Abstract
The fungal feeding, hermaphroditic Bursaphelenchus okinawaensis is a laboratory model to understand the biology of Bursaphelenchus. The extent to which B. okinawaensis can be used to model Bursaphelenchus xylophilus mating was investigated. A chemotaxis assay was conducted to examine whether B. xylophilus and B. okinawaensis produce and respond to volatile sex attractants. Unmated B. xylophilus females were found to attract B. xylophilus males. Similarly, old (sperm depleted) but not young (sperm repleted) B. okinawaensis hermaphrodites attract B. okinawaensis males. Thus, in both species, sperm status corresponds to its ability to attract males. B. xylophilus males also produce a volatile pheromone that attracts both mated and unmated females. A second assay, in which the behavior of males on petri plates in the presence of different females or hermaphrodites of Bursaphelenchus was observed, revealed that B. xylophilus unmated females attract B. okinawaensis males, and B. okinawaensis old hermaphrodites attract B. xylophilus males. These observations suggested that the pheromones of Bursaphelenchus work to some extent across species. Mating behavior through spicule insertion occurs across species, suggesting that postcopulatory mechanisms prevent production of interspecific progeny. The hermaphroditic B. okinawaensis will be a useful model to conduct genetic studies for the understanding of the molecular mechanisms underlying mating behavior in Bursaphelenchus nematodes.
Keywords: behavior, mating behavior, reproductive isolation, sex pheromone, sperm status
Reproductive strategies are a key feature of evolutionary processes and are highly divergent among a taxon. Nematodes are one of the most numerous animal phyla and their reproductive modes can be strikingly different. Although most nematode species are gonochoristic (individuals are either male or female), some species have parthenogenetic (individuals can produce fertile eggs asexually) or hermaphroditic (individuals can produce both kinds of gametes) modes of reproduction. Caenorhabditis elegans is intensively studied in part because it has self-fertilizing hermaphrodites and males, making it efficient for genetic studies (Brenner, 1974). In the genus Caenorhabditis, self-fertilizing species such as C. elegans evolved independently at least three times (Kiontke et al., 2011), and thus have been used to study the evolutionary consequences of their reproductive systems such as reproductive modes, sex determination, and mating behavior.
Nematode mating behavior has been most intensively studied in C. elegans. Briefly, males locate hermaphrodites, and once they contact them, initiate a series of copulatory steps that are coordinated by combination of chemo- and mechanosensory inputs mediated primarily by male specific sensory neurons (Liu and Sternberg, 1995; Barr and Garcia, 2006). Recently, there has been major progress in our understanding the sex pheromones of nematodes using Caenorhabdits nematodes. Srinivasan et al. (2008) showed that a family of water-soluble small molecules, the ascarosides, control sexual attraction in C. elegans (Ludewig and Schroeder, 2013). Ascarosides are also sex pheromones in Panagrellus redivivus (Choe et al., 2012). Hermaphrodite sexual attraction to males is regulated by hermaphrodite sperm status (Kleemann and Basolo, 2007; Morsci et al., 2011), and hermaphrodite sexual attractiveness is regulated by the production of a volatile pheromone (Leighton et al., 2014). Chasnov et al. (2007) reported that C. elegans males are attracted to the volatile of female of the gonochoristic C. brenneri and C. remanei. The volatile sex pheromone that attracts males is thus likely conserved among Caenorhabditis nematodes.
Bursaphelenchus nematodes are of great interest because B. xylophilus is the pathogen causing pine wilt disease, one of the most serious forest diseases in the world (Mamiya, 1988; Futai, 2013). We recently demonstrated the hermaphroditism and genetic tractability of the fungal feeding species B. okinawaensis (Shinya et al., 2014). Mating experiments that used a visible recessive mutation to distinguish self- from cross-progeny showed that B. okinawaensis male mates only with the sperm-depleted old adult hermaphrodites. In the gonochoristic species B. xylophilus, males are attracted to volatile cues from virgin females, while not attracted to the volatile cues from mated or gravid females (Kiyohara, 1982). Also, Liu et al. (2014) carefully observed the mating behavior in B. xylophilus and compared it with C. elegans. One major difference in mating among nematodes is whether the male coils around the female as in B. xylophilus or only contacts the female/hermaphrodite with his copulatory tail as in C. elegans.
Here, we carried out assays of chemotaxis toward volatile cues in B. okinawaensis and B. xylophilus to test the possibility that volatiles from sperm-depleted old hermaphrodites of B. okinawaensis attract males, and to investigate the conservation of the sex pheromone between species. We then observed their mating behavior to test the consistency between the results of volatile chemotaxis assay and their mating behavior.
Materials and Methods
Nematode maintenance and synchronization:
The Ka4 isolate of B. xylophilus (Aikawa and Kikuchi, 2007) and the SH1 strain of B. okinawaensis (Shinya et al., 2014) were used in this study. The nematodes were propagated on the filamentous fungus Botrytis cinerea on malt extract agar medium (MEA; BD Difco, Sparks, MD) plates with 4% agar containing 100 µg/ml chloramphenicol in a 90-mm diameter petri dish at 25°C.
