Abstract
Context/Objective
Exaggerated postprandial lipemia has been reported after spinal cord injury (SCI). We examined metabolite and accompanying pro-inflammatory biomarker responses to repeat feeding of typical high-fat meals in individuals with chronic paraplegia.
Design
Descriptive trial.
Methods
Metabolites (triglycerides, glucose, and insulin) and inflammatory biomarkers (interleukin-6 and high-sensitivity C-reactive protein (hsCRP)) were measured under fasting conditions in 11 recreationally active individuals with chronic (>1 year) paraplegia. Subjects received high-fat meals at time point 0 and again at minute 240. Antecubital venous blood was obtained at time points −30 (fasting), 0 (first meal), 30, 60, 90, 120, 240 (second meal), 360, and 480 minutes. Correlations were examined among the study variables. Exploratory subgroup analysis was performed for subjects with levels of postprandial glucose greater than >200 mg/dl.
Results
Triglycerides showed a significant rise 4 hours after eating. Basal inflammatory markers were elevated, and did not undergo additional change during the testing. Additionally, subjects with excessive postprandial glucose responses showed higher hsCRP levels than those having typical glucose responses both for fasting (11.8 ± 6.5 vs. 2.9 ± 2.7 mg/l, P = 0.064) and postprandial (11.1 ± 4.9 vs. 3.7 ± 3.8 mg/l, P = 0.018) values.
Conclusions
Despite elevations in metabolic response markers, inflammatory markers did not change significantly after consumption of population-representative (i.e. hypercaloric) mixed-nutrient meals. Levels of fasting CRP in the high-risk range are consistent with other reports in persons with SCI and continue to pose concern for their cardiovascular disease risk. The possible association between postprandial metabolic responses and inflammatory states warrants further investigation to identify individual component risks for this secondary health hazard.
Keywords: Feeding, Meal challenge, Glucose, Lipids
Introduction
Persons living with a spinal cord injury (SCI) are reported to undergo accelerated rates of organ system aging,1,2 and in the process sustain an increased risk for early all-cause cardiovascular disease (CVD).3 CVDs are now the primary cause of morbidity in those older than age 60, or living more than 30 years with SCI.4 Causes for this increased risk have yet to be established, although well-established CVD risks of physical inactivity,5 overweight,6 impaired fasting glucose or diabetes,6,7 elevated pro-atherogenic inflammatory cytokines,8,9 and imprudent diet6,10 have all been suggested as component hazards. Evidence confirms the tendency for these individual component risks to cluster after SCI,11 making accelerated CVD a credible health hazard.
Among the most common of CVD risks reported after an SCI is a fasting dyslipidemia, which is typically associated with low blood levels of the cardioprotective high-density lipoprotein cholesterol (HDL-C).3,12,13 While longstanding fasting lipid disorders are often considered a prerequisite for atherogenesis, and the CVD hazard imposed by low HDL-C is forcefully expressed in authoritative risk guidelines,14 almost half of individuals sustaining ‘hard’ CVD events (i.e. myocardial infarction, sudden death, or stroke) have fasting blood lipid profiles that would not predict these episodes.15 This observation has caused investigators to search for more hidden risks as potential instigators for CVD.
While assessment of lipids in the fasting state is a recommended approach for clinical CVD risk assessment,14 the fact remains that humans primarily live in the fed, not the fasted condition. For this reason, considerable interest has been generated in the postprandial period when levels of glucose and triglycerides (TGs) become predictably elevated.16 Postprandial TG elevation in particular poses risks for oxidation to TG-rich lipoproteins, which then through remnant lipoproteins hasten atheroma formation.17 Recent evidence also suggests that the postprandial period is associated with increased cytokine activity, which may be associated with TG appearance or delayed removal, as well as persistently elevated glycemia.18
Nash et al.19 have previously reported an exaggerated postprandial hypertriglyceridemia in persons with SCI. Others reported similar postprandial responses between person with SCI and AB controls using the same prandial challenge of premium ice cream and heavy whipping cream.20 While this particular nutrient intake is commonly used in clinical studies examining prandial challenge, its composition represents a ‘high fat load’ to a greater extent than a typical meal consumed by persons in real life. Additionally, both studies examined a 6-hour interval ensuing a single feeding. In this study we adopted a more representative approach using a fast food breakfast, followed four hours later by re-feeding with a fast-food lunch. This method of prandial testing has been reported,21 better matches the recently reported macronutrient intake that is typical of persons with SCI,6 and elevates postprandial TGs, carbohydrates, and pro-inflammatory cytokines18,22 and is more in line with general eating habits of most people. Thus, the purpose of this study was to examine the degree to which the postprandial period in persons with SCI was associated with prolonged elevation of serum TG and glucose, and whether these responses are associated with elevated levels of pro-atherogenic inflammatory cytokines.
