7.03 | 13.98 | 8.22 |
| Weight (kg) | 86.25 | 17.69 | 85.69 | 15.41 | 86.17 | 17.33 |
| Height (cm) | 162.56 | 10.27 | 165.25 | 8.89 | 162.99 | 10.36 |
| BMI (kg/m2) | 32.70 | 7.01 | 31.45 | 5.69 | 32.50 | 6.82 |
| n | % | n | % | n | % | |
| Country | 278 | 84 | 52 | 16 | 330 | 100 |
| Sex | ||||||
| Female | 143 | 51.4 | 21 | 40.4 | 164 | 49.7 |
| Male | 133 | 47.8 | 31 | 59.6 | 164 | 49.7 |
| Level of education | ||||||
| Primary school | 146 | 52.50 | 32 | 61.50 | 178 | 53.90 |
| Secondary school | 74 | 26.60 | 12 | 23.10 | 86 | 26.10 |
| University | 41 | 14.70 | 5 | 9.60 | 46 | 13.90 |
| Postgraduate | 3 | 1.10 | 2 | 3.80 | 5 | 1.50 |
| Other studies | 12 | 4.30 | 1 | 1.90 | 13 | 3.90 |
| Annual income | ||||||
| <€12,000 | 129 | 46.40 | 31 | 59.60 | 160 | 48.50 |
| €12,000–€20,000 | 74 | 26.60 | 13 | 25.00 | 87 | 26.40 |
| €20,000–€30,000 | 48 | 17.30 | 3 | 5.80 | 51 | 15.50 |
| €30,000–€50,000 | 11 | 4.00 | 4 | 7.70 | 15 | 4.50 |
| >€50,000 | 2 | 0.70 | 1 | 1.90 | 3 | 0.90 |
| Weight change since diagnosis | ||||||
| Unchanged | 46 | 16.50 | 15 | 28.80 | 61 | 18.50 |
| Has lost weight | 75 | 27.00 | 15 | 28.80 | 90 | 27.30 |
| Has gained 0–2 kg | 24 | 8.60 | 6 | 11.50 | 30 | 9.10 |
| Has gained 2–5 kg | 39 | 14.00 | 6 | 11.50 | 45 | 13.60 |
| Has gained 5–10 kg | 38 | 13.70 | 5 | 9.60 | 43 | 13.00 |
| Has gained >10 kg | 54 | 19.40 | 5 | 9.60 | 59 | 17.90 |
| T2DM complications | ||||||
| None | 123 | 44.24 | 32 | 61.54 | 155 | 46.97 |
| Eye disease | 69 | 24.82 | 6 | 11.54 | 75 | 22.73 |
| Kidney problems | 65 | 23.38 | 3 | 5.77 | 68 | 20.61 |
| Heart disease | 79 | 28.42 | 6 | 11.54 | 85 | 25.76 |
| Male impotence | 19 | 6.83 | 3 | 5.77 | 22 | 6.67 |
| Nervous disease | 17 | 6.12 | 1 | 1.92 | 18 | 5.45 |
| Others | 21 | 7.55 | 4 | 7.69 | 25 | 7.58 |
| Frequency of hypoglycemia | ||||||
| One hypoglycemia per week | 27 | 9.7 | 1 | 1.9 | 28 | 8.5 |
| One hypoglycemia per month | 62 | 22.3 | 6 | 11.5 | 68 | 20.6 |
| One hypoglycemia per year | 90 | 32.4 | 8 | 15.4 | 98 | 29.7 |
| Never had an hypoglycemia | 99 | 35.6 | 37 | 71.2 | 136 | 41.2 |
| Last HbA1c level measured (%) | ||||||
| >9 | 46 | 16.50 | 3 | 5.80 | 49 | 14.80 |
| 8–9 | 42 | 15.10 | 6 | 11.50 | 48 | 14.50 |
| 7–8 | 104 | 37.40 | 25 | 48.10 | 129 | 39.10 |
| <6 | 42 | 15.10 | 9 | 17.30 | 51 | 15.50 |
| Frequency of blood glucose monitoring | ||||||
| Three times a day | 46 | 16.50 | 3 | 5.80 | 49 | 14.80 |
| Once a day | 42 | 15.10 | 6 | 11.50 | 48 | 14.50 |
| Three times a week | 104 | 37.40 | 25 | 48.10 | 129 | 39.10 |
| Once a month | 42 | 15.10 | 9 | 17.30 | 51 | 15.50 |
| None | 35 | 12.60 | 9 | 17.30 | 44 | 13.30 |
| Nausea due to T2DM treatment | ||||||
| Yes | 45 | 16.20 | 8 | 15.40 | 53 | 16.1 |
| No | 233 | 83.80 | 44 | 84.60 | 277 | 83.9 |
| Mode of treatment administration | ||||||
| Injections +3/day in relation to meals | 90 | 32.37 | 3 | 5.77 | 93 | 28.18 |
| Injections 2/day with meals | 30 | 10.79 | 4 | 7.69 | 34 | 10.30 |
| Injections 1/day, not related to meals | 86 | 30.94 | 5 | 9.62 | 91 | 27.58 |
| OAD 1–2/day, with meals | 137 | 49.28 | 41 | 78.85 | 178 | 53.94 |
| Physicians’ sociodemographic characteristic | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 42.48 | 10.65 | 39.33 | 9.63 | 41.87 | 10.51 |
| Time since the beginning of practice (years) | 15.43 | 11.35 | 13.35 | 9.69 | 15.02 | 11.06 |
| n | % | n | % | n | % | |
| Country | 178 | 80.5 | 43 | 19.5 | 221 | 100.00 |
| Sex | ||||||
| Female | 105 | 59.00 | 33 | 76.70 | 138 | 62.40 |
| Male | 73 | 41.00 | 10 | 23.30 | 83 | 37.60 |
| Specialty | ||||||
| Primary care | 104 | 58 | 43 | 100.00 | 147 | 66.50 |
| Endocrinologist | 74 | 41.60 | 0 | 0 | 74 | 33.50 |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; OAD, oral antidiabetics; T2DM, type 2 diabetes mellitus; SD, standard deviation.