To collect specific stages, the development of nematodes was synchronized according to Shinya et al. (2014). To obtain unmated B. xylophilus adult females, synchronized L4 females were handpicked with a platinum wire and incubated for 2 d without males on the budding yeast Saccharomyces cerevisiae (strain W303-1A), which were grown on 1/10 MEA with 4% agar containing 100 µg/ml chloramphenicol in a 60-mm diameter petri dish (hereafter called “1/10 MEA-chloramphenicol plates”). For the mated adult B. xylophilus females, gravid adult females were picked from 4-d-old synchronized plates. A gravid adult female of B. xylophilus usually has only one fertilized egg in her uterus, whereas an unmated adult female has no eggs in her uterus. Adult B. xylophilus males were picked from a 3-d-old synchronized plate and incubated for 1 d without females onto the 1/10 MEA-chloramphenicol plate before being assayed. Young B. okinawaensis hermaphrodites (sperm replete) were picked from 3-d-old synchronized plates and incubated for 1 d without males on 1/10 MEA-chloramphenicol plates until assayed. Six-day-old adult B. okinawaensis hermaphrodites after the final molt were prepared according to Shinya et al. (2014) and used as sperm-depleted old adult hermaphrodites. B. okinawaensis males were picked from 3-d-old synchronized plates and incubated for 1 d without hermaphrodites on the 1/10 MEA-chloramphenicol plates before being assayed. The male ratio of B. okinawaensis is quite low (0.1% to 0.4%), but 10 to 20 males were usually found per single synchronized plate.
Worm conditioned media:
Potential chemical cues were obtained as secreted/excreted products (worm conditioned media [WCM]) from B. xylophilus unmated adult females, mated adult females, and adult males, and from B. okinawaensis young adult hermaphrodites, old adult hermaphrodites, and adult males according to Leighton et al. (2014) with a slight modification. We assumed that chemical cues from the different types of the nematodes would be secreted into double-distilled water (ddH2O); thus, chemical cues were captured in WCM. All of these nematodes were collected using the method described above. Before extracting chemical cues, nematodes were soaked in ddH2O in a glass dish for 2 to 3 hr to remove yeast from the nematodes. After this washing step, the nematodes were placed in the inverted cap of a microcentrifuge tube, with one nematode per microliter. The cap was sealed and incubated for 24 hr at 25°C. After chemical cue extraction, chemical cues were immediately used for chemotaxis assay without being frozen.
Chemotaxis assay:
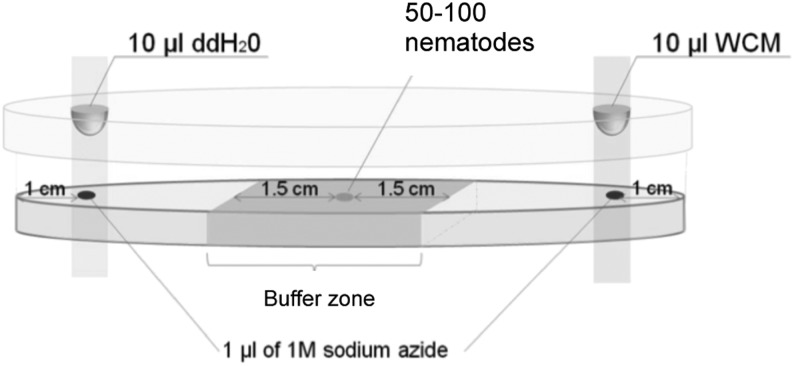
To examine the production and response of volatile sexual attractants, we used a standard assay for attraction to volatile odors. Chemotaxis assays were carried out in a 90-mm diameter petri dish containing 4% agar (Fig. 1). About 10 µl of worm suspension containing 50 to 100 worms were pipetted onto the center of the plate (the buffer zone). Just before the water was completely absorbed, two 1 µl drops of 1 M sodium azide solution were spotted on each far side (1 cm from the edge) of the plate. Subsequently, 10 µl of WCM was pipetted onto the inside lid of the petri dish just above one spot of sodium azide, and 10 µl of ddH2O was pipetted to the other as a control. The WCM exposed to B. xylophilus unmated adult females, mated adult females, and adult males, and from B. okinawaensis young adult hermaphrodites, old adult hermaphrodites, and adult males were used as a chemical cue. Sodium azide was used to paralyze nematodes once they reached the spot of chemical cue or ddH2O; this helps to avoid the confounding effect of behavioral adaptation to chemical cues. A schematic diagram of the chemotaxis assay plate is shown in Fig. 1. The plate was then incubated 6 hr in a small box to eliminate the influence of light. After 6 hr of incubation, only paralyzed nematodes that move out of the 3-cm buffer zone on the assay plate (Fig. 1) were scored for either attracted to the chemical cue emitted from the designated nematodes or from the control, which was water without nematode cue. A chemotaxis index (CI) was calculated as: ([number of nematodes at the test cue zone] − [number of nematodes at the control zone])/([number of nematodes at the test cue zone] + [number of nematodes at the control zone]) to measure the preference toward the test chemical cue. A positive CI indicates attraction, whereas a negative one indicates repulsion. All chemotaxis assays were repeated three times. Dunnett’s test was used to detect differences between nematode cue and ddH2O control (P < 0.01). Statistical significance for CI value of each chemical cue was calculated in comparison to the response to ddH2O (Choe et al., 2012).