Methods
Subjects
Study participants were nine men and two women aged 20–53.6 years with chronic motor-complete SCI (ASIA Impairment Scale A-B) between the T4 and L1 levels. Participants were healthy, community-dwelling individuals with a SCI for longer than 1 year. Exclusion criteria were participation in an endurance training program or a self-initiated program of exercise conditioning; habitual use of steroidal or non-steroidal anti-inflammatory drugs; use of lipid-lowering agents or anti-hyperglycemics; and use of antioxidant vitamins or nutrient supplements. Data from one participant were excluded because of pronounced postprandial hyperinsulinemia suggesting profound insulin resistance. Informed consent was obtained in accordance with Institutional Review Board guidelines. Descriptive characteristics of the study subjects are shown in Table 1.
Table 1 .
Descriptive characteristics of the study subjects
| Range | Mean ± SD | |
|---|---|---|
| Age (years) | 20–54 | 39 ± 13 |
| Level of injury | T4-L1 | N/A |
| Duration of injury (years) | 1.1–33.7 | 10.8 ± 10.5 |
| Body mass (kg) | 60–113 | 83 ± 19 |
| Body mass index | 22.0–44.8 | 29.3 ± 6.7 |
| Body fat (%) | 24–59 | 38 ± 9 |
Anthropometric measurements
Body mass was determined by weighing participants in their wheelchair on a calibrated electronic scale and then subtracting the wheelchair mass. Height was determined by participant report. Body mass index (BMI) was calculated as the quotient of body mass (kg) and the square of height (meters). Body composition (including total body fat and abdominal fat) was determined by a dual-energy X-ray absorptiometry as previously described in persons with SCI by Spungen et al.23
Phlebotomy and postprandial testing
Study participants refrained from caffeine and alcohol intake for 24 hours before testing, and began the procedure following a 10-hour overnight fast. Preparation for phlebotomy included antiseptic placement in a superficial arm vein of a 21-gauge × 1-inch Teflon catheter (Jelco, Smiths Medical, London, UK), which was capped with a multi-sample port and kept patent with sterile physiological (0.9%) saline. Blood samples were obtained 30 minutes before the test meal was consumed (−30 minutes), immediately following food intake (0 minute) and at the following time points thereafter: 30, 60, 90, 120, 180, 240, 300, 360, 420, and 480 minutes.
The first test meal consisted of a fast-food breakfast containing a sausage-egg-muffin sandwich, hash brown potato, and an oral glucose tolerance test drink containing 75 g of dextrose, which was consumed over a 15-minute period. A similar approach to prandial testing using fast-foods has been reported.21 After obtaining the blood sample at the 4-hour time point, participants were fed a second fast-food meal consisting of a quarter pound (pre-cooking weight) hamburger with one slice of American cheese, a medium order of French fried potatoes, and another oral glucose tolerance test drink. Macronutrient composition of the meals is shown in Table 2.
Table 2 .
Macronutrient composition of the test meals
| Kilocalories (kcal) | Fat |
Carbohydrate |
Protein (g) | |||
|---|---|---|---|---|---|---|
| g | % | g | % | |||
| First meal | 820 | 31 | 34 | 119 | 58 | 15 |
| Second meal | 1190 | 45 | 34 | 163 | 54.7 | 33 |
Blood samples were collected in clot lysis activator (serum) and citrated (plasma) Vacutainer tubes. To isolate the serum, the blood samples were centrifuged for 15 minutes at 1500 g. Serum TG and high-sensitivity C – reactive protein (CRP) were measured using the Roche Cobas 6000 Analyzer (Roche Diagnostic Systems, Indianapolis, IN) using manufacturer reagents and procedures. Plasma interleukin (IL)-6 samples were assayed in duplicate using an enzyme-linked immunosorbent assay kit (Quantikine HS6000, R&D System, Minneapolis, MN, USA) following manufacturer's instructions.