The analysis showed that, with the exception of one hypoglycemic event per month, all attributes were significant predictors of choice (P<0.05). Avoiding gaining 3 kg/6 months was the most valued attribute followed by avoiding one hypoglycemia event per month (Table 3). The WTP analysis demonstrated that patients were willing to pay €68.14 to avoid a gain of 3 kg/6 months. In comparison with these, patients were willing to pay up to €54.80 to avoid one hypoglycemic event per month. Monthly WTP to reduce cardiovascular risk was €43.01, €42.74 to avoid nausea, and €24.28 to avoid a 1% increase in HbA1c. Regarding the mode of administration, patients were willing to pay up to €25.65 for one injection less per day. To gain flexibility when it came to injections (irrespective of meals instead of in relation to meals), patients were willing to pay €16.23 per month. Oral antidiabetics were always preferred over injections in relation to meals or irrespective of meals. To reduce the frequency of blood glucose monitoring tests (one less per day), patients were willing to pay €10.19 per month (Table 4).
Table 3.
Patients’ and physicians’ preferences for the treatment attributes
| Attribute | Patients (n=330)
|
Physicians (n=221)
|
Comparison P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated coefficient (β) | 95% CI | P-value | Estimated coefficient (β) | 95% CI | P-value | ||||
| Administration: injection twice a day in relation to meals (reference) | |||||||||
| Injection 1/day in relation to meals | 1.0668 | 0.6722 | 1.4614 | <0.001 | 1.8143 | 1.2933 | 2.3353 | <0.001 | 0.0248 |
| Injection 1/day irrespective of meals | 1.7417 | 1.2378 | 2.2457 | <0.001 | 2.5146 | 1.7628 | 3.2665 | <0.001 | 0.0939 |
| OAD up to 3/day with meals | 2.3210 | 1.9128 | 2.7292 | <0.001 | 2.4662 | 1.9705 | 2.9620 | <0.001 | 0.6707 |
| Blood glucose monitoring (per month) | −0.0141 | −0.0184 | −0.0098 | <0.001 | −0.0346 | −0.0411 | −0.0282 | <0.001 | 0.0000 |
| Additional payment per month | −0.0416 | −0.0478 | −0.0354 | <0.001 | −0.0263 | −0.0328 | −0.0197 | <0.001 | 0.0009 |
| Hypoglycemic event: 1/week (reference) | |||||||||
| Hypoglycemic events: 1/year | −0.0622 | −0.6633 | 0.5390 | <0.001 | 2.6547 | 1.9268 | 3.3826 | <0.001 | 0.1077 |
| Hypoglycemic events: 1/month | 0.9227 | 0.4015 | 1.4439 | 0.8394 | 0.9641 | −0.1329 | 2.0610 | 0.0850 | 0.0002 |
| Hypoglycemic events: none | 0.9976 | 0.5935 | 1.4017 | <0.001 | 1.8995 | 1.3409 | 2.4580 | <0.001 | 0.0103 |
| HbA1c (%) | −0.4695 | −0.5958 | −0.3431 | <0.001 | −0.8329 | −1.0123 | −0.6534 | <0.001 | 0.0012 |
| Weight: gain 3 kg (reference) | |||||||||
| Weight: remains the same | 1.3176 | 1.0028 | 1.6324 | <0.001 | 1.1037 | 0.7358 | 1.4716 | <0.001 | 0.3935 |
| Weight: lose 3 kg | 2.1698 | 1.2180 | 3.1216 | <0.001 | 0.9808 | −0.5174 | 2.4789 | 0.1995 | 0.1904 |
| Weight: lose 6 kg | 1.8155 | 1.3278 | 2.3032 | <0.001 | 1.5389 | 1.0485 | 2.0293 | <0.001 | 0.4416 |
| Mild nausea for up to 3 months (reference) | |||||||||
| Nausea: none | 0.8265 | 0.4049 | 1.2481 | <0.001 | 0.9478 | 0.3506 | 1.5451 | <0.001 | 0.7578 |
| CVR: remains the same (reference) | |||||||||
| CVR: decreases | 0.8318 | 0.3715 | 1.2920 | <0.001 | 1.0206 | 0.3865 | 1.6546 | <0.001 | 0.6496 |
| Additional payment per month | −0.0193 | −0.0235 | −0.0152 | <0.001 | −0.0066 | −0.0122 | −0.0010 | <0.001 | 0.0004 |
Note: Bold values indicate significance value P<0.05.
Abbreviations: CI, confidence interval; CVR, cardiovascular risk; HbA1c, glycated hemoglobin; OAD, oral antidiabetics.
Table 4.
