Abstract
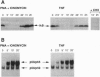
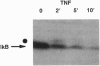
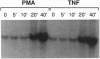
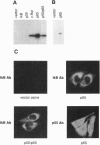
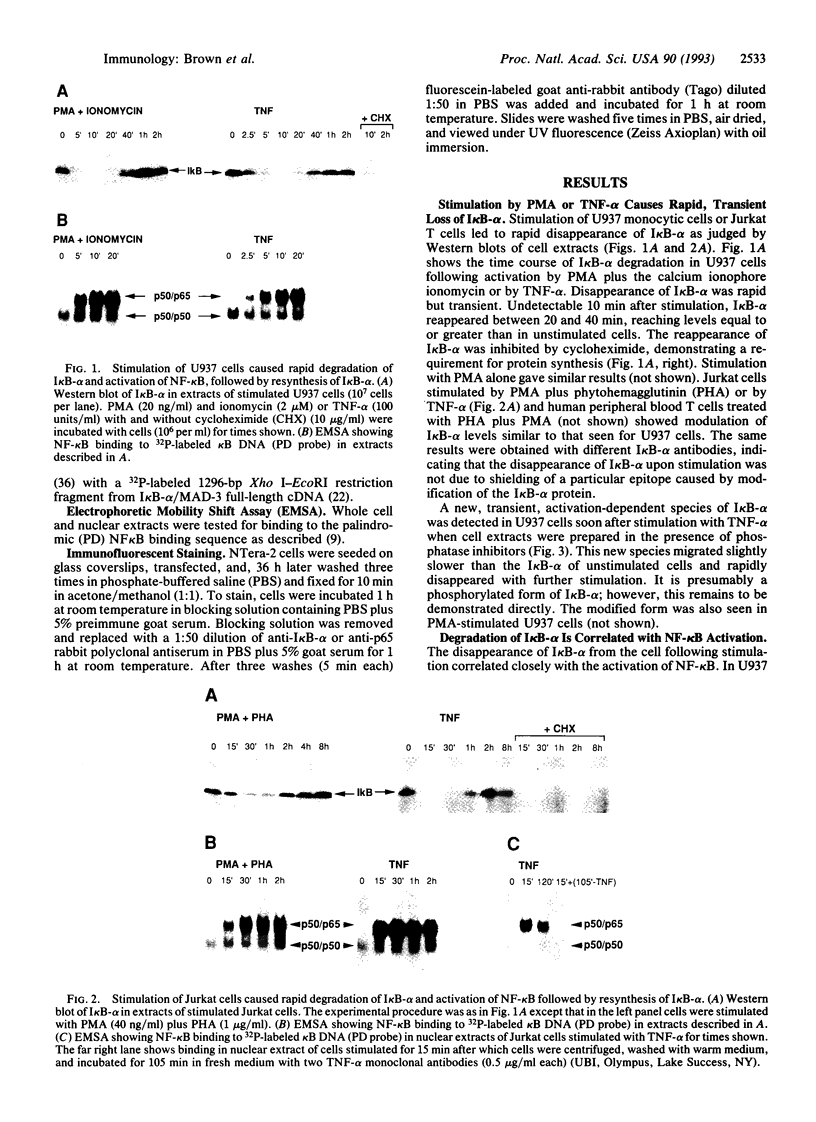
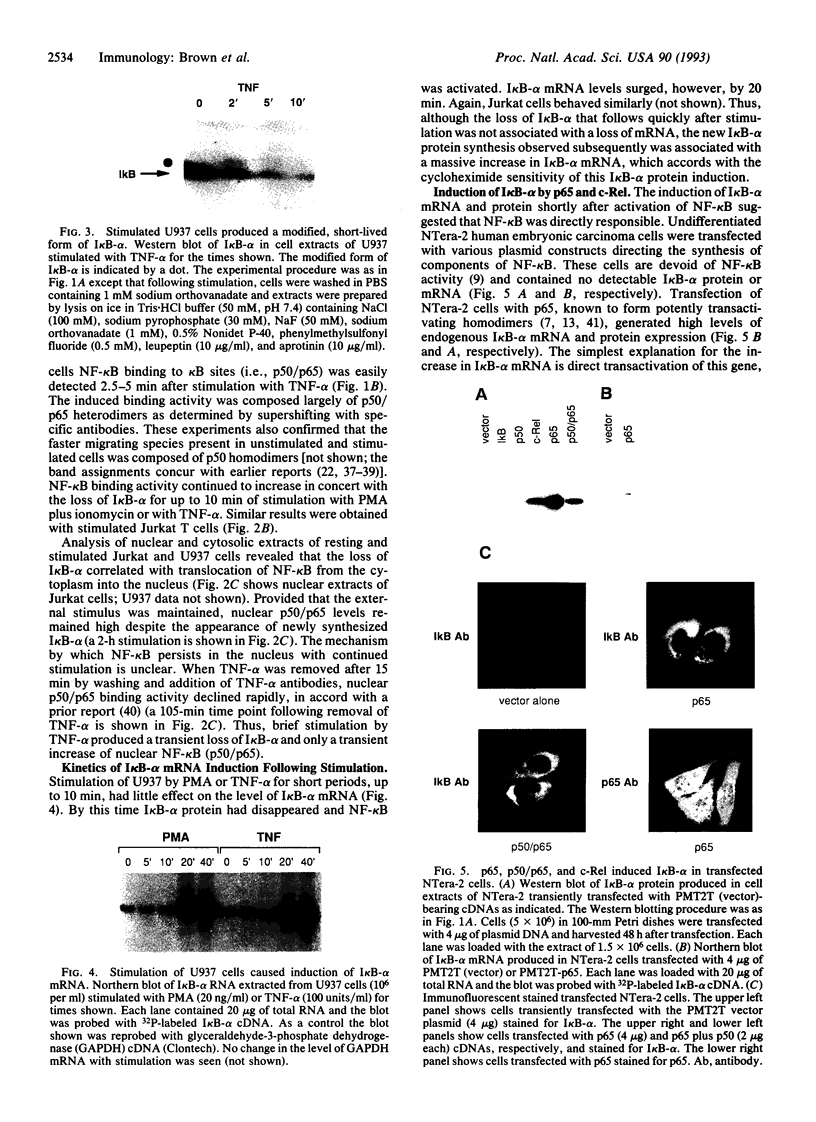
The NK-kappa B transcription factor complex is sequestered in the cytoplasm by the inhibitory protein I kappa B-alpha (MAD-3). Various cellular stimuli relieve this inhibition by mechanisms largely unknown, leading to NF-kappa B nuclear localization and transactivation of its target genes. It is demonstrated here with human T lymphocytes and monocytes that different stimuli, including tumor necrosis factor alpha and phorbol 12-myristate 13-acetate, cause rapid degradation of I kappa B-alpha, with concomitant activation of NF-kappa B, followed by a dramatic increase in I kappa B-alpha mRNA and protein synthesis. Transfection studies reveal that the I kappa B-alpha mRNA and the encoded protein are potently induced by NF-kappa B and by homodimers of p65 and of c-Rel. We propose a model in which NF-kappa B and I kappa B-alpha mutually regulate each other in a cycle: saturating amounts of the inhibitory I kappa B-alpha protein are destroyed upon stimulation, allowing rapid activation of NF-kappa B. Subsequently, I kappa B-alpha mRNA and protein levels are quickly induced by the activated NF-kappa B. This resurgence of I kappa B-alpha protein acts to restore an equilibrium in which NF-kappa B is again inhibited.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Bours V., Burd P. R., Brown K., Villalobos J., Park S., Ryseck R. P., Bravo R., Kelly K., Siebenlist U. A novel mitogen-inducible gene product related to p50/p105-NF-kappa B participates in transactivation through a kappa B site. Mol Cell Biol. 1992 Feb;12(2):685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis N., Bargmann W., Lim M. Y., Bose H., Jr Avian reticuloendotheliosis virus-transformed lymphoid cells contain multiple pp59v-rel complexes. J Virol. 1990 Feb;64(2):584–591. doi: 10.1128/jvi.64.2.584-591.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Ghosh S., Simmons D. L., Tempst P., Liou H. C., Baltimore D., Bose H. R., Jr Rel-associated pp40: an inhibitor of the rel family of transcription factors. Science. 1991 Sep 13;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G., Bours V., Park S., Tomita-Yamaguchi M., Kelly K., Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992 Sep 24;359(6393):339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Scheidereit C., van Loon A. P. Maintenance of NF-kappa B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol. 1991 Jan;11(1):259–266. doi: 10.1128/mcb.11.