Data analysis
Values are presented as mean ± standard deviation. Correlations among blood markers of metabolic and inflammatory responses for area under the curve (AUC) of postprandial time points (i.e. 0–480 minutes), were assessed with the Pearson product–moment correlation coefficient. Changes for each measure across time points were analyzed with univariate analysis of variance with repeated measures, followed by post hoc analysis without adjustment. Level of significance for all analysis was set a priori at α = 0.05. To further explore the influence of prandial glycemia on pro-inflammatory responses, study participants were stratified into two groups based on postprandial glucose levels with a cutoff threshold at ≥200 mg/dl (at any time point). This post-load glycemia level represents an American Diabetes Association standard for provisional diagnosis of diabetes mellitus.24 The grouping variable was added as a between-subjects factor in the repeated measure analysis described above.
Results
Response to feeding
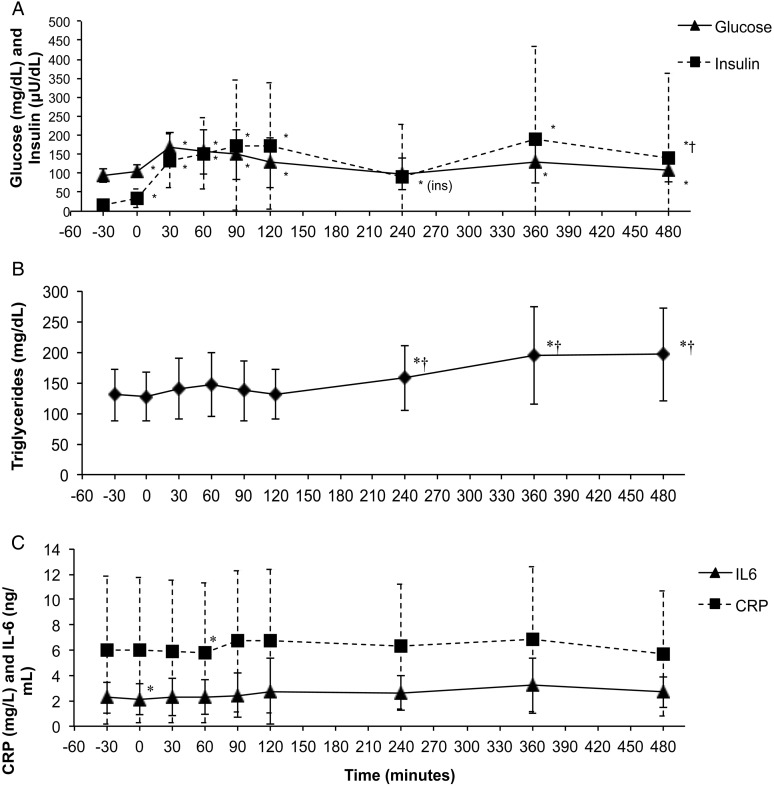
Glucose rose significantly with each feeding and returned to baseline values within 4 hours after the first meal, but not the second. Insulin tracked the changes in postprandial glucose but remained slightly elevated over baseline values (Fig. 1A). TG levels were significantly higher 4 hours after the first feeding, and were further elevated 2 and 4 hours after the second feeding (Fig. 1B).
Figure 1 .
Metabolic and Inflammatory response markers following ingestion of high-fat meals in persons with SCI. Meals were consumed at 0 and 240 minutes. Values are mean ± SD. *Significant difference compared with −30 minutes (P < 0.05). †Significant difference compared with 240 minutes (P < 0.05).
Changes in inflammatory markers following the feedings were small and did not reach statistical significance for the assessed time period (Fig. 1C).
Correlations
The correlation matrix for all inflammatory biomarkers (IL-6 and CRP) and metabolic response markers (TG, glucose and insulin) is presented in Table 3.
Table 3 .
Correlation matrix for postprandial AUC values
| Glucose | Insulin | CRP | IL-6* | ||
|---|---|---|---|---|---|
| Triglycerides | r | 0.359 | 0.269 | 0.363 | −0.053 |
| P | 0.252 | 0.398 | 0.246 | 0.871 | |
| Glucose | r | 1 | 0.661† | 0.197 | −0.596† |
| P | 0.019 | 0.540 | 0.041 | ||
| Insulin | r | 1 | 0.149 | −0.433 | |
| P | 0.643 | 0.160 | |||
| CRP | r | 1 | 0.025 | ||
| P | 0.940 |
*Statistically significant at P < 0.05; CRP, C-Reactive protein; IL-6, interleukin-6.
