Abstract
Background
The results of studies on association between the polymorphisms in the coding region and the promoter of uridine diphosphateglucuronosyl transferase 1A1 (UGT1A1) and neonatal hyperbilirubinemia are controversial. This study aimed to determine whether the UGT1A1 gene polymorphisms of Gly71Arg and TATA promoter were significant risk factors associated with neonatal hyperbilirubinemia.
Material/Methods
The PubMed, Cochrane Library, and Embase databases were searched for papers that describe the association between UGT1A1 polymorphisms and neonatal hyperbilirubinemia. Summary odds ratios and 95% confidence intervals (CI) were estimated based on a fixed-effects model or random-effects model, depending on the absence or presence of significant heterogeneity.
Results
A total of 32 eligible studies and 6520 participants were identified. Among them, 24 studies focused on the association of neonatal hyperbilirubinemia with UGT1A1 Gly71Arg polymorphisms, and a significant difference was found for the comparison of AA vs. AG+GG (OR=3.47, 95% CI=2.29–5.28, P<0.0001). We included 19 studies on the association of neonatal hyperbilirubinemia with UGT1A1 TATA promoter polymorphism, which also found a statistically significant difference between 7/7 and 6/7 + 6/6 (OR=2.24, 95% CI=1.29–3.92, P=0.004).
Conclusions
This meta-analysis demonstrated that UGT1A1 polymorphisms (Gly71Arg and TATA promoter) significantly increase the risk of neonatal hyperbilirubinemia.
MeSH Keywords: Hyperbilirubinemia; Infant, Newborn; Polymorphism, Single Nucleotide
Background
Neonatal hyperbilirubinemia is caused by abnormal metabolism of bilirubin, and is characterized by a syndrome of skin, mucous membrane, and sclera jaundice [1]. While most cases are physiological, when the serum bilirubin concentrations are higher than 12.9 mg/dl in full-term infants and for a prolonged period of time, jaundice is no longer considered physiologic [2,3]. In pathological hyperbilirubinemia, increased production of bilirubin, deficiency in hepatic uptake, impaired conjugation of bilirubin, and/or increased enterohepatic circulation of bilirubin are observed [4]. However, there is no identifiable factor in almost half of cases.
It has been suggested that genetic variation could enhance the risk of neonatal hyperbilirubinemia when coexpressed with other icterogenic conditions [5–7]. Among these, uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) was identified to be associated with neonatal hyperbilirubinemia [8,9]. UGT1A1 is the key rate-limiting enzyme in the liver for bilirubin glucuronidation, which is a clearance mechanism for numerous dietary and environmental chemicals, including bilirubin [10,11]. The polymorphisms of the UGT1A1 coding region or the promoter may produce structural or functional enzymatic deficiencies, leading to intermittent elevation of unconjugated serum bilirubin, resulting in hyperbilirubinemias known as Gilbert’s syndrome (GS) and Crigler-Najjar syndrome (GNS) [12,13]. Numerous polymorphisms of UGT1A1 have been reported in patients with GS and CNS, including Gly71Arg and TATA promoter [14,15].
The Gly71Arg (G71R) of the UGT1A1 gene has been reported as a genetic risk factor for GS, which might reduce the activity of the enzyme, and then cause mild unconjugated hyperbilirubinemia [16–18]. In addition, the TATA promoter polymorphism has been described as another major cause of neonatal hyperbilirubinemia, which is characterized by an increase of total serum bilirubin as a result of poor enzymatic conjugation by glucuronosyltransferase [6,19,20]. TA sequences in the promoter region vary in length from 5 to 8 repeats, and the (TA)7 (UGT1A1*28) and (TA)8 homozygotes, when compared with (TA)6 homozygote, (TA)6/(TA)7 or (TA)6/(TA)8 heterozygotes, have been considered as causes of hyperbilirubinemic syndromes. The (TA)7 homozygote leads to a 70% reduction in UGT1A1 expression as compared to the (TA)6 carriers [21]. Recent data have also shown that the effects of variants in UGT1A1 gene appear to be variable across populations [22–24]. Therefore, we performed a meta-analysis to assess whether the UGT1A1 polymorphisms are associated with neonatal hyperbilirubinemia. We analyzed the Gly71Arg and TATA promoter polymorphisms of UGT1A1 between cases and controls.
Material and Methods
The present meta-analysis was performed according to PRISMA recommendations [25]. The PubMed, Cochrane Library, and Embase databases were searched independently by 2 investigators to retrieve relevant studies published from January 1, 1998 to October 31, 2014. The search criteria “hyperbilirubinemia”, “UDP-glucuronosyl transferase 1A1 (UGT1A1)”, and “polymorphism” were used in text word searches. The “related articles” function was used to broaden the search. The reference lists of the selected articles were also manually examined to find relevant studies that were not discovered during the database searches.
Exclusion criteria
Exclusion criteria were: A) incomplete raw data, B) repetitive reports, and C) material and methods used were not well described or reliable. We used reliability of the methods for patient selection, molecular typing, and statistical analysis as quality variables to accurately assess the quality measures of interest.
Polymorphisms related to neonatal hyperbilirubinemia were divided into 2 groups according to TATA promoter polymorphism and G71R polymorphism in the UGT1A1 gene. All titles, abstracts, and full papers of potentially relevant studies were assessed for eligibility. When several reports from the same study were published, only the most recent or informative one was included in this meta-analysis. The language was restricted to only English.