Fig. 1.
Schematic diagram of volatile chemotaxis assay. WCM, worm conditioned media.
Con- and interspecific mating test:
To investigate the number of progeny produced by cross-mating in conspecific mating and the possibility of interspecific mating between B. xylophilus and B. okinawaensis, mating plates were prepared by placing three adult males of B. xylophilus or B. okinawaensis along with a single 6-d-old B. okinawaensis adult hermaphrodite or unmated B. xylophilus adult female on 1/10 MEA-chloramphenicol plates containing yeast as the nematode food source. Therefore, the mating combinations were (i) B. xylophilus males–B. xylophilus unmated adult female; (ii) B. okinawaensis males–B. okinawaensis 6-d-old adult hermaphrodite; (iii) B. xylophilus males–B. okinawaensis 6-d-old adult hermaphrodite; (iv) B. okinawaensis males–B. xylophilus unmated adult female. These plates were incubated for 24 hr at 25°C. Once eggs were produced on the plate, the hermaphrodites or females were transferred to new plates each day and allowed to lay eggs until they stopped laying eggs. This experiment was performed in 10 biological replicates for each combination.
Male mating behavior assay:
To investigate male mating behavior, especially searching and spicule insertion ability, we carried out the “1-hr assay” described by Wegewitz et al. (2008) with some small modifications. A single male of B. xylophilus or B. okinawaensis was transferred onto the 1/10 MEA-chloramphenicol plates with 1.5-cm diameter yeast spot with 14 females of B. xylophilus or hermaphrodites of B. okinawaensis. We measured the time until the male had a physical contact and exhibited response behavior (first contact) (Fig. 2A,C) and until the male inserted his spicule into the vulva of female or hermaphrodite (spicule insertion; Fig. 2B,D). We considered response behavior to occur when the male stops forward locomotion and pressed his trembling head against his partner’s body. Observations were terminated after spicule insertion. If spicule insertion did not occur, observation was terminated after 1 hr. This experiment was performed in 10 biological replicates. Statistical significance for the time until first contact and until spicule insertion was examined by one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test (P < 0.01).
Fig. 2.
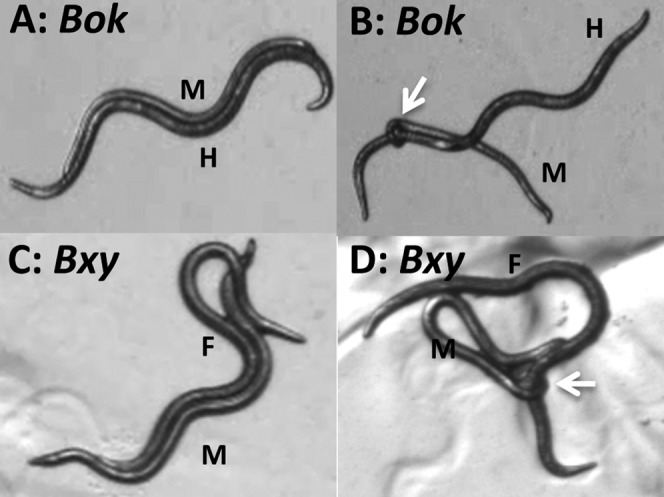
Mating behavior of Bursaphelenchus okinawaensis and B. xylophilus. The images show the stages of (A) pre-mating behavior of B. okinawaensis; (B) spicule insertion behavior of B. okinawaensis; (C) premating behavior of B. xylophilus; and (D) spicule insertion behavior of B. xylophilus. M, male; H, hermaphrodite; F, female. White arrows indicate the position of male spicule inserted into vulva.
Female or hermaphrodite mating behavior:
To test the ability of female or hermaphrodite to search for males, we counted the number of females or hermaphrodites having physical contact with a male in five different time periods. A single male of B. xylophilus or B. okinawaensis was transferred onto the 1/10 MEA-chloramphenicol plates with 1.5-cm diameter yeast spot with 14 females of B. xylophilus or hermaphrodites of B. okinawaensis. The numbers of females or hermaphrodites in physical contact with a male were examined using a time-lapse video recording device at 10, 20, 40, 60, and 120 min after the beginning of incubation. This experiment was performed in five biological replicates. Statistical significance for the number of female or hermaphrodite having physical contact with a male was examined by two-way ANOVA followed by Fisher’s exact test (P < 0.01).
