Abstract
Meloidogyne incognita and Meloidogyne arenaria are important parasitic nematodes of vegetable and ornamental crops. Microplot and greenhouse experiments were conducted to test commercial formulations of the biocontrol agent Pasteuria penetrans for control of M. incognita on tomato and cucumber and M. arenaria on snapdragon. Three methods of application for P. penetrans were assessed including seed, transplant, and post-plant treatments. Efficacy in controlling galling and reproduction of the two root-knot nematode species was evaluated. Seed treatment application was assessed only for M. incognita on cucumber. Pasteuria treatment rates of a granular transplant formulation ranged from 1.5 × 105 endospores/cm3 to 3 × 105 endospores/cm3 of transplant mix applied at seeding. Additional applications of 1.5 × 105 endospores/cm3 of soil were applied as a liquid formulation to soil post-transplant for both greenhouse and microplot trials. In greenhouse cucumber trials, all Pasteuria treatments were equivalent to steamed soil for reducing M. incognita populations in roots and soil, and reducing nematode reproduction and galling. In cucumber microplot trials there were no differences among treatments for M. incognita populations in roots or soil, eggs/g root, or root condition ratings. Nematode reproduction on cucumber was low with Telone II and with the seed treatment plus post-plant application of Pasteuria, which had the lowest nematode reproduction. However, galling for all Pasteuria treatments was higher than galling with Telone II. Root-knot nematode control with Pasteuria in greenhouse and microplot trials varied on tomato and snapdragon. Positive results were achieved for control of M. incognita with the seed treatment application on cucumber.
Keywords: Antirrhinum majus, biological control, Cucumis sativus, cucumber, Meloidogyne, Pasteuria penetrans, Solanum lycopersicum, root-knot nematodes, snapdragon, tomato
In the southeastern United States, root-knot nematodes (RKN; Meloidogyne spp.) are extremely destructive pests, causing significant yield loss in vegetable and ornamental crops. The southern root-knot nematode, M. incognita, and the peanut root-knot nematode, M. arenaria, are found in all vegetable and ornamental producing regions in Florida. Soil fumigation has been commonly used for control of nematodes in Florida vegetable and ornamental production (Rosskopf et al., 2005). However, the phase-out of methyl bromide and increased restrictions on all fumigants limit nematode control options. Crop rotation is ineffective due to the wide host range of Meloidogyne spp. and weed hosts in fields during and between crops (Koenning et al., 1999; Noling and Gilreath, 2003; Kokalis-Burelle and Rosskopf, 2012, 2013). Consequently, the need for new chemical and biological treatments for RKN control is great.
The biological control agent Pasteuria penetrans has shown potential for controlling some species of nematodes (Dickson et al., 1994). Research studying natural populations of Pasteuria on peanuts showed root and pod galls from M. arenaria were reduced by 60% and 95%, respectively, in soil inoculated with P. penetrans at 100,000 endospores/g of soil compared to nontreated control plots. Root and pod galls were reduced by 61% and 82% and 81% and 90%, respectively in 10,000 and 100,000 endospores/g treatments compared to control plots the following year when peanut was replanted into the same plots without additional endospore inoculum (Chen et al., 1996).
Methods for culturing Pasteuria in vitro have been developed, allowing the organism to be mass produced and commercialized as a biopesticide (Gerber and White, 2005). The first isolate of Pasteuria mass produced in vitro was parasitic on sting nematode, Belonolaimus longicaudatus, an important pathogen of turf. Previous research with an in vivo produced isolate of ‘Candidatus Pasteuria usgae’ in field plots demonstrated a reduction of sting nematode 13 mon after inoculation (Giblin-Davis et al., 2003). Recently, in vitro cultured strains of ‘Candidatus Pasteuria usgae’ have successfully controlled sting nematode in the field (Luc et al., 2010a, 2010b).