Data extraction
Two investigators extracted all variables and outcomes of interest independently. Disagreements were resolved through discussion and consensus. Data on first author and year of publication, neonatal hyperbilirubinemia definition, country of study, numbers of cases and controls, and UGT1A1 gene polymorphism genotyping information were extracted (Table 1).
Table 1.
Characteristics of included studies.
| Author (reference) | Year | Country | Characteristics of cases | Control(n) | Case(n) |
|---|---|---|---|---|---|
| Akaba et al. [24] | 1998 | Japan | STB >17 mg/dl | 101 | 42 |
| Maruo et al. [18] | 1999 | Japan | STB >15 mg/dl | 50 | 25 |
| Yamamoto et al. [16] | 2002 | Japan | STB >15 mg/dl in the first 7 days | 49 | 23 |
| Huang et al. [14] | 2002 | Taiwan, China | STB ≥15 mg/dl within 1 week after birth | 218 | 123 |
| Seco et al. [41] | 2002 | Spain | STB >15 mg/dl | 115 | 21 |
| Ulgenalp et al. [4] | 2003 | Turkey | STB >12.9 mg/dl | 35 | 75 |
| Takeuchi et al. [33] | 2004 | Japan | STB level >17 mg/dl | 71 | 68 |
| Huang et al. [28] | 2004 | Taiwan, China | a peak STB ≥20.0 mg/dl in serum within 10 d of birth | 100 | 72 |
| Sutomo et al. [34] | 2004 | Malaysia | STB >15 mg/dl at day 3 | 36 | 32 |
| Kanai et al. [51] | 2005 | Japan | STB >15 mg/dl at day 4 and thereafter | 116 | 29 |
| Yusoff et al. [48] | 2005 | Malaysia | STB >15 mg/dl | 50 | 55 |
| Ferraris et al. [15] | 2006 | Italy | STB >17 mg/dl | 83 | 53 |
| Babaoglu et al. [21] | 2006 | Tukey | STB >17 mg/dl | 32 | 74 |
| Muslu et al. [36] | 2006 | Turkey | STB >15mg/dl, <7d | 55 | 107 |
| Farheen et al. [26] | 2006 | India | UCB ≥1.2 mg/dl | 95 | 95 |
| Wong et al. [9] | 2007 | Malaysia | STB >15 mg/dl at age 1–2 days or >17 mg/dl at age 3 days and onward | 125 | 74 |
| Watchko et al. [6] | 2009 | America | STB >95% high-risk zone | 299 | 153 |
| Chang et al. [27] | 2009 | Taiwan, China | STB >5.9 mg/dl beyond 28 days of age | 90 | 35 |
| Prachukthum et al. [39] | 2009 | Thailand | STB >95% as defined by the Bhutani nomogram | 86 | 91 |
| Agrawal et al. [7] | 2009 | India | STB >18 mg/dl | 50 | 69 |
| Ergin et al. [19] | 2010 | Turkey | STB ≥17 mg/dl | 54 | 50 |
| Kilic et al. [40] | 2010 | Turkey | STB >12.9 mg/dl, or TSB >8.8 mg/dl at day 14 | 23 | 47 |
| Narter et al. [38] | 2011 | Turkey | STB >15 mg/dl in the first 10 days | 70 | 39 |
| Chou et al. [11] | 2011 | Taiwan, China | STB >15 mg/dl | 508 | 180 |
| Long et al. [1] | 2011 | China | STB >95% as defined by the Bhutani nomogram | 105 | 112 |
| Sato et al. [31] | 2012 | Japan | Full-term and breast-fed neonates, STB >10 mg/dl at day 1, >16 mg/dl at day 3, and >20 mg/dl at day 6 | 345 | 56 |
| Silva et al. [35] | 2012 | India | STB >15 mg/dl in the first 5 days | 180 | 126 |
| Tiwari et al. [50] | 2013 | India | STB >95% as defined by the Bhutani nomogram | 100 | 100 |
| Wong et al. [9] | 2013 | Malaysia | STB >15 mg/dl | 263 | 52 |
| Travan et al. [32] | 2014 | Italy | STB >20 mg/dl | 70 | 70 |
| Tiwari et al. [30] | 2014 | India | STB >95% high-risk zone, newborns of ≤2 weeks’ of age | 218 | 113 |
| Silva et al. [29] | 2014 | India | STB ≥15 mg/dl, ≥35 weeks | 171 | 124 |
STB – serum total bilirubin; UCB – serum unconjugated bilirubin.
Quality assessment
The included studies were assessed independently by the 2 reviewers using the Newcastle-Ottawa Scale (NOS) [26]. The NOS employs a star rating system to assess quality from 3 broad perspectives of the study: (1) selection of the study groups, (2) comparability of the groups, and (3) identification of the exposure (for case-control studies) or outcome of interest (for cohort studies). Scores ranged from 0 to 9 stars, and studies with 7 or more stars were considered to be of high quality.
Statistical analysis
The statistical analysis was performed using meta-analysis software called “Comprehensive Meta Analysis”. The strength of the association between UGT1A1 gene polymorphisms and neonatal hyperbilirubinemia risk was calculated with the OR and respective 95% CIs. The significance of the pooled OR was determined by the Z test, and P-values of less than 0.05 were considered significant. Chi-square test was used for assessing the Hardy-Weinberg equilibrium (HWE) of genotypes in the control group of each study. Statistical heterogeneity among studies was assessed with the I2 statistics. This value ranges from 0% (complete consistency) to 100% (complete inconsistency). If the I2 value was more than 50%, the random-effects model was chosen to calculate the pooled OR; otherwise, the fixed-effects model was used. All of the results were presented as forest plots. In the sensitivity analysis, we removed each study sequentially and performed meta-analysis with the rest repeatedly to show how conclusions might be affected. The presence of publication bias was assessed by a visual inspection of a funnel plot and Egger’s linear regression test.