†Values were Ln transformed when departing form normality.
Exploratory analysis
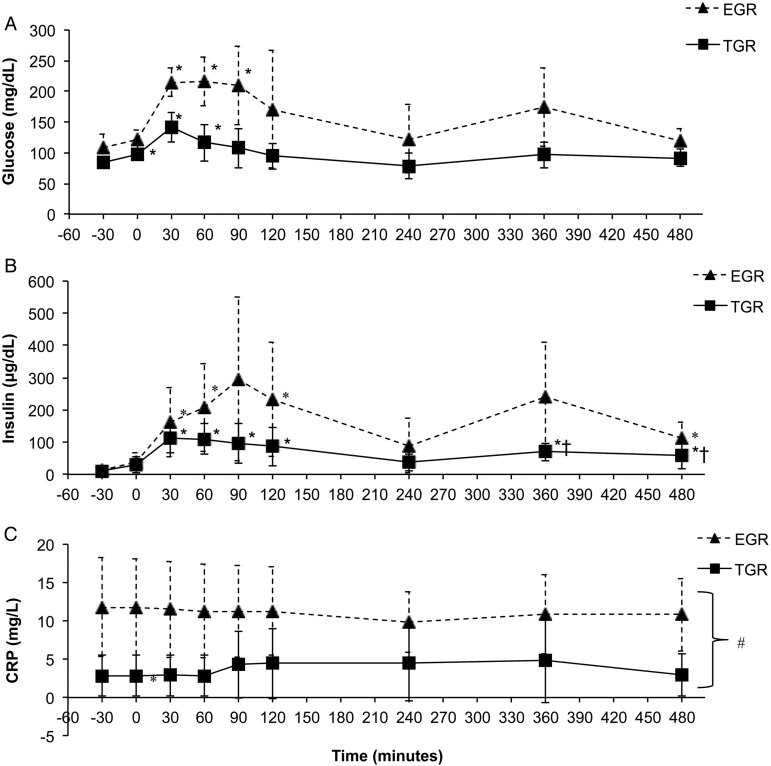
Exploratory analysis revealed two subgroups of participants. One subgroup experienced an exaggerated glucose response (EGR) reaching glucose levels >200 mg/dl during the testing, while another subgroup had a more typical glucose response (TGR), i.e. glucose levels remained <200 mg/dl. Significant interactions for these subgroups in glucose and insulin response over time were observed, and simple effects for each subgroup are presented in Fig. 2A. In addition, basal CRP values were significantly higher in the EGR vs. the TGR group (EGR = 11.2 ± 4.08 TGR = 3.61 ± 4.08 P = 0.016, Fig. 2C).
Figure 2 .
Glucose (A), insulin (B), and CRP (C) responses to ingestion of high-fat meals in persons with SCI. Subjects were stratified by exaggerated glucose response (EGR) and typical glucose response (TGR). Meals were consumed at 0 and 240 minutes. Values are mean ± SD. *Significant difference compared to −30 minutes (P < 0.05). †Significant difference compared to 240 minutes (P < 0.05). #Significant main effect for group (P < 0.05).
Discussion
The key finding of this study is that postprandial glucose, insulin, and TGs all increased in response to multiple feedings with population-representative (i.e. hypercaloric) mixed-nutrient meals, while biomarkers of inflammation did not significantly change over time.
All-cause CVD represents an accelerated health risk for all persons with chronic SCI,25,26 although the basis for hastening of disease remains obscure. While the component risk of fasting dyslipidemia has been extensively documented in the population,3,12,27 more recent interest has focused on the postprandial period, where excessive prandial challenge is known to stimulate lipid oxidation, remnant lipoprotein evolution, and ultimately atheroma formation.17,28,29 Inflammation plays an important role in this process, and recent evidence suggests that the postprandial period is associated with increased cytokine activity,30 which is possibly related to TG appearance or delayed clearance. Exaggerated postprandial TG response to a single dosing of a high-fat liquid meal (92% of calories derived from fat) has been reported in persons with SCI.19 Others reported TG levels for person with SCI that while on average higher at all measurement time points were not statistically different from AB controls and also showed a similar postprandial response between the two groups.20 As a high-fat liquid nutrient challenge is unrepresentative of a typical diet and feeding regimen, we therefore administered two fast food meals (4 hours apart), which is similar in caloric level and macronutrient composition to intake for this population.6 This study was the first to use a meal that represented more typical composition, and an anticipated re-feeding four hours later.