Patients’ and physicians’ willingness to pay for diabetes treatment attributes
| Attribute | Unit change | Patients
|
Physicians
|
||||
|---|---|---|---|---|---|---|---|
| Monthly WTP (€) | 95% CI
|
Monthly WTP (€) | 95% CI
|
||||
| Inferior (€) | Superior (€) | Inferior (€) | Superior (€) | ||||
| Hypoglycemic events | Avoid one hypoglycemic event/month | 54.80* | 23.29 | 82.26 | 287.18 | 160.31 | 1,387.21 |
| Avoid one hypoglycemic event/week | 51.59* | 37.79 | 64.26 | 141.42 | 13.95 | 613.62 | |
| Avoid one hypoglycemic event/year | 3.87 | 0.34 | 24.81 | 114.18 | 35.29 | 409.49 | |
| Weight | Avoid gaining 3 kg/6 months | 68.14 | 54.55 | 85.08 | 166.87 | 88.63 | 843.09 |
| Reduce 6 kg/6 months | 25.75** | 8.18 | 48.00 | 65.80 | 9.34 | 411.52 | |
| Reduce 3 kg/6 months | 44.07** | 7.07 | 84.80 | 18.59 | 2.80 | 835.62 | |
| CVR | Decrease CVR | 43.01 | 20.46 | 62.83 | 154.30 | 98.13 | 434.19 |
| Nausea | Avoid nauseas | 42.74 | 23.89 | 61.77 | 143.30 | 73.39 | 543.62 |
| HbA1c | Avoid increasing HbA1c by 1% | 24.28 | 18.41 | 30.31 | 125.92 | 73.30 | 622.75 |
| Mode of administration | OAD instead of injections in relation to meals | 30.16 | 25.33 | 34.86 | 69.07 | 44.99 | 100.39 |
| One injection less per day | 25.65 | 17.09 | 35.91 | 26.66 | 11.79 | 45.89 | |
| Administration irrespective of meals instead of in relation to meals | 16.23 | 9.84 | 22.59 | 24.82 | 16.07 | 33.55 | |
| OAD instead of injections irrespective of meals | 13.93 | 7.63 | 20.55 | 1.84 | 0.27 | 22.25 | |
| Blood glucose monitoring | One blood glucose monitoring test less/day | 10.19 | 7.18 | 13.91 | 39.55 | 27.61 | 56.18 |
| One blood glucose monitoring test less/month | 0.34 | 0.24 | 0.46 | 1.32 | 0.92 | 1.87 | |
Note:
Difference not statistically significant (P=0.8394).
Difference not statistically significant (P=0.3826).
Abbreviations: CI, confidence interval; CVR, cardiovascular risk; HbA1c, glycated hemoglobin; OAD, oral antidiabetics; WTP, willingness to pay.
Potentially better clinical outcomes are preferred over poorer ones when all other things are kept constant. Nevertheless, in this study, there was a slight disordering of the WTP for the frequency of hypoglycemias and the weight attributes, where avoiding one hypoglycemia per month was valued more than avoiding one per week and losing 3 kg was valued more than losing 6 kg/6 months. However, these anomalies were not statistically significant (P>0.05).
Neither sex, being over 65 years old, having incomes above €12,000 a year, frequency of blood glucose control, frequency of hypoglycemias, HbA1c value nor obesity were significant conditioning factors (P>0.05) for patients’ preferences. Having elapsed more than 10 years since the diagnosis and receiving injectable treatment were conditioning factors for patients’ preferences for the convenience attributes (Table S1).
Physicians’ results
Of the 221 professionals who participated in the study, the majority were Spaniards (81%). The sex distribution varied between countries, the proportion of women being 59.0% and 76.7% in Spain and Portugal, respectively. The mean age of physicians was 41.9 (SD: 10.5) years, being slightly younger in Portugal (39.3 [9.6] vs 42.5 [10.6]). Although in Portugal all the participants were primary-care doctors with a mean time since the beginning of practice of 13.3 (9.7) years, in Spain, family physicians represented 58%, whereas the other 42% were endocrinologists, with a mean experience of treating patients with T2DM of 15.4 (11.3) years (Table 2).
Regarding antidiabetic attributes, data analyses showed that, with the exception of one hypoglycemic event per year and a bodyweight reduction of 3 kg/6 months, all attributes were significant predictors of choice (P<0.05). Avoiding one hypoglycemic event per week was the most valuated attribute followed by avoiding gains in bodyweight of 3 kg/6 months and decreasing cardiovascular risk (Table 3).
For the attribute hypoglycemic events, physicians were willing to pay €287.18 to avoid one hypoglycemic event per week. In comparison with these, for the weight attribute, physicians were willing to pay €166.87 to avoid gaining 3 kg. Monthly WTP to reduce cardiovascular risk was €154.30, €143.30 to avoid nausea, and €125.92 to avoid a 1% increase in HbA1c. Regarding the mode of administration, physicians were willing to pay up to €69.07 to reduce the number of injections to one less per day. To gain flexibility at the time of injections (irrespective of meals instead of in relation to meals), physicians were willing to pay €26.66. Oral antidi-abetics were always preferred over injections in relation to meals, although the orals were considered comparable to injections irrespective of meals. Findings showed that physicians were willing to pay €39.55 to reduce the frequency of blood glucose monitoring tests (one less per day) (Table 4).
Neither sex, country of origin, medical specialty nor having more than 10 years practicing the specialty were significant conditioning factors for physicians’ preferences (P>0.05) (Table S1).
Comparison between patients’ and physicians’ preferences
Considering the utility values obtained for the convenience and clinical attributes, the 16 scenarios were ordered from the most preferred to the last. These analysis demonstrated that both physicians and patients clearly preferred a similar treatment: a medication that is administered by injection, once a day and irrespective of meals; that requires blood glucose monitoring three times per week; maintains HbA1c between 6% and 7%; reduces 6 kg/6 months; decreases cardiovascular risk; does not produce nausea; and is only associated to one hypoglycemic event per year. Tables 5 and 6 described the preferred scenarios for both patients’ and physicians’.
Table 5.