Results
Literature search
The initial literature search retrieved 322 relevant articles. We excluded 289 articles for not investigating the topic of interest or insufficient data after carefully screening the titles and abstracts. All studies included were in accordance with NOS scale and were therefore defined as high-quality studies. A total of 32 articles with 2455 cases of neonatal hyperbilirubinemia and 4065 controls were included in the meta-analysis. The characteristics of the included studies are summarized in Table 1. A review of the data extraction revealed 100% agreement between the 2 reviewers.
Main analysis
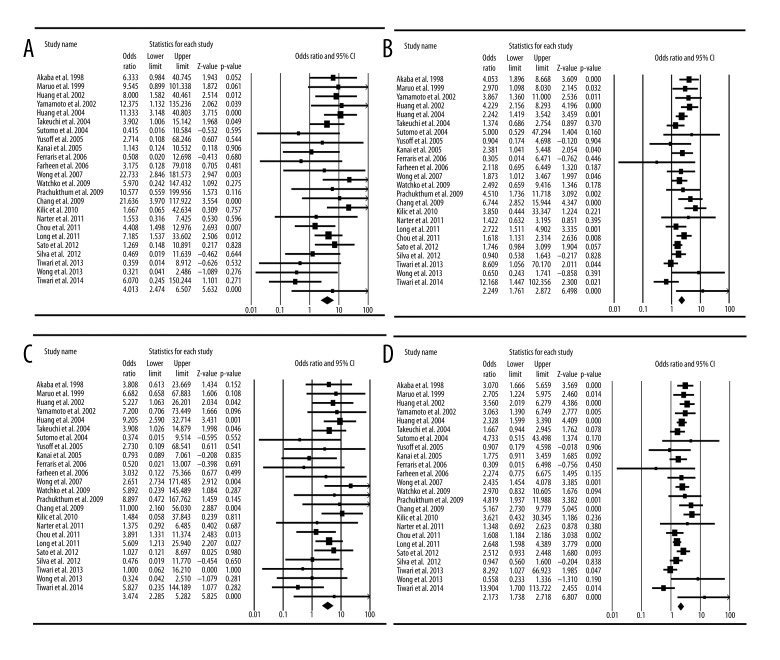
Finally, 24 studies focused on the relationship between G71R UGT1A1 polymorphism and neonatal hyperbilirubinemia (Table 2). Table 2 and Table 3 list the genotyped and allele distributions of the G71R for the cases and controls. Although most research has been in East Asian populations, the A allele does not appear to be different among races. The genotype frequencies of the G/A polymorphism were 80.7% (GG), 17.7% (GA), and 1.6% (AA) in controls, and 72.0% (GG), 23.0% (GA), and 5.0% (AA) in hyperbilirubinemia neonates. The A allele frequencies in the control group was 0.104. For allele level comparison, the A allele was found to be associated with a risk of hyperbilirubinemia in terms of the frequency of allele comparison (A vs. G: OR=2.17; 95% CI=1.74–2.72, P <0.0001). For a dominant model of the A allele, the AG + AA genotypes were associated with the risk for hyperbilirubinemia (AG + AA vs. GG, OR=2.25, 95% CI=1.76–2.87, P<0.0001). For a recessive model of the A allele, the AA homozygote genotype was associated with susceptibility to hyperbilirubinemia (AA vs. AG+GG, OR=3.47, 95% CI =2.29–5.28, P <0.0001, Heterogeneity=0.058) (Figure 1C). For the extreme genotype, the AA genotype was associated with the risk for hyperbilirubinemia (AA vs. GG, OR=4.01, 95% CI=2.47–6.51, P<0.0001, Heterogeneity=0.224) (Figure 1A) (Table 3).
Table 2.
Allele frequencies of Gly71Arg UGT1A1 polymorphisms in cases of neonatal hyperbilirubinemia and controls.