The objectives of this research were to determine if in vitro-produced P. penetrans could provide control of M. incognita on tomato (Solanum lycopersicum) and cucumber (Cucumis sativus), and M. arenaria on snapdragon (Antirrhinum majus) in greenhouse and field microplot trials in Florida. Because many vegetable crops are propagated as transplants, the opportunity exists to introduce biocontrol agents into the transplant mix, increasing the potential to provide early season protection against invasion by nematodes (Kokalis-Burelle et al., 2006). Transplant mix applications were tested on tomato and snapdragon, and a seed treatment formulation was tested on cucumber due to its large seed size. Previous research has shown that post-plant application of Pasteuria improves nematode control (Hewlett et al., 2007). This project evaluated three methods of treatment application; seed, transplant, and post-plant. Treatments were evaluated for their efficacy in controlling galling and reproduction of M. incognita on tomato and cucumber and M. arenaria on snapdragon.
Materials and Methods
General methods
Field soil used for greenhouse and microplot trials was either steamed (greenhouse) or fumigated with Telone II (microplots) prior to use. M. incognita and M. arenaria were isolated from pure cultures maintained on tomato (S. lycopersicum ‘Rutgers’) in the greenhouse at the U.S. Horticultural Research Laboratory, Fort Pierce, FL, and were added to soil as described below. Treatments (excluding the untreated control and chemical control) consisted of varying rates of in vitro produced P. penetrans endospores, supplied by Pasteuria Bioscience, Inc. (Alachua, FL) (now Syngenta Crop Protection, LLC). Treatment rates ranged from 5 × 104 endospores/cm3 of transplant mix to 3 × 105 endospores/cm3 applied at seeding. Additional post-plant applications of 1.5 × 105 endospores/cm3 of soil were applied as a liquid formulation to soil post-transplant for greenhouse and microplot trials. Greenhouse trials were conducted in new 7.6-liter plastic pots filled with steam-sterilized field soil (classified in the Oldsmar series; sandy, siliceous, hyperthermic Alfic Arenic Alaquod). Field microplots were newly constructed of plastic drums which were 58-cm diameter and 90-cm depth buried in soil. Bottoms of drums were removed before burial to allow for drainage and drums were filled with Oldsmar sand from the farm site. Both greenhouse and microplot experiments were fertilized with a slow-release fertilizer (Osmocote® Smart-Release®14-14-14) and were scouted for insects and foliar diseases. Insects were controlled with sticky traps and Safer® Insecticidal Soap. Greenhouse benches were equipped with plexiglass dividers to separate pots and prevent cross contamination of treatments during watering.
Nematode inoculum
Nematode eggs were extracted from ‘Rutgers’ tomato roots using the NaOCl method (Hussey and Barker, 1973). Eggs were collected and the final concentration was adjusted to approximately 1000 eggs/ml water. Soil for greenhouse trials was infested with nematode eggs by pipetting 1 ml of egg suspension into soil and mixing the soil before placing in pots. For microplot experiments, 5 ml of nematode inoculum containing 1000 eggs/ml was decanted into plots and watered into soil. Nematode inoculum was applied to soil in all experiments prior to treatment application. Untreated soil was infested with nematodes and left untreated. Steam treated soil for greenhouse trials was infested with nematodes and then steamed for 1 hr. For microplot trials, nematode inoculum was applied to plots prior to fumigation with Telone II.
Greenhouse and field microplot experiments
Tomato and snapdragon transplants were grown in potting mix inoculated with P. penetrans at seeding and were transplanted into nematode infested soil at the first true leaf stage, 6 to 7 wk after seeding. For greenhouse trials, plants were maintained in the greenhouse for 8 to 10 wk. For microplot trials, plants were transplanted into microplots at the stage described above. Post-plant treatment applications were made approximately 2 wk after transplanting.
For all tomato trials treatments were: untreated control, steamed control (greenhouse) or Telone II (microplot), 1.5 × 105 endospores/cm3 transplant mix, 3 × 105 endospores/cm3 transplant mix, 1.5 × 105 endospores/cm3 transplant mix + 1.5 × 105 endospores/cm3 of soil post-plant, 3 × 105 endospores/cm3 transplant mix + 1.5 × 105 endospores/cm3 of soil post-plant, and 1.5 × 105 endospores/cm3 of soil post-plant alone.