Metabolic response markers examined in this study adhered to expected tendencies with glucose rising post-challenge and returning to baseline values within 4 hours (Fig. 1A). Insulin response generally followed the same trajectories as those observed for glucose although values remained slightly elevated over baseline levels 4 hours after initial feeding (Fig. 1A). TG levels were significantly increased 240 minutes after the first feeding and were further elevated at 360 minutes and at 480 minutes (i.e. 2 and 4 hours after the second feeding, Fig. 1B). The delayed appearance of plasma TG is an anticipated feature of food intake, and is expected to occur more slowly than glucose appearance. The response to the first feeding is consistent with previous reports20,31,32 while the response to the second finding presents novel information. Others have observed excessive rises in TG after 120 minutes compared with control in patients with SCI19 premature CVD33 or obesity.34 While the change in TG levels did not reach statistical significance until 240 minutes after the first meal, changes observed after 120 minutes did not appear excessive, and after the second feeding there was no further increase from 360 to 480 minutes. However, due to lack of a control group this is not conclusive evidence for the lack of an excessive postprandial TG. Moreover, the extended TG elevation accompanying a second feeding may delay TG removal necessary to lessen oxidation to TG-rich lipoproteins, whose remnant lipoproteins carry high risk for atherosclerosis the longer they remain in general circulation.35
Postprandial TG responses of persons with SCI have been reported to be related to visceral abdominal fat,20 although this observation was based upon a feeding challenge that was both liquid in composition and abnormally high in saturated fat. Others have reported similar associations for non-disabled individuals.30,36,37 To the contrary, no such associations between TG and either BMI or body mass were observed in this study, although the test meal was both lower in total fat than previously studied and solid in composition. We believe this explains the slower rise in serum TG than previously reported in persons with SCI undergoing prandial challenge.19,20 While our postprandial TG and glucose responses appeared to be independent of anthropometrics (although this did not include direct measures of visceral fat), insulin AUC was significantly associated with both BMI and body mass. Similar significant correlations between insulin response and visceral fat or waist–hip ratio have been reported in non-disabled individuals having wide ranges of body composition.36,37
In this study we found no significant IL-6 and CRP responses to prandial challenge. Others38 have also reported no significant changes in IL-6 and CRP after a high fat meal (51 g of fat) but saw increased values for IL-1β 6–8 hours post-challenge. Still others have identified significant increases in IL-6 using a 50–65 g fat intake challenge in persons with premature CVD, type 2 diabetes compared with healthy controls.18,33 Similarly, metabolic disease states such as insulin resistance39 and the metabolic syndrome40 are associated with elevated CRP levels in persons with SCI. Despite the non-significant increases in IL-6, IL-6, and glucose postprandial responses were negatively correlated (Table 1). This is contrary to some reports that identify correlations between elevated fasting41,42 and post-challenge43 glucose and CRP levels even absent of overt metabolic disease. Insulin, on the other hand, has been reported to have direct or indirect anti-inflammatory properties, most likely via a glucose lowering effect.29,44–46 Our data did show a negative (though non-significant) correlation of insulin and IL-6 (r = −0.433, P = 0.160) but does do not support a pro-inflammatory effect of glucose in this sample. Future studies employing more complex analysis (i.e. beyond simple correlations) will have to detangle the interrelations among metabolic and inflammatory response markers in this population.
As a consequence of the large variability, we observed in baseline and post-feeding glucose and insulin values an exploratory analysis was performed that identified two subsets of participants with postprandial glucose levels greater than 200 mg/dl (EGR, n = 4) or less (TGR, n = 7). The EGR subset was significantly older and had higher BMI compared with TGR (Table 4). Significant interactions for these groups in glucose and insulin response over time were observed and simple effects for each group are presented in (Fig. 2A). No group × time interaction was observed for either inflammatory biomarker, or for TG. However, CRP (but not IL-6) values were consistently higher for the EGR group in line with the known association between glucose and CRP41–43 (but not IL-6). It is noteworthy that both subgroups had average CRP values in the high-risk range CVD according to the American Heart Association47 but levels were almost three times greater in the EGR versus TGR demonstrating a possible compounding effect of SCI and postprandial hyperglycemia. Interestingly, only two of the participants in the EGR and 1 in the TGR exhibited fasting hyperglycemia (i.e. >100 mg/dl) suggesting that postprandial glucose response may be a more sensitive predictor of inflammation than fasting glucose levels in this population. A sustained elevation of CRP concentration as observed in this study has been reported by other investigators examining persons with chronic SCI8,9,47,48 and has been reviewed by several authors.8,11 This feature of pro-inflammatory activity is a recognized risk factor for, and potential instigator of cardiovascular and pulmonary disease,49–51 and is elevated in non-disabled individuals with insulin resistance and MetS, the latter showing correlation between severity of insulin resistance and inflammatory stress biomarkers.52–55 As these conditions are seen at elevated prevalence after SCI the associations among cardioendocrine control, inflammation, and long-term health prognosis is worthy of additional study.