| Author | Country | Control (n) | Case (n) | A allele frequencies in control group | ||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | GG | GA | AA | |||
| Akaba et al. 1998 | Japan | 76 | 23 | 2 | 18 | 21 | 3 | 0.134 |
| Maruo et al. 1999 | Japan | 35 | 14 | 1 | 11 | 11 | 3 | 0.16 |
| Yamamoto et al. 2002 | Japan | 33 | 15 | 1 | 8 | 12 | 3 | 0.173 |
| Huang et al. 2002 | Taiwan, China | 153 | 62 | 3 | 63 | 46 | 14 | 0.156 |
| Takeuchi et al. 2004 | Japan | 48 | 20 | 3 | 41 | 17 | 10 | 0.183 |
| Huang et al. 2004 | Taiwan, China | 80 | 18 | 2 | 35 | 30 | 7 | 0.11 |
| Sutomo et al. 2004 | Malaysia | 35 | 1 | 0 | 28 | 4 | 0 | 0.014 |
| Kanai et al. 2005 | Japan | 80 | 31 | 5 | 14 | 14 | 1 | 0.177 |
| Yusoff et al. 2005 | Malaysia | 47 | 3 | 0 | 52 | 3 | 0 | 0.03 |
| Ferraris et al. 2006 | Italy | 81 | 2 | 0 | 53 | 0 | 0 | 0.012 |
| Farheen et al. 2006 | India | 90 | 5 | 0 | 85 | 9 | 1 | 0.026 |
| Wong et al. 2007 | Malaysia | 93 | 31 | 1 | 45 | 18 | 11 | 0.132 |
| Watchko et al. 2009 | America | 295 | 4 | 0 | 148 | 4 | 1 | 0.007 |
| Chang et al. 2009 | Taiwan, China | 68 | 20 | 2 | 11 | 17 | 7 | 0.133 |
| Prachukthum et al. 2009 | Thailand | 80 | 6 | 0 | 68 | 19 | 4 | 0.035 |
| Kilic et al. 2010 | Turkey | 22 | 1 | 0 | 40 | 7 | 0 | 0.022 |
| Chou et al. 2011 | Taiwan, China | 367 | 135 | 6 | 111 | 61 | 8 | 0.145 |
| Long et al. 2011 | China | 81 | 22 | 2 | 62 | 39 | 11 | 0.124 |
| Sato et al. 2012 | Japan | 236 | 103 | 6 | 31 | 24 | 1 | 0.167 |
| Silva et al. 2012 | India | 141 | 39 | 0 | 100 | 26 | 0 | 0.108 |
| Tiwari et al. 2013 | India | 99 | 1 | 0 | 92 | 8 | 0 | 0.005 |
| Wong et al. 2013 | Malaysia | 226 | 22 | 15 | 47 | 4 | 1 | 0.099 |
| Tiwari et al. 2014 | India | 217 | 1 | 0 | 107 | 5 | 1 | 0.002 |
| Narter et al. 2011 | Turkey | 47 | 19 | 4 | 23 | 13 | 3 | 0.24 |
| Total | 2730 | 598 | 53 | 1293 | 412 | 90 | 0.104 | |
Table 3.
Meta-analysis of the genotyped and allele distributions of Gly71Arg UGT1A1 polymorphisms for the cases and controls.
| GG | AA | GA+AA | GG+AG | AA | G | A | |
|---|---|---|---|---|---|---|---|
| Model | / | Fixed | Fixed | / | Fixed | / | Random |
| Heterogeneity (I2) | / | 22.4% | 49.3% | / | 5.8% | / | 55.5% |
| OR (95%CI) | / | 4.01 (2.47 to 6.51) | 2.25 (1.76 to 2.87) | / | 3.47 (2.29 to 5.28) | / | 2.17 (1.74 to 2.72) |
| P | / | <0.0001 | <0.0001 | / | <0.0001 | / | <0.0001 |
| Figure | / | Figure 1A | Figure 1B | / | Figure 1C | / | Figure 1D |
OR – odds ratio; 95%CI – 95% confidence interval.
Figure 1.
Meta-analysis of UGT1A1 Gly71Arg polymorphism and neonatal hyperbilirubinemia. (A) Comparison of A/A vs. G/G; (B) comparison of A/A+G/A vs. G/G; (C) comparison of A/A vs. G/G+G/A; (D) comparison of A allele vs. G allele.
Quantitative synthesis showed significant differences in the comparisons of GG vs. AA+GA (OR=2.25, P<0.0001, 95% CI=1.76–2.87, Heterogeneity=0.493) (Figure 1B). In addition, comparing the A allele to the G allele in the G71R polymorphism also showed a significant difference (OR=2.17, P <0.0001, 95% CI=1.74–2.72, Heterogeneity=0.555) (Figure 1D).
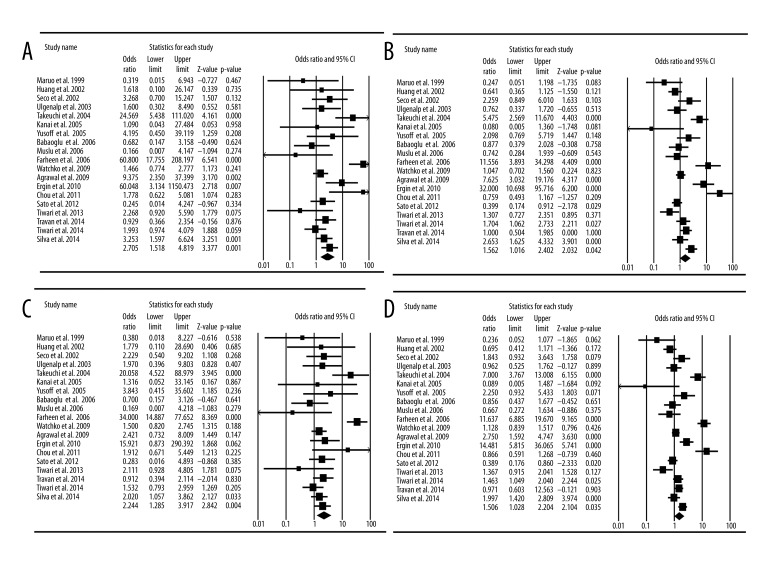
Nineteen studies focused on the relationships between TATA promoter polymorphism and neonatal hyperbilirubinemia (Table 4). Table 4 and Table 5 list the genotyped and allele distributions of the TATA promoter polymorphisms for the cases and controls. The genotype frequencies of the TATA polymorphisms were 63.4% (6/6), 31.0% (6/7), and 5.6% (7/7) in controls, and 51.8% (6/6), 32.9% (6/7), and 15.3% (7/7) in hyperbilirubinemia neonates. The (TA)7 allele frequencies in the control group was 0.216. For allele level comparison, the (TA)7 allele was associated with an increased risk of hyperbilirubinemia in terms of the frequency of allele comparison ((TA)7 vs. (TA)6, OR=1.51, 95% CI=1.03–2.20, P=0.035, Heterogeneity=89.2%) (Figure 2D). For a dominant model of the 6/6 allele, the 6/7+7/7 genotypes were associated with the risk for hyperbilirubinemia (6/7+7/7 vs. 6/6, OR=1.56, 95%CI=1.02–2.40, P=0.042, Heterogeneity=85.1%) (Figure 2B). For the 7/7 allele, the homozygote genotype was also associated with hyperbilirubinemia (6/7 + 6/6 vs. 7/7, OR=2.24, 95% CI=1.29–3.92, P=0.004, Heterogeneity=73.4%) (Figure 2C). For the extreme genotype, the 7/7 genotype was associated with the risk for hyperbilirubinemia (7/7 vs. 6/6, OR=2.71, 95% CI=1.52–4.82, P=0.001, heterogeneity=70.8%) (Figure 2A, Table 5). Analysis of these studies indicated that the TATA promoter polymorphism also increased the risk of neonatal hyperbilirubinemia.