For all snapdragon trials treatments were: untreated control, steamed control (greenhouse) or Telone II (microplot), 5 × 104 endospores/cm3 transplant mix, 1.5 × 105 endospores/cm3 transplant mix, 3 × 105 endospores/cm3 transplant mix, 5 × 104 endospores/cm3 transplant mix + 1.5 × 105 endospores/cm3 of soil post-plant, 1.5 × 105 endospores/cm3 transplant mix + 1.5 × 105 endospores/cm3 of soil post-plant, 3 × 105 endospores/cm3 transplant mix + 1.5 × 105 endospores/cm3 of soil post-plant, and 1.5 × 105 endospores/cm3 of soil post-plant alone.
For all cucumber trials, treatments were: untreated control, steamed control (greenhouse) or Telone II (microplot), seed treatment at 106 spores/seed, seed treatment at 106 spores/seed + post-plant treatment of 1.5 × 105 endospores/cm3 of soil, post-plant treatment of 1.5 × 105 endospores/cm3 of soil alone.
Disease evaluation
All plants were evaluated for plant growth and galling by root-knot nematodes after 12 wk. Root galling was assessed using a root gall index based on a scale of 1 to 10 where 1 = no galls and 10 = severe galling (Bridge and Page, 1980). At the end of experiments plant growth measurements including stem diameter at the crown, shoot height, shoot fresh weight, and root fresh weight were recorded. Root condition ratings were also performed and were based on a 0 to 5 scale with 0 = white, healthy roots, 5 = completely discolored, necrotic roots. Nematode juveniles (J2) were extracted from both roots and soil using the Baermann funnel technique. Nematode reproduction (Rf) was calculated as Rf = Pf/Pi, where Pi = initial inoculum level and Pf = eggs extracted at the end of the experiment using the NaOCl method (Hussey and Janssen, 2002).
Statistical analysis
A randomized complete block design with five replications was used for all experiments. All experiments were conducted twice and data from both experiments were subjected to a t test and combined when no significant differences were found between tests. Data were subjected to one-way analysis of variance using general linear model analysis. Means were separated based on least significant difference (Fisher’s protected LSD) procedures.
Results
Tomato
In greenhouse tomato trials, M. incognita J2 populations in roots were reduced by steam treatment compared to two Pasteuria treatments (Table 1). However, the untreated control and steam treatment did not differ, and the number of J2 isolated from tomato roots at the end of the greenhouse trial was low overall. M. incognita J2 populations in soil were higher, but the same statistical trends held true as those for J2 populations in roots. Tomato root weight was lowest in the steam treatment, as were the number of nematode eggs isolated per gram of root, which both differed between the untreated control (UTC) and at least one Pasteuria treatment. Nematode reproduction, root disease, and nematode galling were all reduced by steam, however, the Pasteuria treatments did not differ from the untreated control for those variables (Table 1).
Table 1.
Meloidogyne incognita juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on tomato in the greenhouse.