Physicians’ and patients’ preferences for the convenience attributes
| Patients’ preferred treatment | Mode of administration | Blood glucose monitoring | Additional payment/month (€) | Physicians’ preferred treatment |
|---|---|---|---|---|
| 1 | Injection 1/day irrespective of meals | Three times per week | 0 | 1 |
| 2 | OAD up to 3/day with meals | No need for testing | 20 | 2 |
| 3 | Injection 1/day in relation to meal | No need for testing | 0 | 3 |
| 4 | OAD up to 3/day with meals | Three times a day | 0 | 9 |
| 5 | Injection 1/day irrespective of meals | Once a day | 20 | 5 |
| 6 | OAD up to 3/day with meals | Once a day | 50 | 6 |
| 7 | Injection 1/day irrespective of meals | No need for testing | 50 | 4 |
| 8 | Injection 2/day in relation to meals | Once a day | 0 | 11 |
| 9 | Injection 2/day in relation to meals | Three times per week | 20 | 10 |
| 10 | Injection 1/day in relation to meals | Three times a day | 20 | 12 |
| 11 | Injection 1/day in relation to meals | Three times per week | 50 | 7 |
| 12 | OAD up to 3/day with meals | Three times per week | 100 | 8 |
| 13 | Injection 2/day in relation to meals | Three times a day | 50 | 16 |
| 14 | Injection 1/day in relation to meals | Once a day | 100 | 13 |
| 15 | Injection 1/day irrespective of meals | Three times a day | 100 | 15 |
| 16 | Injection 2/day in relation to meals | No need for testing | 100 | 14 |
Abbreviation: OAD, oral antidiabetics.
Table 6.
Physicians’ and patients’ preferences for the clinical attributes
| Patients’ preferred treatment | No of hypoglycemic events | HbA1c | Weight change/6 months | Nauseas/3 months | CVR | Additional payment per month (€) | Physicians’ preferred treatment |
|---|---|---|---|---|---|---|---|
| 1 | 1/year | 6%–7% | Lose 6 kg | None | Decreases | 0 | 1 |
| 2 | 1/week | <6% | Lose 3 kg | None | Decreases | 20 | 4 |
| 3 | None | 7%–8% | Lose 6 kg | None | The same | 20 | 5 |
| 4 | 1/month | <6% | Lose 6 kg | Mild nausea | Decreases | 50 | 3 |
| 5 | None | >8% | The same | None | Decreases | 50 | 7 |
| 6 | None | 6%–7% | Lose 3 kg | Mild nausea | Decreases | 100 | 6 |
| 7 | 1/month | >8% | Lose 3 kg | None | The same | 0 | 14 |
| 8 | 1/week | 7%–8% | The same | Mild nausea | Decreases | 0 | 13 |
| 9 | 1/year | <6% | The same | None | The same | 100 | 2 |
| 10 | None | <6% | Gain 3 kg | Mild nausea | The same | 0 | 8 |
| 11 | 1/month | 6%–7% | The same | Mild nausea | The same | 20 | 10 |
| 12 | 1/year | 7%–8% | Lose 3 kg | Mild nausea | The same | 50 | 9 |
| 13 | 1/year | >8% | Gain 3 kg | Mild nausea | Decreases | 20 | 11 |
| 14 | 1/week | 6%–7% | Gain 3 kg | None | The same | 50 | 15 |
| 15 | 1/month | 7%–8% | Gain 3 kg | None | Decreases | 100 | 12 |
| 16 | 1/week | >8% | Lose 6 kg | Mild nausea | The same | 100 | 16 |
Abbreviations: CVR, cardiovascular risk; HbA1c, glycated hemoglobin; No, number.
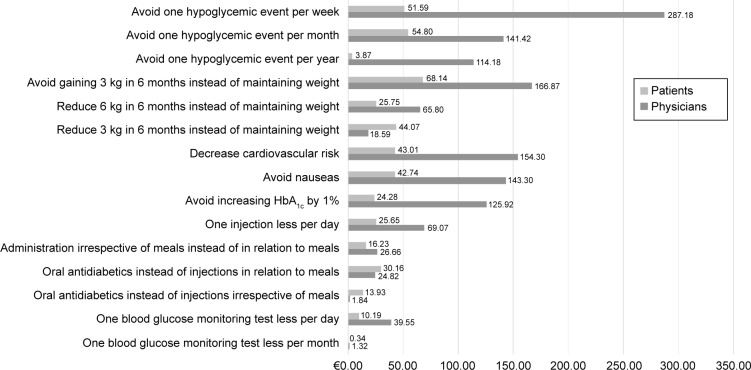
Statistically significant differences (P<0.05) were found in the preferences between physicians and patients regarding the attributes: mode of administration (injections once a day in relation to meals), blood glucose monitoring, hypoglycemic events (one per month), hypoglycemic events (none), HbA1c, and additional payment per month (Table 3). Physicians were willing to pay approximately three times more than patients for almost all attributes. Patients valued more than physicians reducing bodyweight by 3 kg in 6 months and the attributes of the mode of administration, specifically those regarding receiving oral antidiabetics instead of injected medication either in relation to or irrespective of meals (Figure 3).
Figure 3.
Comparison of physicians’ and patients’ monthly WTP for diabetes treatment attributes.
Abbreviations: HbA1c, glycated hemoglobin; WTP, willingness to pay.
Discussion
Results presented in this manuscript demonstrate that patients were willing to pay for improvements in their T2DM treatment, in order to gain additional benefits, just as stated by Bogelund et al23 and Jendle et al24 in their previous published studies. As that the evidence provided is unique for the studied population it can be considered paramount. Moreover, the information regarding patients’ and physicians’ preferences should be taken into account by health care decision makers given that participants were representative of both Spanish and Portuguese population. These outcomes can be considered as robust and may become important tools in the outcomes research area since they were product of a study that followed a rigorous methodology for its statistical analysis.