Table 4.
Allele frequencies of TATA promoter UGT1A1 polymorphisms in cases of neonatal hyperbilirubinemia and controls.
| Author | Country | Control (n) | Case (n) | (TA) 7 allele frequencies in control group | ||||
|---|---|---|---|---|---|---|---|---|
| 6/6 | 6/7 | 7/7 | 6/6 | 6/7 | 7/7 | |||
| Maruo et al. 1999 | Japan | 37 | 11 | 2 | 23 | 2 | 0 | 0.15 |
| Huang et al. 2002 | Taiwan, China | 165 | 52 | 1 | 102 | 20 | 1 | 0.124 |
| Seco et al. 2002 | Spain | 61 | 46 | 8 | 7 | 11 | 3 | 0.270 |
| Ulgenalp et al. 2003 | Turkey | 14 | 19 | 2 | 35 | 32 | 8 | 0.329 |
| Takeuchi et al. 2004 | Japan | 57 | 12 | 2 | 29 | 14 | 25 | 0.113 |
| Kanai et al. 2005 | Japan | 96 | 20 | 0 | 29 | 0 | 0 | 0.086 |
| Yusoff et al. 2005 | Malaysia | 43 | 6 | 1 | 41 | 10 | 4 | 0.08 |
| Babaoglu et al. 2006 | Tukey | 18 | 11 | 3 | 44 | 25 | 5 | 0.266 |
| Muslu et al. 2006 | Turkey | 47 | 7 | 1 | 95 | 12 | 0 | 0.082 |
| Farheen et al. 2006 | India | 32 | 53 | 10 | 4 | 15 | 76 | 0.384 |
| Watchko et al. 2009 | America | 129 | 127 | 28 | 66 | 62 | 21 | 0.322 |
| Agrawal et al. 2009 | India | 25 | 21 | 4 | 8 | 49 | 12 | 0.29 |
| Ergin et al. 2010 | Turkey | 48 | 6 | 0 | 10 | 34 | 6 | 0.056 |
| Chou et al. 2011 | Taiwan, China | 392 | 107 | 9 | 147 | 27 | 6 | 0.123 |
| Sato et al. 2012 | Japan | 254 | 81 | 10 | 49 | 7 | 0 | 0.146 |
| Tiwari et al. 2013 | India | 37 | 53 | 10 | 31 | 50 | 19 | 0.365 |
| Travan et al. 2014 | Italy | 26 | 30 | 14 | 26 | 31 | 13 | 0.414 |
| Tiwari et al. 2014 | India | 101 | 93 | 24 | 38 | 57 | 18 | 0.323 |
| Silva et al. 2014 | India | 89 | 63 | 19 | 36 | 63 | 25 | 0.295 |
| Total | 1671 | 818 | 148 | 820 | 521 | 242 | 0.216 | |
Table 5.
Meta-analysis of the genotyped and allele distributions of TATA promoter UGT1A1 polymorphisms for the cases and controls.
| (TA) 6/6 | (TA) 7/7 | (TA) 6/7 + (TA) 7/7 | (TA) 6/6 + (TA) 6/7 | (TA) 7/7 | (TA) 6 | (TA) 7 | |
|---|---|---|---|---|---|---|---|
| Model | / | Random | Random | / | Random | / | Random |
| Heterogeneity (I2) | / | 70.8% | 85.1% | / | 73.4% | / | 89.2% |
| OR (95%CI) | / | 2.71 (1.52 to 4.82) | 1.56 (1.02 to 2.40) | / | 2.24 (1.29 to 3.92) | / | 1.51 (1.03 to 2.20) |
| P | / | 0.001 | 0.042 | / | 0.004 | / | 0.035 |
| Figure | / | Figure 2A | Figure 2B | / | Figure 2C | / | Figure 2D |
OR – odds ratio; 95%CI – 95% confidence interval.
Figure 2.
Meta-analysis of TATA promoter UGT1A1 polymorphism and neonatal hyperbilirubinemia. (A) Comparison of 7/7 vs. 6/6; (B) comparison of 7/7+6/7 vs. 6/6; (C) comparison of 7/7 vs. 6/6+/7; (D) comparison of 7 allele vs. 6 allele.