Results
Female or hermaphrodite volatile pheromone:
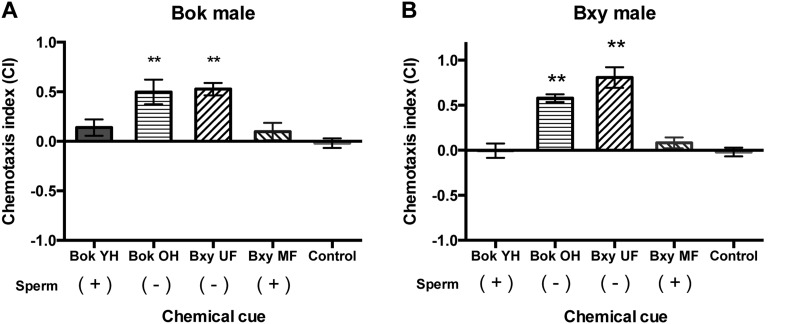
B. okinawaensis males were attracted to the volatile odors of sperm-depleted old adult B. okinawaensis hermaphrodites (P < 0.01). By contrast, chemical cue of sperm-replete young adult hermaphrodites did not show significant attraction of the conspecific males (Fig. 3A). Similarly, B. xylophilus males were attracted to the volatile of the unmated females having no sperm (P < 0.0001), while the chemical cue of mated females having sperm injected by prior mating did not significantly attract conspecific males (Fig. 3B). In this chemotaxis assay, we also investigated the interspecific effect of the volatile compounds produced by females or hermaphrodites in sexual attraction. As a result, interspecific attractions were found only in the combination of B. okinawaensis males–B. xylophilus unmated female (P < 0.01) and B. xylophilus males–B. okinawaensis old hermaphrodite (P < 0.001). In other words, the chemical cue of B. okinawaensis old hermaphrodites and B. xylophilus unmated females attracted males in both species. No significant attraction was observed using the chemical cue of B. okinawaensis young hermaphrodites and B. xylophilus mated females in any combinations.
Fig. 3.
Effect of chemotaxis on males of A) Bursaphelenchus okinawaensis (Bok) by the chemical cue of young hermaphrodites (YH) and old hermaphrodites (OH) of Bok or unmated female (UF) and mated female (MF) of B. xylophilus (Bxy) as compared to the water control; B) Bxy by the chemical cue of YH and OH of Bok or UF and MF of Bxy as compared to the water control. Chemotaxis index (CI) = ([number of nematodes at the test cue zone] − [number of nematodes at the control zone])/([number of nematodes at the test cue zone] + [number of nematodes at the control zone]). Sperm status of hermaphrodites or females are shown as (+) or (−). Sperm (+) indicates hermaphrodite having self-produced sperm or female having sperm supplied by male, while sperm (−) indicates hermaphrodite or female have no sperm at the preparation of the WCM. Error bars indicate standard error. **Indicates significant difference (P < 0.01) in CI between the tested chemical cue and water control based on Dunnett’s test.
Male volatile pheromone:
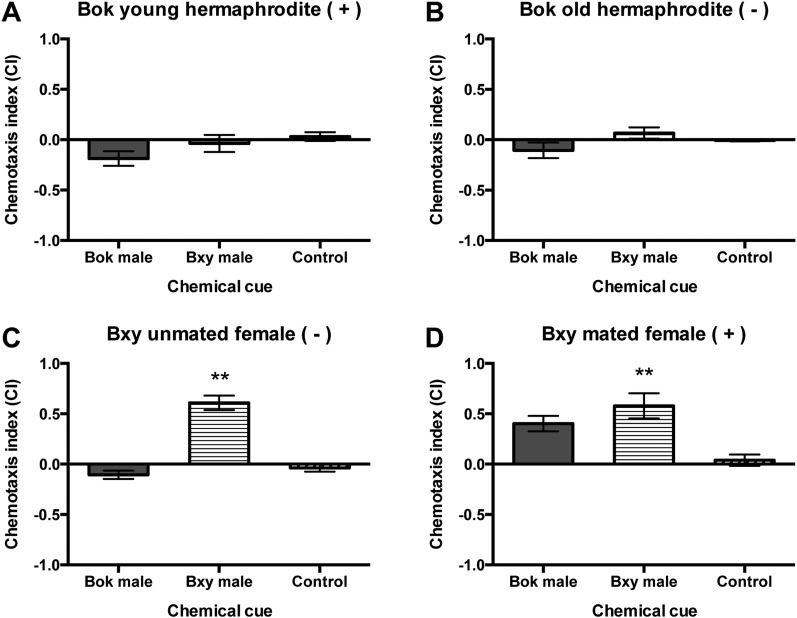
The chemical cue of B. xylophilus male showed species-specific sexual attraction, whereas B. okinawaensis hermaphrodites were not attracted to of B. okinawaensis males (Fig. 4). In B. xylophilus, both unmated and mated females were significantly attracted to the chemical cue of conspecific males (P < 0.001 and P < 0.01, respectively) (Fig. 4C,D). However, B. okinawaensis hermaphrodites were neither attracted to the chemical cues of B. okinawaensis males nor of B. xylophilus males (Fig. 4A,B). The sensitivity of B. xylophilus female to the conspecific male volatile was not affected by their sperm status.
Fig. 4.