One of the main outcomes presented is that Spanish and Portuguese patients valued avoid gaining 3 kg/6 months the most followed by avoiding hypoglycemias. These results are in line with those presented in the previous studies. Mohamed et al27 performed an analysis of preferences for oral antidiabetics in Sweden and Germany and demonstrated that bodyweight gain was the most important attribute, followed by glucose control. Porzsolt et al10 showed that, to German physicians and patients, weight loss was as important as improvements in blood glucose control. Moreover, Jendle et al26 confirmed that patients placed great value on treatments that improved their glucose medication while avoiding gains in bodyweight and involved the administration of fewer injections. These consistent findings might be explained by the fact that patients are aware of the consequences of obesity on glucose control and other diabetes comorbidities such as cardiovascular risk.
Regarding the mode of administration, it is clear that patients preferred oral antidiabetics to injected medications. Hauber et al50 showed that patients with T2DM were willing to pay for improvements in dosing, efficacy, and safety, being this WTP dependent on the therapy currently being received. However, in the current study, although patients were willing to pay up to €30.16 to receive oral antidiabetics instead of injections in relation to meals, the preferred hypothetical treatment was the one administered once a day as an injection irrespective of meals associated with blood glucose monitoring three times per day and without additional cost. An explanation for these results might be that patients value oral antidiabetics almost the same as injections irrespective of meals and because flexible dosing might have a positive impact on HRQoL, which can potentially enhance adherence and could contribute to improve long-term outcomes, as described by Evans et al.51
In this study, there was a slight disordering on the WTP for the frequency of hypoglycemias and the weight attributes, although these differences were not statistically significant. Poulos et al,11 Mohamed et al,27 and Hauber et al50 among others, have described similar inconsistencies in their publications, indicating that T2DM patients frequently fail to make a distinction among some levels of a given antidiabetic attribute.
Another important finding is that physicians’ in Spain and Portugal rated avoiding one hypoglycemic event per week, avoiding gaining 3 kg/6 months, and decreasing cardiovascular risk as being more valuable T2DM treatment attributes than avoiding a 1% increase in HbA1c. Physicians’ preferences for T2DM treatment attributes are rarely discussed in the literature, and far less frequently the WTP for such a characteristic. Only two publications on these subjects have been identified and their results concurred with the ones presented in the current study. Porzsolt et al10 examined the German physicians’ and patients’ relative preferences for glucose control, side effects, convenience, and weight change attributes. Their results demonstrated that physicians considered bodyweight change to be at least as important as blood glucose monitoring. Moreover, Poulos et al11 studied physicians’ preferences for extra glycemic effects of T2DM treatments in United States and United Kingdom. Their results showed that glucose control (HbA1c) was the most important attribute followed by reducing cardiovascular risk and change in bodyweight.
The outcomes previously described are not surprising at all, since educational programs for health care providers have created an awareness of the risk of hypoglycemia, and the risk, during tight glycemic management of diabetes, of its association with an increase in cardiovascular events and its relation to other detrimental effects such as lessening of HRQoL, treatment satisfaction and, ultimately, adherence to treatment.52 Furthermore, given that cardiovascular disease is the major cause of death in patients with T2DM and obesity is a risk factor for both conditions, it is only logical that those attributes are highly valued by health care providers.53 Weight loss is considered as a key factor in the management of T2DM and the reduction of mortality associated with the disease.54 As a result, health care providers consider weight gain as extremely detrimental to diabetic patients’ health, thereby suggesting that avoiding even a relatively small weight gain is more important than reducing weight.55
Both patients and physicians in Spain and Portugal were willing to pay for the health benefits associated with better diabetes treatment, avoiding hypoglycemia and weight gain being paramount. These results comply with the Prospect Theory of Kahneman and Tversky, which proposes that in general people’s aversion for losses is greater than attraction for benefits.56 Mühlbacher and Juhnke12 stated that for chronic conditions such as T2DM and especially in studies that utilized DCE there is usually a higher agreement level between patients’ and physicians’ responses. However, these similarities depend on the type of attribute being assessed. Attributes focused on outcomes (HbA1c and hypoglycemia) are usually more highly valued by physicians, while patients add less value to attributes that can negatively affect their HRQoL. Concurring with the previous statement, in this study differences between physicians’ and patients’ preferences were only statistically significant for HbA1c, frequency of hypoglycemia, injections in relation to meals, frequency of blood glucose monitoring, and payment, these attributes being less valued by patients than by physicians in all cases. Although in their study Porzsolt et al10 did not statistically compare the results of patients’ and physicians’ preferences, their findings were in line with the ones presented in this study. Physicians preferred the attributes related to efficiency while patients preferred the convenience and HRQoL improving attributes.
Regarding the payment attribute, physicians were willing to pay up to three times more than patients for almost all attributes with the exception of receiving oral antidiabetics instead of injected medications. These differences have also been described in other publications12,57 and can be explained based on the distinct perspectives of patients and physicians. Patients were asked to choose which treatment they preferred for themselves, while physicians were asked which treatment they would prefer to prescribe. Moreover, health care providers make decisions drawing on scientific information and are aware of the real value of each characteristic of a therapeutic measure, which might help increase the WTP for such attributes.58 On the other hand, given the current economic situation in both Spain and Portugal, together with the high level of reimbursement of antidiabetic treatments, it could lead to a lower patients’ WTP.
Study limitations
The use of DCE and conditional logit models is the recommended approach for measuring participants’ preferences and determining WTP.20,30 However, DCE is a technique for measuring stated preferences and even though it resembles the real consumer’s choice, there is always a gap between stated and revealed preferences.15,20 For this reason, caution should be paid given the probability that respondents presented different preferences in this survey from the ones they would state in a real-life situation.