Discussion
Our meta-analysis showed that both UGT1A1 G71R and TATA promoter polymorphisms are risk factors for developing hyperbilirubinemia in white, black and Asian neonates, which was consistent with some previous studies but conflicted with others. Homozygous or heterozygous G71R and (TA)7 polymorphism were frequent not only in hyperbilirubinemia patients but also in healthy subjects [27,28]. It has been reported that the high frequency of G71R and (TA) insertion of the UGT1A1 gene are associated with a high incidence of neonatal hyperbilirubinemia [29–31]. Sato et al. found that the influence of G71R polymorphism might be overcome by adequate breastfeeding [32]. However, contrary to these findings, Mezzacappa et al. did not find any significant effect of the variants on bilirubin levels among the newborns [20,33–37].
An in vitro study verified that the UGT1A1 G71R mutant could decrease UGT1A1 enzymatic activity, which could cause moderately delayed bilirubin elimination [38]. Therefore, neonates carrying the G71R UGT1A1 variant may be at risk for hyperbilirubinemia [18]. However, some studies found no effect of the polymorphism [36,39] Therefore, the mechanism of the G71R polymorphism requires further research [40,41].
The (TA) insertion in the promoter also has been considered be associated with hyperbilirubinemia [42]. The A (TA7) TAA allele was reported to be frequently present in GS [43]. The extra TA reduced expression of the enzyme, resulting in decreased bilirubin glucuronidation activity [44]. The SNP could reduce the promoter activity, which leads to unconjugated nonhemolytic hyperbilirubinemia [45,46].
The data strongly suggest that UGT1A1 promoter (TA)7 polymorphism influences serum total bilirubin values by increasing heme catabolism as well as decreasing bilirubin conjugation [23]. In analogous studies, the (TA)7 variant was associated with modestly higher total serum bilirubin levels and (TA)7 polymorphism in the promoter developed prolonged indirect hyperbilirubinemia [7,47,48]. However, some other studies have failed to demonstrate a clinically significant effect of UGT1A1 TATA promoter variations on hyperbilirubinemia risk [23,49], such as a southern Brazil study that found the (TA)7 promoter polymorphism of UGT1A1 had no association with hyperbilirubinemia [50]. Ultimately, our research showed that (TA)7 promoter polymorphism was associated with increased risk of neonatal hyperbilirubinemia. Some studies have found a synergic effect with the (TA)7TAA promoter and G71R variants on the level of plasma bilirubin [51]. By the pathway of influence of the metabolism of heme oxygenase, we can speculate that neonates carrying the Gly71Arg or (TA)7TAA polymorphisms have decreased UGT1A1 activity, which may directly or indirectly increase COHbc and decrease serum conjugated bilirubin fractions [52].
The most important limitation of this meta-analysis is the inconsistency of the baseline characteristics (e.g., age, sex, and concomitant disease) between the case and control groups, which might increase the selection bias.
Conclusions
Our meta-analysis suggests that Gly71Arg and (TA)7 promoter polymorphisms in the UGT1A1 gene significantly increase risk of neonatal hyperbilirubinemia.
Footnotes
Source of support: Self financing
References
- 1.Long J, Zhang S, Fang X, et al. Neonatal hyperbilirubinemia and Gly71Arg mutation of UGT1A1 gene: a Chinese case-control study followed by systematic review of existing evidence. Acta Paediatr. 2011;100:966–71. doi: 10.1111/j.1651-2227.2011.02176.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen WX, Wong VC, Wong KY. Neurodevelopmental outcome of severe neonatal hemolytic hyperbilirubinemia. J Child Neurol. 2006;21:474–79. doi: 10.1177/08830738060210061301. [DOI] [PubMed] [Google Scholar]
- 3.Wong V, Chen WX, Wong KY. Short- and long-term outcome of severe neonatal nonhemolytic hyperbilirubinemia. J Child Neurol. 2006;21:309–15. doi: 10.1177/08830738060210040301. [DOI] [PubMed] [Google Scholar]
- 4.Ulgenalp A, Duman N, Schaefer FV, et al. Analyses of polymorphism for UGT1*1 exon 1 promoter in neonates with pathologic and prolonged jaundice. Biol Neonate. 2003;83:258–62. doi: 10.1159/000069487. [DOI] [PubMed] [Google Scholar]
- 5.Sarici SU, Saldir M. Genetic factors in neonatal hyperbilirubinemia and kernicterus. Turk J Pediatr. 2007;49:245–49. [PubMed] [Google Scholar]
- 6.Watchko JF, Lin Z, Clark RH, et al. Complex multifactorial nature of significant hyperbilirubinemia in neonates. Pediatrics. 2009;124:e868–77. doi: 10.1542/peds.2009-0460. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal SK, Kumar P, Rathi R, et al. UGT1A1 gene polymorphisms in North Indian neonates presenting with unconjugated hyperbilirubinemia. Pediatr Res. 2009;65:675–80. doi: 10.1203/PDR.0b013e31819ed5de. [DOI] [PubMed] [Google Scholar]