Table 4 .
Descriptives for postprandial glucose response groups
| Postprandial glucose response group |
Independent sample t-test | ||
|---|---|---|---|
| EGR (n = 4) | TGR (n = 7) | P | |
| Age (years) | 49 ± 4 | 32 ± 12 | 0.014* |
| Level of injury | T5–L1 | T4-10 | N/A |
| Duration of injury (years) | 14.4 ± 15 | 8 ± 6 | 0.345 |
| Body mass (kg) | 89 ± 21 | 78 ± 19 | 0.349 |
| Body mass index | 34 ± 7 | 26 ± 5 | 0.037* |
| Body fat (%) | 42 ± 10 | 33 ± 7 | 0.082 |
| HOMA-IR | 4.8 ± 3.4 | 2.4 ± 2.0 | 0.142 |
Values are mean ± SD; HOMA_IR, homeostatic model assessment-insulin resistance.
*Statistically significant at P < 0.05.
Study limitations
Several factors limit this study findings. The dataset was derived from a relatively small test population, did not have a control group and as a first study we did not place limits on body mass. This made isolation of individual component risks challenging, but also made significant findings more compelling. Clinically the participants were free of infection and musculoskeletal injury that might otherwise elevate inflammatory biomarkers, although occult or subclinical conditions cannot be entirely ruled out. The possibility that subclinical bacteria and musculoskeletal injury contribute to CVD risk after SCI – beyond that sustained from vascular depots – has been suggested, and remains a potentially novel non-cardiac/vascular CVD risk in this population. This study did not individualize caloric intake based upon participant body mass, although without prescriptive diet this would have been impractical in a mixed-nutrient test meal.
Conclusions
Persons with SCI presented with fasting levels of CRP in the high-risk range that changed little with feeding and pose concern for their CVD risk. CRP values were even higher for those who exhibited an exaggerated blood glucose response to feeding. The possible association between postprandial metabolic responses and inflammatory states warrants further investigation to identify individual component risks for this secondary health hazard.
Disclaimer statements
Contributors There are no contributors beyond the authors.
Funding This research is supported by a grant from the United States Department of Education, National Institute for Disability and Rehabilitation Research (#H133G080150).
Conflicts of interest We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Ethics approval Signed informed consent was obtained from all subjects before the start of the study, which was approved by the University of Miami Medical Sciences Subcommittee for the Protection of Human Subjects.
References
- 1.Adkins RH. Research and interpretation perspectives on aging related physical morbidity with spinal cord injury and brief review of systems. NeuroRehabilitation 2004;19(1):3–13. [PubMed] [Google Scholar]
- 2.Charlifue S, Jha A, Lammertse D. Aging with spinal cord injury. Phys Med Rehabil Clin N Am 2010;21(2):383–402. [DOI] [PubMed] [Google Scholar]
- 3.Nash MS, van de Ven I, van Elk N, Johnson BM. Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch Phys Med Rehabil 2007;88(1):70–5. [DOI] [PubMed] [Google Scholar]
- 4.Whiteneck GG, Charlifue S, Frankel Ha, Fraser M, Gardner B, Gerhart K, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Spinal Cord 1992;30(9):617–30. [DOI] [PubMed] [Google Scholar]
- 5.Nash MS. Exercise as a health-promoting activity following spinal cord injury. J Neurol Phys Ther 2005;29(2):87–103, 106. [DOI] [PubMed] [Google Scholar]
- 6.Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med 2009;32(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77. [DOI] [PubMed] [Google Scholar]
- 8.Nash MS, Gintel SL, Mendez AJ, Hamm LF, Lewis JE, Groah SL. Elevated CRP and vascular disease after SCI: inflammatory epiphenomenon or pathologic agent? J Spinal Cord Med 2006;29(3):252. [Google Scholar]
- 9.Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005;86(6):1176–81. [DOI] [PubMed] [Google Scholar]