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Rashid D., Davis N., Bose H. R., Jr, Verma I. M. Direct association of pp40/I kappa B beta with rel/NF-kappa B transcription factors: role of ankyrin repeats in the inhibition of DNA binding activity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4333–4337. doi: 10.1073/pnas.89.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. A., Weinberg J. B., Greene W. C. Nuclear expression of the 50- and 65-kD Rel-related subunits of nuclear factor-kappa B is differentially regulated in human monocytic cells. J Clin Invest. 1992 Jul;90(1):121–129. doi: 10.1172/JCI115824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Link E., Kerr L. D., Schreck R., Zabel U., Verma I., Baeuerle P. A. Purified I kappa B-beta is inactivated upon dephosphorylation. J Biol Chem. 1992 Jan 5;267(1):239–246. [PubMed] [Google Scholar]
- Mercurio F., Didonato J., Rosette C., Karin M. Molecular cloning and characterization of a novel Rel/NF-kappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA Cell Biol. 1992 Sep;11(7):523–537. doi: 10.1089/dna.1992.11.523. [DOI] [PubMed] [Google Scholar]
- Meyer R., Hatada E. N., Hohmann H. P., Haiker M., Bartsch C., Röthlisberger U., Lahm H. W., Schlaeger E. J., van Loon A. P., Scheidereit C. Cloning of the DNA-binding subunit of human nuclear factor kappa B: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):966–970. doi: 10.1073/pnas.88.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P. J., Gilmore T. D. The C terminus of the NF-kappa B p50 precursor and an I kappa B isoform contain transcription activation domains. Nucleic Acids Res. 1992 May 25;20(10):2453–2458. doi: 10.1093/nar/20.10.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Klement J. F., Coleman T. A., Maher M., Chen C. H., Rosen C. A. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992 May;6(5):745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Tewari M., Dobrzanski P., Mohn K. L., Cressman D. E., Hsu J. C., Bravo R., Taub R. Rapid induction in regenerating liver of RL/IF-1 (an I kappa B that inhibits NF-kappa B, RelB-p50, and c-Rel-p50) and PHF, a novel kappa B site-binding complex. Mol Cell Biol. 1992 Jun;12(6):2898–2908. doi: 10.1128/mcb.12.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Sex, maps, and imprinting. Cell. 1991 Jan 11;64(1):1–3. doi: 10.1016/0092-8674(91)90199-9. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Baeuerle P. A. The 65-kD subunit of NF-kappa B is a receptor for I kappa B and a modulator of DNA-binding specificity. Genes Dev. 1990 Nov;4(11):1975–1984. doi: 10.1101/gad.4.11.1975. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Schreck R., Baeuerle P. A. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991 Jul;10(7):1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- Zabel U., Henkel T., Silva M. S., Baeuerle P. A. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993 Jan;12(1):201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]