- 8.Watchko JF, Lin Z. Exploring the genetic architecture of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med. 2010;15:169–75. doi: 10.1016/j.siny.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Wong FL, Wang MK, Boo NY, et al. Rapid detection of the UGT1A1 single nucleotide polymorphism G211A using real-time PCR with Taqman minor groove binder probes. J Clin Lab Anal. 2007;21:167–72. doi: 10.1002/jcla.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 11.Chou HC, Chen MH, Yang HI, et al. 211 G to a variation of UDP-glucuronosyl transferase 1A1 gene and neonatal breastfeeding jaundice. Pediatr Res. 2011;69:170–74. doi: 10.1203/PDR.0b013e31820263d2. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer D, Klinker H. Crigler-Najjar syndrome type II in a caucasian patient resulting from two mutations in the bilirubin uridine 5′-diphosphate-glucuronosyltransferase (UGT1A1) gene. J Hepatol. 2002;36:706–7. doi: 10.1016/s0168-8278(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 13.Kadakol A, Ghosh SS, Sappal BS, et al. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297–306. doi: 10.1002/1098-1004(200010)16:4<297::AID-HUMU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Huang CS, Chang PF, Huang MJ, et al. Relationship between bilirubin UDP-glucuronosyl transferase 1A1 gene and neonatal hyperbilirubinemia. Pediatr Res. 2002;52:601–5. doi: 10.1203/00006450-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Ferraris A, D’Amato G, Nobili V, et al. Combined test for UGT1A1–3279T-->G and A(TA)nTAA polymorphisms best predicts Gilbert’s syndrome in Italian pediatric patients. Genet Test. 2006;10:121–25. doi: 10.1089/gte.2006.10.121. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto A, Nishio H, Waku S, et al. Gly71Arg mutation of the bilirubin UDP-glucuronosyltransferase 1A1 gene is associated with neonatal hyperbilirubinemia in the Japanese population. Kobe J Med Sci. 2002;48:73–77. [PubMed] [Google Scholar]
- 17.Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341–49. doi: 10.1542/pir.32-8-341. [DOI] [PubMed] [Google Scholar]
- 18.Maruo Y, Nishizawa K, Sato H, et al. Association of neonatal hyperbilirubinemia with bilirubin UDP-glucuronosyltransferase polymorphism. Pediatrics. 1999;103:1224–27. doi: 10.1542/peds.103.6.1224. [DOI] [PubMed] [Google Scholar]
- 19.Ergin H, Bican M, Atalay OE. A causal relationship between UDP-glucuronosyltransferase 1A1 promoter polymorphism and idiopathic hyperbilirubinemia in Turkish newborns. Turk J Pediatr. 2010;52:28–34. [PubMed] [Google Scholar]
- 20.Mezzacappa MA, Facchini FP, Pinto AC, et al. Clinical and genetic risk factors for moderate hyperbilirubinemia in Brazilian newborn infants. J Perinatol. 2010;30:819–26. doi: 10.1038/jp.2010.48. [DOI] [PubMed] [Google Scholar]
- 21.Babaoglu MO, Yigit S, Aynacioglu AS, et al. Neonatal jaundice and bilirubin UDP-glucuronosyl transferase 1A1 gene polymorphism in Turkish patients. Basic Clin Pharmacol Toxicol. 2006;98:377–80. doi: 10.1111/j.1742-7843.2006.pto_341.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamisako T. What is Gilbert’s syndrome? Lesson from genetic polymorphisms of UGT1A1 in Gilbert’s syndrome from Asia. J Gastroenterol Hepatol. 2004;19:955–57. doi: 10.1111/j.1440-1746.2004.03524.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan M, Hammerman C, Rubaltelli FF, et al. Hemolysis and bilirubin conjugation in association with UDP-glucuronosyltransferase 1A1 promoter polymorphism. Hepatology. 2002;35:905–11. doi: 10.1053/jhep.2002.32526. [DOI] [PubMed] [Google Scholar]
- 24.Akaba K, Kimura T, Sasaki A, et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int. 1998;46:21–26. doi: 10.1080/15216549800203512. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Jiang Z, Li C, Shu M. Prediction of heart rate variability on cardiac sudden death in heart failure patients: A systematic review. Int J Cardiol. 2014;174:857–60. doi: 10.1016/j.ijcard.2014.04.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farheen S, Sengupta S, Santra A, et al. Gilbert’s syndrome: High frequency of the (TA)7 TAA allele in India and its interaction with a novel CAT insertion in promoter of the gene for bilirubin UDP-glucuronosyltransferase 1 gene. World J Gastroenterol. 2006;12:2269–75. doi: 10.3748/wjg.v12.i14.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang PF, Lin YC, Liu K, et al. Prolonged unconjugated hyperbiliriubinemia in breast-fed male infants with a mutation of uridine diphosphate-glucuronosyl transferase. J Pediatr. 2009;155:860–63. doi: 10.1016/j.jpeds.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Huang MJ, Kua KE, Teng HC, et al. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res. 2004;56:682–89. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- 30.D’Silva S, Colah RB, Ghosh K, Mukherjee MB. Combined effects of the UGT1A1 and OATP2 gene polymorphisms as major risk factor for unconjugated hyperbilirubinemia in Indian neonates. Gene. 2014;547:18–22. doi: 10.1016/j.gene.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari PK, Bhutada A, Agarwal R, et al. UGT1A1 gene variants and clinical risk factors modulate hyperbilirubinemia risk in newborns. J Perinatol. 2014;34:120–24. doi: 10.1038/jp.2013.140. [DOI] [PubMed] [Google Scholar]