Effect of chemotaxis on hermaphrodites of A) young (YH) and B) old hermaphrodites (OH) of Bursaphelenchus okinawaensis (Bok) or females of C) unmated (UF), and D) mated females (MF) of B. xylophilus (Bxy) by the chemical cue of male of Bok or Bxy as compared to the water control. Chemotaxis index (CI) was calculated as in Fig. 3. A positive CI indicates attraction, while a negative one indicates repulsion. (+) or (−) in each set indicates sperm status of hermaphrodites or females which used for chemotaxis assay. Sperm (+) indicates hermaphrodite having self-produced sperm or female having sperm supplied by male, while sperm (−) indicates hermaphrodite or female have no sperm at the beginning of chemotaxis assay. Error bars indicate standard error. **Indicates significant difference (P < 0.01) in CI between the tested chemical cue and water control based on Dunnett’s test.
The number of progeny after con- and interspecific mating:
To determine the efficiency of mating within and between species, animals were allowed to mate for a defined period of time and counted their progeny. Specifically, the number of progeny produced by 24 hr of mating was counted. B. okinawaensis hermaphrodites produced 57.7 ± 21.8 progeny after mating with B. okinawaensis males through 7 d, although most of the eggs were laid in the first 4 d. B. xylophilus females produced 48.2 ± 7.7 progeny by 3 d after mating with B. xylophilus male. In interspecific combinations (B. okinawaensis males × B. xylophilus female and B. xylophilus males × B. okinawaensis hermaphrodite), no progeny were observed. However, in both cases, aspects of mating behavior, such as response and vulva location behavior, were sometimes observed.
Male mating behavior:
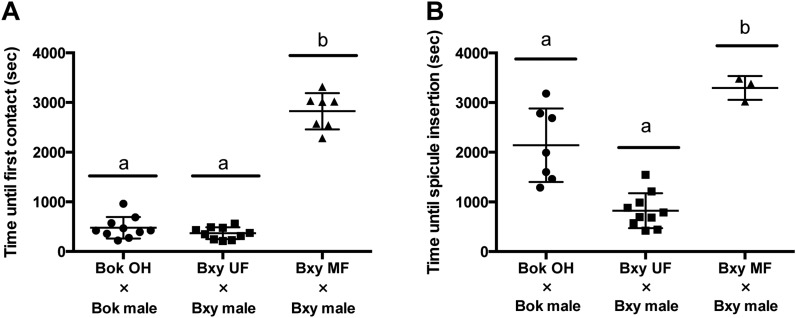
To investigate male mating behavior in detail, the 1-hr-assay described above was performed. In this test, the time until the first contact and until the spicule insertion was measured. B. okinawaensis males did not show any mating behavior such as response and vulva location to the young hermaphrodite, and therefore, the results for young hermaphrodites are not shown in Fig. 5. For old hermaphrodites, the time until first contact was 478 ± 205 sec and until spicule insertion was 2,142 ± 685 sec. Spicule insertion was successful in 8 of 10 trials. In B. xylophilus, significant differences between unmated and mated female were observed in both the time until first male–female contact and spicule insertion (Fig. 5A,B). The time until first contact was significantly less for unmated female than for mated females (P < 0.01). When paired with unmated females, males successfully inserted their spicules in all 10 trials, where spicule insertion was observed in 4 of 10 trials if males were paired with mated females (P < 0.05). Moreover, the time until spicule insertion was significantly less for unmated females (N = 10) than for mated females (N = 4). These results suggest that males were more strongly attracted to the unmated female and achieved their first spicule insertion earlier than with mated female. These results are consistent with the results of the chemotaxis assays.
Fig. 5.
Efficiency of some steps of mating behavior. Scatter plot of the observed time (A) until the first contact and (B) the first spicule insertion in the conspecific mating partners. Data are presented as mean ± standard deviation (SD). This assay was carried out with a single male and 14 hermaphrodites or females on a plate. We considered the first contact to occur when the male stops forward locomotion and pressed his trembling head against his partner’s body and the first contact to occur when the male wrapped around the vulva and kept the posture at least 1 min. Horizontal bars at the top indicate the result of statistical analysis using Tukey’s honestly significant difference (HSD) test. Identical letters indicate no significant difference at P < 0.01. YH, young hermaphrodite; OH, old hermaphrodite; UF, unmated female; MF, mated female.