In addition, there is some uncertainty associated with the interpretation of the exercise by patients and how it may affect the results. Although the selection of the choice levels was performed according to the ISPOR recommendations, some of the attributes might not match the currently available treatments and in some instances the presented scenario was less favorable than the treatment patients were already taking. Nonetheless, all participants received a clear explanation before answering the questionnaire stating that all presented treatments were hypothetical and in no case were to be compared against their current treatment.
Another limitation is that some of the WTP 95% CI can be considered wide which could suggest heterogeneity in the responses. This finding could be due to the use of the conditional logit model. This model assumes that the measured utility is equal across all respondents and choice questions, and therefore does not recognize unobserved systematic relationships in preferences among respondents (preference heterogeneity).59,60 Nevertheless, the conditional logit model has the ability to provide efficient estimators.40 Finally, results obtained from this study should be interpreted in the context where they were obtained. Future research regarding preferences of patients in the subpopulations with conditioning characteristic is warranted.
Conclusion
Both patients and physicians in Spain and Portugal are willing to pay for the health benefits associated with improved diabetes treatment, the most important being to avoid both hypoglycemia and gaining bodyweight. Decreased cardiovascular risk and weight reduction became the third most valued attributes of antidiabetics for physicians and patients, respectively.
Physicians and patients had different preferences regarding the attributes mode of administration (injections once a day in relation to meals), blood glucose monitoring, hypoglycemic events (one per month), hypoglycemic events (none), HbA1c, and additional payment per month. Patients valued more than physicians reducing bodyweight by 3 kg in 6 months and the attributes of the mode of administration, specifically those regarding receiving oral antidiabetics instead of injected medication either in relation to or irrespective of meals.
Supplementary material
Table S1.
Patients’ and physicians’ conditioning factors P-values results obtained by the multinomial model
| Convenience attributes (P-value) | Clinical attributes (P-value) | |
|---|---|---|
| Patients’ characteristics | ||
| Sex | 0.1706 | 0.2626 |
| Being >65 years | 0.2726 | 0.9263 |
| Having incomes above €12,000 a year | 0.1898 | 0.6045 |
| Blood glucose control 1/day | 0.8984 | 0.9375 |
| Blood glucose control 3/day | 0.4034 | 0.2662 |
| Blood glucose control 1/month | 0.1763 | 0.8459 |
| No need for glucose control | 0.5483 | 0.1529 |
| Hypoglycemia 1/month | 0.3977 | 0.6653 |
| Hypoglycemia 1/month | 0.8749 | 0.5689 |
| Never had hypoglycemia | 0.3856 | 0.8026 |
| HbA1c 8%–9% | 0.5366 | 0.8342 |
| HbA1c 7%–8% | 0.9104 | 0.7592 |
| HbA1c <6% | 0.4873 | 0.7888 |
| HbA1c unknown | 0.5150 | 0.8570 |
| Obesity | 0.5173 | 0.1160 |
| More than 10 years since the diagnosis | 0.0281 | 0.7682 |
| Receiving injectable treatment | 0.0394 | 0.7395 |
| Physicians’ characteristics | ||
| Sex | 0.5614 | 0.0847 |
| Medical specialty | 0.3180 | 0.3051 |
| Time since beginning of practice longer than 10 years | 0.0717 | 0.7243 |
| Country | 0.4044 | 0.1023 |
Note: Bold values indicate significance value P<0.05.
Abbreviation: HbA1c, glycated hemoglobin.
Acknowledgments
This project was sponsored by Novo Nordisk A/S.
Footnotes
Disclosure
CM, RF, PFC, CP, MB, JR, EE, JL, LL, IL, CM, JNP, DOB, SP, and MTC worked on this study, which was funded by Novo Nordisk A/S. ARA is an employee of Novo Nordisk Europe and CC works at Novo Nordisk Lisbon (an affiliate of Novo Nordisk Europe). The authors report no other conflicts of interest in this work.
References
- 1.American Diabetes Association Standards of Medical Care in diabetes –2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Valdés S, Rojo-Martínez G, Soriguer F. Evolution of prevalence of type 2 diabetes in adult Spanish population. Med Clin (Barc) 2007;129(9):352–355. doi: 10.1157/13109554. [DOI] [PubMed] [Google Scholar]
- 3.Soringer F, Goday A, Bosch-comas A, Bordiu E, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 2012;55:88–93. doi: 10.1007/s00125-011-2336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Observatório Nacional da Diabetes Diabetes: Factos e Números 2012. [Accessed: November, 2013]. Available from: http://www.ulsm.min-saude.pt/ResourcesUser/Documentos/i018361.pdf.