- 32.Sato H, Uchida T, Toyota K, et al. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J Hum Genet. 2013;58:7–10. doi: 10.1038/jhg.2012.116. [DOI] [PubMed] [Google Scholar]
- 33.Travan L, Lega S, Crovella S, et al. Severe neonatal hyperbilirubinemia and UGT1A1 promoter polymorphism. J Pediatr. 2014;165:42–45. doi: 10.1016/j.jpeds.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi K, Kobayashi Y, Tamaki S, et al. Genetic polymorphisms of bilirubin uridine diphosphate-glucuronosyltransferase gene in Japanese patients with Crigler-Najjar syndrome or Gilbert’s syndrome as well as in healthy Japanese subjects. J Gastroenterol Hepatol. 2004;19:1023–28. doi: 10.1111/j.1440-1746.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- 35.Sutomo R, Talib NA, Yusoff NM, et al. Screening for G71R mutation of the UGT1A1 gene in the Javanese-Indonesian and Malay-Malaysian populations. Pediatr Int. 2004;46:565–69. doi: 10.1111/j.1442-200x.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 36.D’Silva S, Colah RB, Ghosh K, Mukherjee MB. G71R mutation of the UGT1A1 gene is not associated with neonatal hyperbilirubinemia in India. J Matern Fetal Neonatal Med. 2012;25:1833–34. doi: 10.3109/14767058.2011.644362. [DOI] [PubMed] [Google Scholar]
- 37.Muslu N, Turhan AB, Eskandari G, et al. The frequency of UDP-glucuronosyltransferase 1A1 promoter region (TA)7 polymorphism in newborns and it’s relation with jaundice. J Trop Pediatr. 2007;53:64–68. doi: 10.1093/tropej/fml067. [DOI] [PubMed] [Google Scholar]
- 38.Clarke DJ, Moghrabi N, Monaghan G, et al. Genetic defects of the UDP-glucuronosyltransferase-1 (UGT1) gene that cause familial non-haemolytic unconjugated hyperbilirubinaemias. Clin Chim Acta. 1997;266:63–74. doi: 10.1016/s0009-8981(97)00167-8. [DOI] [PubMed] [Google Scholar]
- 39.Narter F, Can G, Ergen A, et al. Neonatal hyperbilirubinemia and G71R mutation of the UGT1A1 gene in Turkish patients. J Matern Fetal Neonatal Med. 2011;24:313–16. doi: 10.3109/14767058.2010.490889. [DOI] [PubMed] [Google Scholar]
- 40.Prachukthum S, Nunnarumit P, Pienvichit P, et al. Genetic polymorphisms in Thai neonates with hyperbilirubinemia. Acta Paediatr. 2009;98:1106–10. doi: 10.1111/j.1651-2227.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 41.Kilic I, Koseler A, Cakaloz I, Atalay E. Screening for G71R mutation of the UDP-glucuronosyltransferase 1 (UGT1A1) gene in neonates with pathologic and prolonged hyperbilirubinemia in Turkey. Int J Clin Pharmacol Ther. 2010;48:504–8. doi: 10.5414/cpp48504. [DOI] [PubMed] [Google Scholar]
- 42.Seco ML, del Rio E, Barcelo MJ, et al. [Interest in the study of genetic variants of the promoter region of the UGT1A1 gene in neonatal jaundice]. An Esp Pediatr. 2002;56:139–43. [in Spanish] [PubMed] [Google Scholar]
- 43.Hsieh TY, Shiu TY, Huang SM, et al. Molecular pathogenesis of Gilbert’s syndrome: decreased TATA-binding protein binding affinity of UGT1A1 gene promoter. Pharmacogenet Genomics. 2007;17:229–36. doi: 10.1097/FPC.0b013e328012d0da. [DOI] [PubMed] [Google Scholar]
- 44.Raijmakers MT, Jansen PL, Steegers EA, Peters WH. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J Hepatol. 2000;33:348–51. doi: 10.1016/s0168-8278(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 45.Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–75. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 46.Strassburg CP, Manns MP. Jaundice, genes and promoters. J Hepatol. 2000;33:476–79. doi: 10.1016/s0168-8278(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 47.Roy-Chowdhury N, Deocharan B, Bejjanki HR, et al. Presence of the genetic marker for Gilbert syndrome is associated with increased level and duration of neonatal jaundice. Acta Paediatr. 2002;91:100–1. doi: 10.1080/080352502753458058. [DOI] [PubMed] [Google Scholar]
- 48.Monaghan G, McLellan A, McGeehan A, et al. Gilbert’s syndrome is a contributory factor in prolonged unconjugated hyperbilirubinemia of the newborn. J Pediatr. 1999;134:441–46. doi: 10.1016/s0022-3476(99)70201-5. [DOI] [PubMed] [Google Scholar]
- 49.Yusoff S, Van Rostenberghe H, Yusoff NM, et al. Frequencies of A(TA)7TAA, G71R, and G493R mutations of the UGT1A1 gene in the Malaysian population. Biol Neonate. 2006;89:171–76. doi: 10.1159/000088844. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho CG, Castro SM, Santin AP, et al. Polymorphic variants of UGT1A1 in neonatal jaundice in southern Brazil. J Trop Pediatr. 2010;56:366–67. doi: 10.1093/tropej/fmp131. [DOI] [PubMed] [Google Scholar]
- 51.Tiwari PK, Sethi A, Basu S, et al. Heme oxygenase-1 gene variants and hyperbilirubinemia risk in North Indian newborns. Eur J Pediatr. 2013;172:1627–32. doi: 10.1007/s00431-013-2091-7. [DOI] [PubMed] [Google Scholar]
- 52.Kanai M, Kijima K, Shirahata E, et al. Neonatal hyperbilirubinemia and the bilirubin uridine diphosphate-glucuronosyltransferase gene: the common -3263T > G mutation of phenobarbital response enhancer module is not associated with the neonatal hyperbilirubinemia in Japanese. Pediatr Int. 2005;47:137–41. doi: 10.1111/j.1442-200x.2005.02030.x. [DOI] [PubMed] [Google Scholar]