Female or hermaphrodite mating behavior:
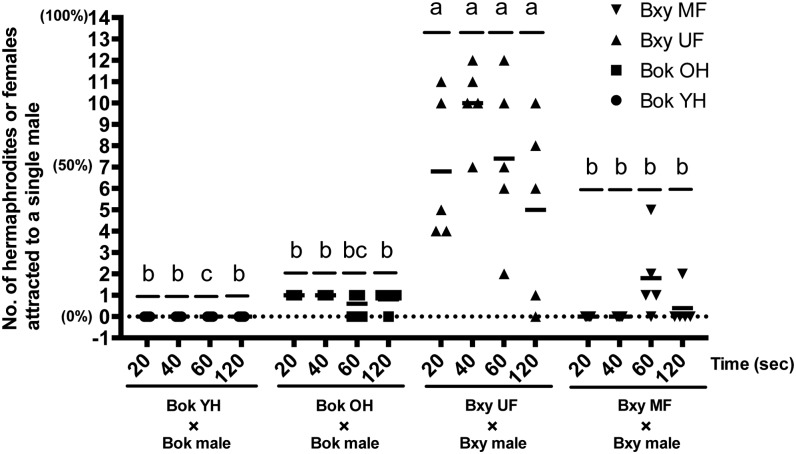
The two-way ANOVA showed that the number of females attracted to a conspecific single male was significantly affected by the nematode combination (F[3, 64] = 69.91, P < 0.01). On the other hand, it was not significantly affected by the incubation period (F[3, 64] = 1.67, P > 0.01) and the interaction between the incubation period and the nematode combination (F[9, 64] = 1.93) (P > 0.01). A significant difference was observed between B. xylophilus unmated female and the others in all time periods (P < 0.01). During the first 40 min, the number of B. xylophilus unmated females having physical contact with male gradually increased. At 40 min after the start of observation, 7 to 12 of 14 females were attracted to a single male and got entangled (Figs. 6,7). For B. okinawaensis, males had contact with at most one single old hermaphrodite. During our observations, B. xylophilus unmated females were clearly attracted to a single male (Fig. 7), whereas no attraction of B. okinawaensis hermaphrodite to a single male was observed.
Fig. 6.
The number of hermaphrodites of B. okinawaensis or females of B. xylophilus attracted to a conspecific single male. The assay was carried out with a single male and 14 hermaphrodites or females on a plate. At four different time points (20 min, 40 min, 60 min, and 120 min), the number of hermaphrodites or females having a physical contact to a single male was counted. Each symbol represents the actual number of hermaphrodites or females attracted to a single male in each plate. The figures in the parentheses indicate percentages. Horizontal bars indicate the average number of five independent measurements. Significant difference between nematode combinations at each of the four time points was analyzed by two-way analysis of variance (ANOVA) followed by Fisher’s exact test (P < 0.01). Identical letters at the top indicate no significant difference. YH, young hermaphrodite; OH, old hermaphrodite; UF, unmated female; MF, mated female.
Fig. 7.
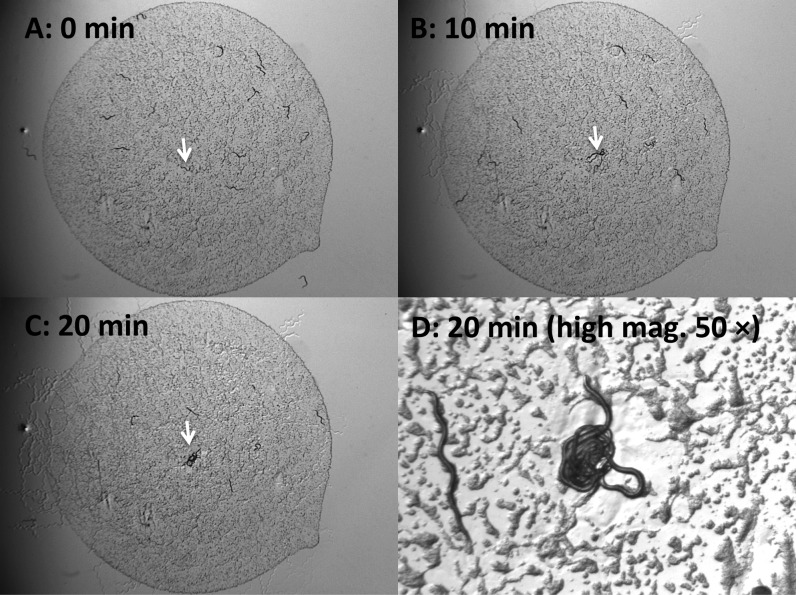
Time-lapse images of B. xylophilus unmated female attraction to a single B. xylophilus male. The assay was carried out with a single male and 14 hermaphrodites or females on a plate. The images show the unmated female attraction after (A) 0 min; (B) 10 min; (C) 20 min; (D) 20 min with high magnification (40×) from the start of the observations. White arrows indicate the position of male.
Discussion
Our chemotaxis assay showed that old B. okinawaensis hermaphrodites and unmated B. xylophilus females produce a volatile sex pheromone. Moreover, our results indicate that the production of the pheromone coincides with the sperm status of hermaphrodites or females (Fig. 3). The sex pheromone produced by hermaphrodites or females is not species specific: it attracts males of different species to the same extent it does its own males. Therefore, the main components of hermaphrodite or female pheromone are likely the same in these two species. These results are consistent with previous reports of Caenorhabditis volatile sex pheromones. Leighton et al. (2014) reported that a hermaphrodite volatile pheromone attracts males, and the production of pheromone is regulated by hermaphrodite sperm status, in particular by oocyte–soma communication. Chasnov et al. (2007) showed that female sex pheromone from C. brenneri and C. remanei attracted males of different species within the same genus. The Bursaphelenchus genus was assigned to Clade IV by Blaxter et al. (1998), and is phylogenetically distant from Caenorhabditis genus, in Blaxter Clade V. Therefore, volatile pheromone regulation mechanism by sperm status, and possibly by oocyte–soma communication, might be widely conserved among nematodes.