- 5.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimated of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García Pérez LE, Alvarez M, Dilla T, Gil-Guillen V, Orozco Beltran D. Adherence to therapies in patients with Type 2 diabetes. Diabetes Ther. 2013;4:175–798. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin A, Hoffman C, Stevens A, Douglas A, Bloomgarden Z. Determinants of adherence to diabetes treatment. J Diabetes. 2015 Jan 7; doi: 10.1111/1753-0407.12264. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Lizán L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient reported outcomes as cost determinant in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–1664. doi: 10.2147/PPA.S67253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porzsolt F, Clouth J, Deutschmann M, Hippler HJ. Preferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes. 2010;8:125. doi: 10.1186/1477-7525-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulos C, Gonzalez JM, Lee L, et al. Physicians preferences for extra glycemic effects of type 2 diabetes treatments. Diabetes Ther. 2013;4:443–459. doi: 10.1007/s13300-013-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11:163–180. doi: 10.1007/s40258-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 13.Johnson FR, Hauber B, Ozdemir S, Stiegel CA, Hass S, Sands BE. Are gastroenterologists less tolerant of treatment risk than patients? Benefit-risk preferences in Crohn’s disease management. J Manag Care Pharm. 2010;16(8):606–628. doi: 10.18553/jmcp.2010.16.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association Strategies for improving care. Sec 1 in Standards of Medical Care in Diabetes 2015. Diabetes Care. 2015;38(Suppl 1):S5–S7. doi: 10.2337/dc15-S004. [DOI] [PubMed] [Google Scholar]
- 15.Viney R, Lancsar E, Louviere J. Discrete choice experiments to measure consumer preferences for health and healthcare. Expert Rev Pharmacoecon Outcomes Res. 2002;2(4):89–96. doi: 10.1586/14737167.2.4.319. [DOI] [PubMed] [Google Scholar]
- 16.Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. 2003;2(1):55–64. [PubMed] [Google Scholar]
- 17.Scott A. Identifying and analysing dominant preferences in discrete choice experiments: an application in health care. J Econ Psychol. 2002;23:383–398. [Google Scholar]
- 18.Johnson FR, Mohamed AF, Özdemir S, Marchall DA, Phillips KA. How does cost matter in health care Discrete Choice Experiments? Health Econ. 2011;20:323–330. doi: 10.1002/hec.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in heath economics: a review of the literature. Health Econ. 2012;21:145–172. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- 20.Bridges JFP, Hauber AB, Marshall D, et al. Conjoint Analysis applications I Health: a Checklist of the ISPOR Good Research Practices for Conjoint analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 22.Aristides M, Weston AR, FitzGerald P, Le Reun C, Manidakis N. Patient preference and willingness-to-pay for Humalog Mix25 relative to Humulin 30/70: a multicountry application of a discrete choice experiment. Value Health. 2004;7(4):442–454. doi: 10.1111/j.1524-4733.2004.74007.x. [DOI] [PubMed] [Google Scholar]
- 23.Bogelund M, Vilsboll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark – a discrete choice experiment. Curr Med Res Opin. 2011;27(11):2175–2183. doi: 10.1185/03007995.2011.625404. [DOI] [PubMed] [Google Scholar]
- 24.Jendle J, Torffvit O, Ridderstrale M, Lammert M, Ericsson A, Bogelund M. Willingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetes. Curr Med Res Opin. 2010;26(4):917–923. doi: 10.1185/03007991003657867. [DOI] [PubMed] [Google Scholar]
- 25.Jendle J, Ridderstrale M, Torfvitt O, Ericsson A, Larsen S. Willingness-to-pay for benefits associated with basal insulin treatment of type 2 diabetes. J Med Econ. 2012;15(2):261–263. doi: 10.3111/13696998.2011.644408. [DOI] [PubMed] [Google Scholar]
- 26.Jendle J, Torffvit O, Ridderstrale M, et al. Willingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme results. J Med Econ. 2012;15(Suppl 2):1–5. doi: 10.3111/13696998.2012.703633. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed AF, Zhang J, Johnson FR, et al. Avoidance of weight gain is important for oral type 2 diabetes treatments in Sweden and Germany: patients preferences. Diabetes Metab. 2013;39:397–403. doi: 10.1016/j.diabet.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Van Everdingen YM, Waarts E. The effect of national culture on the adoption of innovations. Mark Letters. 2003;14(3):217–232. [Google Scholar]
- 29.Javidan M, House RJ, Dorfman PW, et al. Conceptualizing and measuring cultures and their consequences: a comparative review of GLOBE’s and Hofstede’s approaches. J Int Bus Stud. 2006;37:897–914. [Google Scholar]
- 30.Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 31.Aizaki H. Basic functions for supporting an implementation of choice experiments in R. J Stat Softw. 2012;50(2):1–24. [Google Scholar]
- 32.Bech M, Kjaer T, Laurdsen J. Does the number of choice set matter? Results from a web survey applying a discrete choice experiment. Health Econ. 2011;20:273–286. doi: 10.1002/hec.1587. [DOI] [PubMed] [Google Scholar]
- 33.National Institute of Statistics of Spain (population, 2012) Population by Reference Date, Sex and Age. [Accessed: November, 2013]. Available from: http://www.ine.es.
- 34.National Institute of Statistics of Portugal (Censos, 2011) População residente, segundo a dimensão dos lugares, população isolada, embarcada, corpo diplomático e sexo, por idade (ano a ano) Available from: http://censos.ine.pt/xportal/xmain?xpid=CENSOS&xpgid=censos_quadros_populacao.
- 35.Interactive Consultation NHS [Accessed: Novemberm, 2013]. Available from: http://www.msssi.gob.es/estadEstudios/estadisticas/sisInfSanSNS/home.htm.