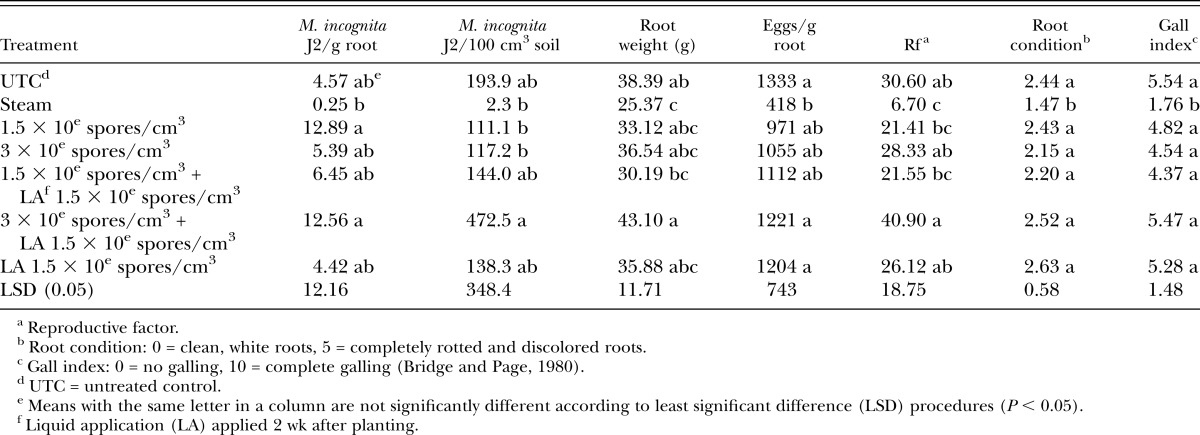
There were no differences in M. incognita J2 in roots or soil, eggs/g root, or nematode reproduction (Rf) in tomato microplot experiments (Table 2). Root weight in the Telone treatment was higher than the UTC and the majority of Pasteuria treatments (Table 2). Telone had healthier root condition ratings compared with several Pasteuria treatments and had lower gall index values than the untreated control and all Pasteuria treatments except the 300k spore/cc transplant treatment (Table 2).
Table 2.
Meloidogyne incognita juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on tomato in microplots.
Cucumber
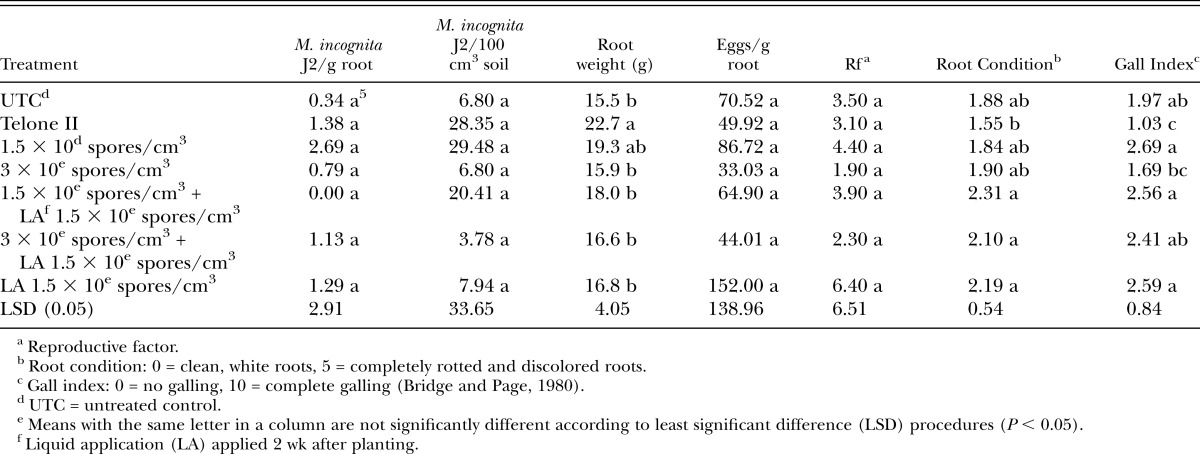
In contrast with results on tomato, all Pasteuria treatments in the greenhouse trials were comparable to the steam treatment control for reducing M. incognita populations in cucumber roots and soil, reducing nematode reproduction and gall index values, and improving root condition (Table 3). Root weights were similar among all treatments although numerically higher in the untreated control, most probably due to heavier galling than with the other treatments.
Table 3.
Meloidogyne incognita juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on cucumber in the greenhouse.