Another striking result from our chemotaxis assay is the difference in male volatile pheromones between B. xylophilus and B. okinawaensis. The presence of a male volatile sex pheromone in B. xylophilus was shown by Kiyohara et al. (1982). However, the species specificity of male volatile pheromone and sperm status specificity remained unknown. Our study showed that volatile pheromone of B. xylophilus males has species-specific sexual attraction, whereas B. okinawaensis hermaphrodites were not attracted to the one of B. okinawaensis males. Both mated and unmated B. xylophilus females were attracted to male pheromone. Therefore, the sensitivity to the male pheromone in females is not likely regulated by her sperm status. The number of reports describing male sex pheromones in nematodes is limited compared with female sex pheromones (Greet, 1964; Anya, 1976; Somers et al., 1977; Duggal, 1978; Garcia-Rejon et al., 1985; Choe et al., 2012). Choe et al. (2012) reported that the gonochoristic species Panagrellus redivivus males produce a soluble sex pheromone (ascaroside dhas#18) that attracts females, consistent with the results of Duggal (1978); however, there is no evidence for a male volatile pheromone in this species.
Con- and interspecific cross-mating assays were performed to determine the efficiency of mating within and between species. In B. okinawaensis, old adult hermaphrodites depleted of self-produced sperm were used as mating partners of males because we previously reported that B. okinawaensis young hermaphrodites replete with self-produced sperm did not produce progeny by cross-mating (Shinya et al., 2014). Because B. okinawaensis males mate only with sperm depleted hermaphrodites, any progeny produced after cross-mating likely result from sperm injected by one or a few sperm transfers in a very short time period. In B. xylophilus, unmated females were used for the mating experiment. During a 24 hr incubation with males, copulation behavior was observed at least several times, and it is thus likely that sperm were supplied several times. In this experiment, the possibility of interspecific mating was also investigated. In both interspecific combinations, i.e., B. okinawaensis males × B. xylophilus females and B. xylophilus males × B. okinawaensis hermaphrodites, no progeny was observed after 24 hr incubation. However, in both cases, copulatory behavior was observed at high frequency. Also, our chemotaxis assay showed that the volatile pheromone of hermaphrodites or females attracted males of different species. These results suggest that the production of interspecific progeny is prevented by postcopulatory mechanisms rather than premating behavior such as the production of and response to sex pheromone. Reproductive isolation involves several barriers. In Caenorhabditis nematodes, the steps of sperm activation, spermathecal targeting, ovulation, sperm completion, and fertilization have been known as a postcopulatory reproductive barriers in the interspecific reproduction (Hill and L’Hernault, 2001). Reproductive isolation among B. xylophilus strains and between B. xylophilus and B. mucronatus has been reported (Bolla and Boschert, 1993). However, the molecular mechanisms underlying the reproductive isolation among Bursaphelenchus species remain unknown. Further genetic and molecular studies might help elucidate the nature of the reproductive barrier(s) among Bursaphelenchus nematodes.
We observed mating behavior in detail to confirm the consistency between the result of volatile chemotaxis assay and their real mating behavior. First, the time until the first contact and until the spicule insertion were measured to investigate the ability to search for mating partner. The chemical attractiveness should reflect this searching ability. These results are fully consistent with the results of our chemotaxis assay. The time until first contact was short in the combinations of B. okinawaensis males–B. okinawaensis old hermaphrodite and B. xylophilus males–B. xylophilus unmated female. B. okinawaensis males did not show any mating behavior such as response and vulva location to the young hermaphrodite. Even if a B. okinawaensis male had a physical contact with young hermaphrodite accidentally, the male did not stop his forward locomotion. In the combination of B. xylophilus males–B. xylophilus mated female, the time until first contact was significantly longer than that in the unmated female. B. xylophilus males and unmated females were attracted to each other, whereas males were not attracted to mated females. Therefore, one-way attractiveness would delay the time until they found a mating partner, and B. xylophilus males were able to insert their spicules with mated females, suggesting that B. xylophilus mated female have a local mating cue to induce male copulation behavior. Only in the combination of B. xylophilus males with B. xylophilus unmated females was strong attraction of females to male observed. When a male finds a mating partner, he stops his forward locomotion and starts premating behavior such as vulva location behavior. At that time, most of the unmated females on the plate were aggregated onto a single male. On the other hand, B. okinawaensis males always had either no physical contact or had physical contact with only a single old hermaphrodite, suggesting that B. okinawaensis hermaphrodites are not attracted to B. okinawaensis males. Chemotaxis assays showed that B. xylophilus females were attracted to volatile pheromones of conspecific males, whereas B. okinawaensis hermaphrodites were not attracted to the chemical cue of B. okinawaensis males. Their preference for volatile sex pheromone likely reflects their mating behavior. In the gonochoristic species, mating is necessary for reproduction. On the other hand, hermaphroditic species can reproduce without mating by male and the mating should be one of the options. Therefore, the presence of a hermaphroditic reproductive mode is expected to relax purifying selection for male mating (Garcia et al., 2007). B. okinawaensis males might lack the ability to produce a sex attractant for hermaphrodites as an evolutionary consequence.