- 36.Administraçao Central do Sistema de Saúde . Estudo de necessidade previsionais de recursos humanos em saúde – Médicos. Lisboa: Administraçao Central do Sistema de Saúde; 2009. [Accessed: November, 2013]. Available from: http://www.acss.min-saude.pt/Portals/0/Relat%C3%B3rio%20Final_M%C3%A9dicos.pdf. [Google Scholar]
- 37.de Bekker-Grob E, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare. A practical guide. Patient 2015. 2015 Mar 1; doi: 10.1007/s40271-015-0118-z. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization . How to Construct a Discrete Choice Experiment for Health Workforce Recruitment and Retention in Remote and Rural Areas: A User Guide with Case Studies. Geneva: World Health Organization; 2012. Available from: http://www.capacityplus.org/files/resources/discrete-choice-experiment-user-guide.pdf. [Google Scholar]
- 39.Croissant Y. mlogit: Multinomial Logit Model. Cran R project.org; 2011. [Accessed: July, 2014]. Available from: http://CRAN.R-project.org/packagemlogit. [Google Scholar]
- 40.McFadden D. Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Frontiers in Econometrics. New York: Academic Press; 1974. pp. 105–142. [Google Scholar]
- 41.Trope Y, Liberman N. Construal-level theory of psychological distance. Psychol Rev. 2010;117(2):440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Löckenhoff CE. Age, time and decision making: from processing speed to global time horizons. Ann NY Acad Sci. 2011;1235:44–56. doi: 10.1111/j.1749-6632.2011.06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. Diabetes Care. 2004;27(8):2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 44.Mandeville KL, Lagarde M, Hanson K. The use of discrete choice experiments to inform health workforce policy: a systematic review. BMC Health Serv Res. 2014;14:367. doi: 10.1186/1472-6963-14-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ. 2007;16:827–840. doi: 10.1002/hec.1197. [DOI] [PubMed] [Google Scholar]
- 46.Guimaraes C, Marra CA, Gill S, et al. A discrete choice experiment evaluation of patient’s preferences for different risk, benefit and delivery attributes of insulin therapy for diabetes management. Patient Prefer Adherence. 2010;4:433–440. doi: 10.2147/PPA.S14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brame R, Paternoster R, Mazerolle P, Piquero A. Testing for the equality of Maximum likelihood regression coefficients between two independent equations. J Quant Criminol. 1998;14(3):245–261. [Google Scholar]
- 48.Paternoster R, Brame R, Mazerolle P, Piquero A. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36(4):859–866. [Google Scholar]
- 49.International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: guideline for good clinical practice E6(R1) 1996. [Accessed: January, 2015]. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. [PubMed]
- 50.Hauber AB, Han S, Yang JC, et al. Effect of pill burden on dosing preferences, willingness to pay and likely adherence among patients with type 2 diabetes. Patients Prefer Adherence. 2013;7:937–949. doi: 10.2147/PPA.S43465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans M, Jensen HH, Bogelubd M, et al. Flexible insulin dosing improves health related quality of life (HRQOL): a time trade off survey. J Med Econ. 2013;16(11):1357–1365. doi: 10.3111/13696998.2013.846262. [DOI] [PubMed] [Google Scholar]
- 52.Shafiee G, Mohajeri-Teharani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11:17. doi: 10.1186/2251-6581-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorber D. Importance of cardiovascular disease and risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndrome Obes. 2014;7:169–183. doi: 10.2147/DMSO.S61438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilding JPH. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68:682–691. doi: 10.1111/ijcp.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matza LS, Yurgin N, Boye KS, Malley K, Shorr JM. Obese versus non obese patients with type 2 diabetes: patient-reported outcomes and utility of weight change. Curr Med Res Opin. 2007;23(9):2051–2062. doi: 10.1185/030079907X219454. [DOI] [PubMed] [Google Scholar]
- 56.Kahneman D, Tversky A. Prospect Theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–192. [Google Scholar]
- 57.Deal K, Keshavjee K, Troyan S, Kyba R, Holbrook AM. Physicians and patients willingness to pay for electronic cardiovascular disease management. Int J Med Inform. 214(83):517–528. doi: 10.1016/j.ijmedinf.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Mol PGM, Arnardottir AH, Straus SMJ, et al. Understanding drug preferences, different perspectives. Br J Clin Pharmacol. 2014. Available from: http://onlinelibrary.wiley.com/doi/10.1111/bcp.12566/pdf. [DOI] [PMC free article] [PubMed]
- 59.Hauber B, Gonzalez JM, Groothuis-Outdshoorn KGM, et al. Conjoint analysis statistical analysis: an ISPOR Conjoint Analysis Good Research Practices Task Force Report. Draft for review. Available from: http://www.ispor.org/taskforces/documents/ISPOR-Conjoint-Analysis-Statistical-Analysis-GRP-TF-Report-DRAFT-for-REVIEW.pdf.
- 60.Clark MD, Determan D, Petrou S, Moro D, Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Phamaco Economics. 2014;32:883–902. doi: 10.1007/s40273-014-0170-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Patients’ and physicians’ conditioning factors P-values results obtained by the multinomial model
| Convenience attributes (P-value) | Clinical attributes (P-value) | |
|---|---|---|
| Patients’ characteristics | ||
| Sex | 0.1706 | 0.2626 |
| Being >65 years | 0.2726 | 0.9263 |
| Having incomes above €12,000 a year | 0.1898 | 0.6045 |
| Blood glucose control 1/day | 0.8984 | 0.9375 |
| Blood glucose control 3/day | 0.4034 | 0.2662 |
| Blood glucose control 1/month | 0.1763 | 0.8459 |
| No need for glucose control | 0.5483 | 0.1529 |
| Hypoglycemia 1/month | 0.3977 | 0.6653 |
| Hypoglycemia 1/month | 0.8749 | 0.5689 |
| Never had hypoglycemia | 0.3856 | 0.8026 |
| HbA1c 8%–9% | 0.5366 | 0.8342 |
| HbA1c 7%–8% | 0.9104 | 0.7592 |
| HbA1c <6% | 0.4873 | 0.7888 |
| HbA1c unknown | 0.5150 | 0.8570 |
| Obesity | 0.5173 | 0.1160 |
| More than 10 years since the diagnosis | 0.0281 | 0.7682 |
| Receiving injectable treatment | 0.0394 | 0.7395 |
| Physicians’ characteristics | ||
| Sex | 0.5614 | 0.0847 |
| Medical specialty | 0.3180 | 0.3051 |
| Time since beginning of practice longer than 10 years | 0.0717 | 0.7243 |
| Country | 0.4044 | 0.1023 |
Note: Bold values indicate significance value P<0.05.
Abbreviation: HbA1c, glycated hemoglobin.