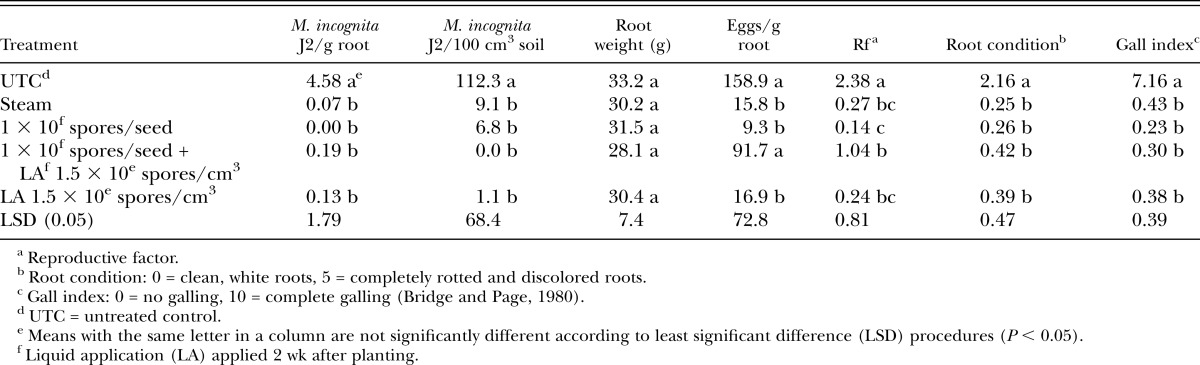
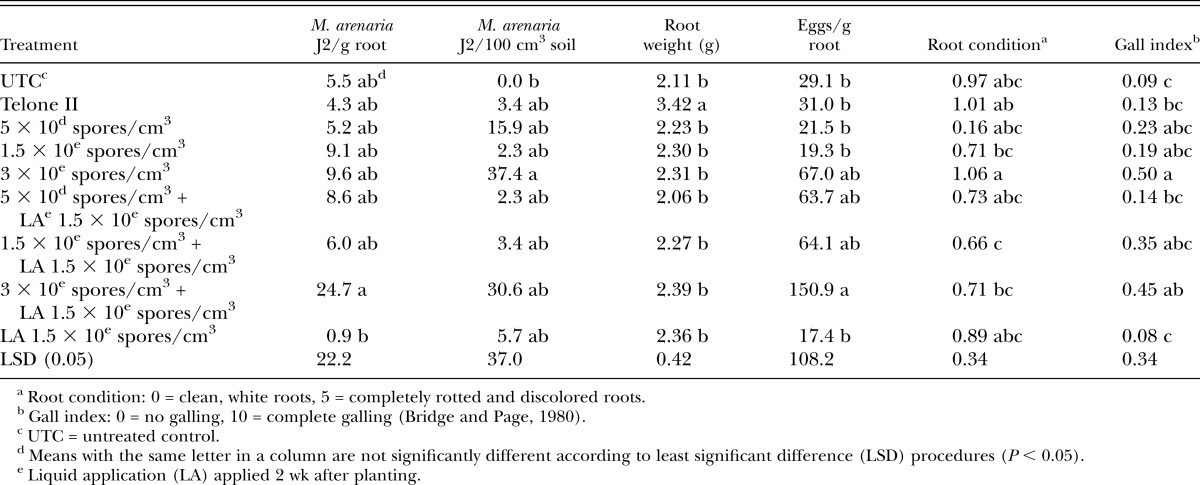
In cucumber microplot trials, there were no differences among treatments for M. incognita populations in roots or soil, eggs per gram root tissue or root condition ratings (Table 4). Nematode reproductive index values were low across all treatments; however, only the Telone II treatment was significantly lower than the UTC (Table 4). However, galling for all Pasteuria treatments was higher than galling in the Telone II control (Table 4). There were no positive effects of the additional post-plant liquid treatment for any trials with the exception of cucumber microplots where addition of the post-plant treatment reduced nematode Rf compared with seed treatment alone (Table 4).
Table 4.
Meloidogyne incognita juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on cucumber in microplots.
Snapdragon
In greenhouse snapdragon trials, there were no differences among treatments for M. arenaria J2 isolated from roots (Table 5). M. arenaria J2 isolated from soil were only reduced with the steam control treatment, and all Pasteuria treatments were similar to the UTC. Galling was high on all treatments and the untreated control did not differ from the steam treatment for gall index ratings (Table 5). The lowest rate of Pasteuria significantly increased the number of eggs/g root compared to several other treatments including the untreated control, and had the second-highest rate of galling (Table 5).