The hermaphroditic B. okinawaensis is a laboratory model for aspects of the Bursaphelenchus biology. Here it has been demonstrated that the volatile pheromone produced by B. okinawaensis hermaphrodite and B. xylophilus is shared between species and precopulatory behavior is highly similar. The hermaphroditic B. okinawaensis seems useful for genetic analysis to understand the mating behavior of Bursaphelenchus nematodes as well as the evolution of mating behavior among nematodes.
Literature Cited
- Aikawa T, Kikuchi T. Estimation of virulence of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) based on its reproductive ability. Nematology. 2007;9:371–377. [Google Scholar]
- Anya AO. Studies on the reproductive physiology of nematodes: The phenomenon of sexual attractions and the origin of the attractants in Aspiculuris tetraptera. International Journal of parasitology. 1976;6:109–117. doi: 10.1016/0020-7519(76)90076-x. [DOI] [PubMed] [Google Scholar]
- Barr MM, Garcia LR. 2006. Male mating behavior (June 19, 2006). The C. elegans Research Community, ed. WormBook. doi/10.1895/wormbook.1.78.1, http://www.wormbook.org.
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Bolla RI, Boschert M. Pinewood nematode species complex—interbreeding potential and chromosome number. Journal of Nematology. 1993;25:227–238. [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proceedings of the National Academy of Sciences. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A, Chuman T, von Reuss SH, Dossey AT, Yim JJ, Ajredini R, Kolawa AA, Kaplan F, Alborn HT, Teal PEA, Schroeder FC, Sternberg PW, Edison AS. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proceedings of the National Academy of Sciences. 2012;109:20949–20954. doi: 10.1073/pnas.1218302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal CL. Sex attraction in the free living nematode Panagrellus redivivus. Nematologica. 1978;24:213–221. [Google Scholar]
- Futai K. Pine wood nematode, Bursaphelenchus xylophilus. Annual Review of Phytopathology. 2013;51:61–83. doi: 10.1146/annurev-phyto-081211-172910. [DOI] [PubMed] [Google Scholar]
- Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175:1761–1771. doi: 10.1534/genetics.106.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rejon L, Sanchez-Moreno M, Verdejo S, Monteoliva M. Site of sex pheromone production in Ascaris suum (Nematoda) Canadian Journal of Zoology. 1985;63:664–665. [Google Scholar]
- Greet DN. Observations on sexual attraction and copulation in the nematode Panagrolaimus rigidus (Schneider) Nature. 1964;204:96–97. [Google Scholar]
- Hill KL, L'Hernault SW. Analyses of reproductive interactions that occur after heterospecific matings within the genus Caenorhabditis. Developmental Biology. 2001;232:105–114. doi: 10.1006/dbio.2000.0136. [DOI] [PubMed] [Google Scholar]
- Kiontke KC, Félix MA, Ailion M, Rockman MV, Braendle C, Pénigault JB, Fitch DH. A phylogeny and molecular barcodes for Caenorhabditis with numerous new species from rotting fruits. BMC Evolutionary Biology. 2011;11:339. doi: 10.1186/1471-2148-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara T. Sexual attraction in Bursaphelenchus xylophilus. Japanese Journal of Nematology. 1982;11:7–12. [Google Scholar]
- Kleemann GA, Basolo AL. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Animal Behaviour. 2007;74:1339–1347. [Google Scholar]
- Leighton DHW, Choe A, Wu S, Sternberg PW. Communication between oocytes and somatic cells regulates volatile pheromone production in C. elegans. Proceedings of the National Academy of Sciences. 2014;111:17905–17910. doi: 10.1073/pnas.1420439111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BJ, Hu JF, Liu ZY, Xu L, Lu Q, Li YX, Zhang XY. Behavioural features of Bursaphelenchus xylophilus in the mating process. Nematology. 2014;16:895–902. [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Ludewig AH, Schroeder FC. 2013. Ascaroside signaling in C. elegans (January 18, 2013). The C. elegans Research Community, ed. WormBook. doi/10.1895/wormbook.1.155.1, http://www.wormbook.org.
- Mamiya Y. History of pine wilt disease in Japan. Journal of Nematology. 1988;20:219–226. [PMC free article] [PubMed] [Google Scholar]
- Morsci NS, Haas LA, Barr MM. Sperm status regulates sexual attraction in Caenorhabditis elegans. Genetics. 2011;189:1341–1346. doi: 10.1534/genetics.111.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya R, Hasegawa K, Chen A, Kanzaki N, Sternberg PW. 2014 doi: 10.1534/g3.114.012385. Evidence of hermaphroditism and sex ratio distortion in the fungal feeding nematode Bursaphelenchus okinawaensis. G3 4:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JA, Shorey HH, Gaston LK. Sex pheromone communication in the nematode, Rhabditis pellio. Journal of Chemical Ecology. 1977;3:467–474. [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegewitz V, Schulenburg H, Streit A. Experimental insight into the proximate causes of male persistence variation among two strains of the androdioecious Caenorhabditis elegans (Nematoda) BMC Ecology. 2008;8:12. doi: 10.1186/1472-6785-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]