Table 5.
Meloidogyne arenaria juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on snapdragon in the greenhouse.
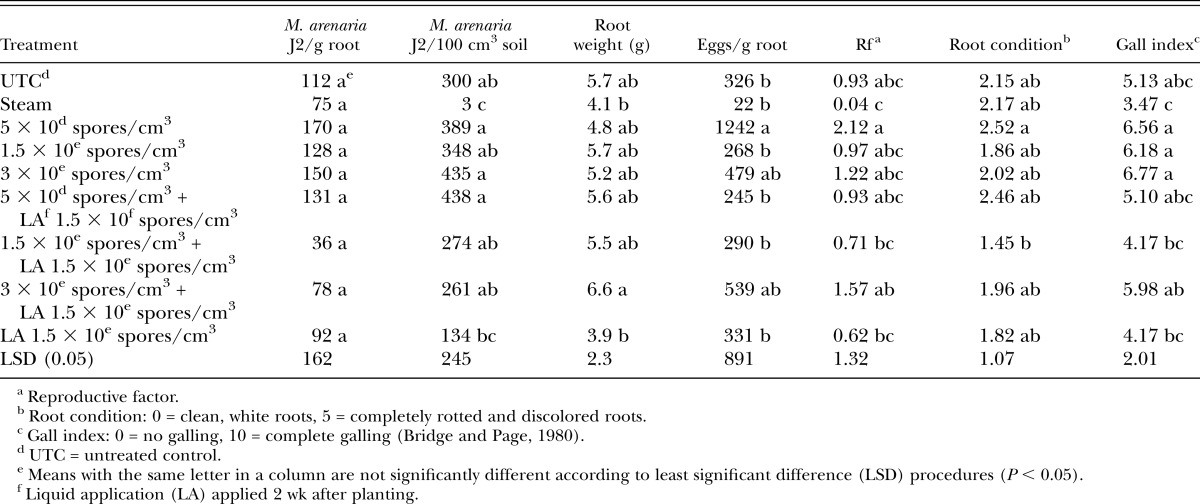
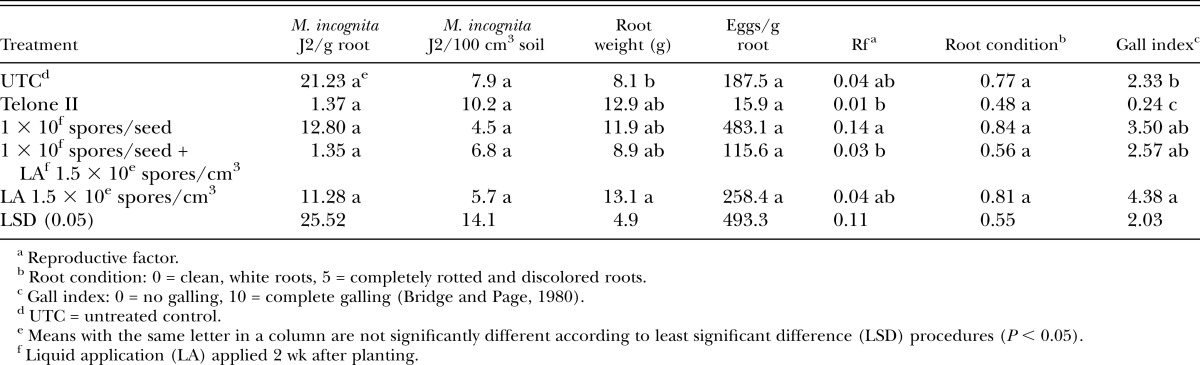
In microplot snapdragon trials, few differences were seen among treatments for nematode populations in roots and soil (Table 6). It is unusual that no nematode J2 were isolated from soil for the untreated controls. A significant increase was seen in the number of nematode eggs isolated from roots in the treatment receiving the high rate of Pasteuria + the liquid post-plant application (Table 6). However, overall galling was low for these experiments with no differences seen in galling between the untreated control and Telone II treatment.
Table 6.
Meloidogyne arenaria juveniles (J2) in roots and soil, plant root weight, plant root condition, and nematode gall index values for both experiments on snapdragon in microplots.
Discussion
P. penetrans is being marketed as a highly specific and safe biological nematicide. The developed strain of P. penetrans tested here is a parasite of the root-knot nematodes M. arenaria, and M. incognita, is nontoxic to humans and other mammals, and does not contribute to pollution of soil or water. There are currently no alternative biopesticide treatments comparable to P. penetrans. In contrast, the alternative conventional chemical pesticides currently registered in the United States including Telone (1,3-dichloropropene) and Paladin (dimethyl disulfide) face obstacles to their use which include large buffer zones, restrictions on use in some soils, and offensive odor. In addition, most chemical fumigants are facing stringent restrictions on their use due to environmental concerns, classification as class B carcinogens, and high potential for ground water contamination.
Initial studies on in vitro produced Pasteuria by Hewlett et al. (2003) found that although an 87% reduction in total nematode eggs was observed on tomato seedlings treated with in vitro produced spores compared to the untreated controls, there were no significant differences in galling, the number of egg masses, or eggs of M. arenaria between treatments. As in the studies presented here, Hewlett et al. (2003) saw a high level of variability among replicates for most treatments. This variability was attributed to the small volume of soil used in the experiments, which resulted in a short distance for nematodes traveling to the root tips, decreasing the exposure time for nematodes to Pasteuria spores in the soil. Hewlett et al. (2003) did not find significant difference in the percentage of females infected with Pasteuria between in vitro and in vivo produced spores leading them to conclude that spores produced in vitro were similar to those produced in vivo with regard to attachment and infection of the nematodes. Later work by Hewlett et al. (2006) found a significant reduction in galling by M. incognita on Hostas spp. in plots treated with 105 in vitro produced Pasteuria endospores/cm3 of soil compared to the untreated control. The nematode suppression at this endospore density was similar to other reports (Chen and Dickson, 1998) for suppression achieved with P. penetrans endospores produced in vivo.
In the studies presented here, rates were mostly comparable, or higher, than those used in previous research by Hewlett et al. (2003, 2006) with the exception of one treatment on snapdragon. Therefore, lack of suppression with some treatments cannot be attributed to application rate, as rates used in this research were comparable to the previous studies. Results of the current research on efficacy of P. penetrans for control of M. incognita on tomato and cucumber and M. arenaria on snapdragon were mixed. Although the number of M. incognita J2 isolated from tomato roots in greenhouse trials were low, gall index values were moderate for the UTC and Pasteuria treatments, and low in the steamed soil control, indicating adequate inoculum density for symptom development and efficacy of the steam treatment in reducing symptom development. Low root weights in the steam treatment were consistent with both lower gall index values (galled roots are often heavier than healthy roots) and a general reduction in plant growth typically observed in a sterilized medium. Compared with the greenhouse studies, tomato microplot studies had higher root weights in the Telone II control, which were associated with larger, healthier, and less galled roots, compared to most other treatments. These results illustrate the importance of considering gall index values in conjunction with root weight values in order to determine if increased root weight is due to an increase in root galling or an increase in healthy root growth. Other factors that may play an important role in variability of efficacy are possible differences between in vivo and in vitro produced spores. Further research directly comparing these two spore types is necessary.
Results of cucumber microplot trials were not as positive as results of the greenhouse trials on cucumber. The one positive effect of the post-plant liquid treatment, a reduction in Rf compared with seed treatment alone, did occur in cucumber microplots. The remainder of the data did not indicate enhanced control with addition of the post-plant application. The reduction in efficacy in the microplot trials may be due to increased antagonism from native microbial populations compared with the greenhouse studies. It may be necessary to reduce native soil microbial populations with a chemical treatment such as chloropicrin in order to optimize nematode control in the field with Pasteuria, but further studies would need to be done to confirm this.
Literature Cited
- Bridge J, Page SLJ. Estimation of root-knot infestation levels in roots using a rating chart. Tropical Pest Management. 1980;26:296–298. [Google Scholar]
- Chen ZX, Dickson DW. Review of Pasteuria penetrans: Biology, ecology, and Biological Control Potential. Journal of Nematology. 1998;30:313–340. [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW, McSorley R, Mitchell DJ, Hewlett TE. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. Journal of Nematology. 1996;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Oostendorp M, Giblin-Davis RM, Mitchell DJ. 1994. Control of plant-parasitic nematodes by biological antagonists. Pp. 575–601 in D. Rosen, F. D. Bennett, and J. L. Capinera, eds. Pest management in the subtropics, biological control—A Florida perspective. Andover, Hampshire, UK: Intercept.
- Gerber JF, White JH. 2005. Materials and methods for the efficient production of Pasteuria. U.S. Patent 6,919,197 B2.
- Giblin-Davis RM, Williams DS, Bekal S, Dickson DW, Becker JO, Preston JF. ‘Candidatus Pasteuria usgae’ sp. nov., an obligate endoparasite of the phytoparasitic nematode, Belonolaimus longicaudatus. International Journal of Systematic and Evolutionary Microbiology. 2003;53:197–200. doi: 10.1099/ijs.0.02292-0. [DOI] [PubMed] [Google Scholar]
- Hewlett TE, Griswold ST, Smith KS. Biological control of Meloidogyne incognita using in-vitro produced Pasteuria penetrans in a microplot study. Journal of Nematology. 2006;38(2):274. [Google Scholar]
- Hewlett TE, Griswold ST, Smith KS. 2007. Efficacy of in-vitro Pasteuria spp. parasitizing two nematode species. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions. Pp. 38–1.
- Hewlett TE, Smith KS, Griswold ST, Crow WT. 2003. Comparison of the efficacy of Pasteuria penetrans endospores produced in vivo and in vitro for the control of Meloidogyne arenaria. Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions. Pp. 121.1–121.3.
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Hussey RS, Janssen GJ. 2002. Root-knot nematodes: Meloidogyne species. Pp. 43–70 in J. L. Starr, R. Cook, and J. Bridge, eds. Plant resistance to plant parasitic nematodes. Wallingford, UK: CABI Publishing.
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Kokalis-Burelle N, Kloepper JW, Reddy MS. Plant growth-promoting rhizobacteria as transplant amendments and their effects on indigenous rhizosphere microorganisms. Applied Soil Ecology. 2006;31:91–100. [Google Scholar]
- Kokalis-Burelle N, Rosskopf EN. Susceptibility of several common subtropical weeds to Meloidogyne arenaria, M. incognita, and M. javanica. Journal of Nematology. 2012;44:142–147. [PMC free article] [PubMed] [Google Scholar]
- Kokalis-Burelle N, Rosskopf EN. Susceptibility of several floriculture crops to three common species of Meloidogyne in Florida. Nematropica. 2013;43:164–170. [Google Scholar]
- Luc JE, Crow WT, McSorley R, Giblin-Davis RM. Suppression of Belonolaimus longicaudatus with in vitro-produced Pasteuria sp. endospores. Nematropica. 2010a;40(2):217–225. [Google Scholar]
- Luc JE, Wenjing P, Crow WT, Giblin-Davis RM. Effects of formulation and host nematode density on the ability of in vitro-produced Pasteuria endospores to control its host Belonolaimus longicaudatus. Journal of Nematology. 2010b;42(2):87–90. [PMC free article] [PubMed] [Google Scholar]
- Noling JW, Gilreath JP. Role of weed hosts in population enhancement of root-knot nematode. Journal of Nematology. 2003;35:356. [Google Scholar]
- Rosskopf EN, Chellemi DO, Kokalis-Burelle N, Church GT. 2005. Alternatives to methyl bromide: A Florida perspective. Online. Plant Health Progress doi: 10.1094/PHP-2005-1027-01-RV.