- 10.Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia 1992;30(12):880–9. [DOI] [PubMed] [Google Scholar]
- 11.Myers J. Cardiovascular disease after SCI: prevalence, instigators, and risk clusters. Top Spinal Cord Inj Rehabil 2009;14(3):1–14. [Google Scholar]
- 12.Bauman W, Spungen A, Zhong Y, Rothstein J, Petry C, Gordon S. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Spinal Cord 1992;30(10):697–703. [DOI] [PubMed] [Google Scholar]
- 13.Brenes G, Dearwater S, Shapera R, LaPorte R, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil 1986;67(7):445–50. [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359(21):2195–207. [DOI] [PubMed] [Google Scholar]
- 16.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation 1979;60(3):473–85. [DOI] [PubMed] [Google Scholar]
- 17.Cohn JS. Postprandial lipemia and remnant lipoproteins. Clin Lab Med 2006;26(4):773–86. [DOI] [PubMed] [Google Scholar]
- 18.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 2002;39(7):1145–50. [DOI] [PubMed] [Google Scholar]
- 19.Nash MS, DeGroot J, Martinez-Arizala A, Mendez AJ. Evidence for an exaggerated postprandial lipemia in chronic paraplegia. J Spinal Cord Med 2005;28(4):320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmons RR, Garber CE, Cirnigliaro CM, Moyer JM, Kirshblum SC, Galea MD, et al. The influence of visceral fat on the postprandial lipemic response in men with paraplegia. J Am Coll Nutr 2010;29(5):476–81. [DOI] [PubMed] [Google Scholar]
- 21.Bray GA, Most M, Rood J, Redmann S, Smith SR. Hormonal responses to a fast-food meal compared with nutritionally comparable meals of different composition. Ann Nutr Metabol 2007;51(2):163–71. [DOI] [PubMed] [Google Scholar]
- 22.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106(16):2067–72. [DOI] [PubMed] [Google Scholar]
- 23.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407. [DOI] [PubMed] [Google Scholar]
- 24.CLASSIFICATION AND DIAGNOSIS I. Standards of Medical Care in Diabetes. Diabetes Care 2005;28:S5. [Google Scholar]
- 25.Bauman WA, Spungen AM, Raza M, Rothstein J, Zhang R, Zhong Y, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med 1992;59(2):163–8. [PubMed] [Google Scholar]
- 26.Cowan RE, Nash MS. Cardiovascular disease, SCI and exercise: unique risks and focused countermeasures. Disabil Rehabil 2010;32(26):2228–36. [DOI] [PubMed] [Google Scholar]
- 27.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76. [DOI] [PubMed] [Google Scholar]
- 28.Cohn JS. Postprandial lipemia: emerging evidence for atherogenicity of remnant lipoproteins. Can J Cardiol 1998;14(Suppl B):18B–27B. [PubMed] [Google Scholar]
- 29.Funada J, Sekiya M, Hamada M, Hiwada K. Postprandial elevation of remnant lipoprotein leads to endothelial dysfunction. Circ J 2002;66(2):127–32. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn P, Despres JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity Silver Spring 2006;14(10):1747–54. [DOI] [PubMed] [Google Scholar]
- 31.Tsai W, Li Y, Lin C, Chao T, Chen J. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci 2004;106(3):315–20. [DOI] [PubMed] [Google Scholar]
- 32.Beisswenger P, Brown W, Ceriello A, Le N, Goldberg R, Cooke J, et al. Meal_induced increases in C_reactive protein, interleukin_6 and tumour necrosis factor _ are attenuated by prandial basal insulin in patients with Type 2 diabetes. Diabetic Med 2011;28(9):1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundman P, Eriksson MJ, Silveira A, Hansson LO, Pernow J, Ericsson CG, et al. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation (C-reactive protein, interleukin-6, soluble adhesion molecules, von Willebrand factor, and endothelin-1). Am J Cardiol 2003;91(9):1128–31. [DOI] [PubMed] [Google Scholar]
- 34.Nabeno-Kaeriyama Y, Fukuchi Y, Hayashi S, Kimura T, Tanaka A, Naito M. Delayed postprandial metabolism of triglyceride-rich lipoproteins in obese young men compared to lean young men. Clinica Chimica Acta 2010;411(21):1694–9. [DOI] [PubMed] [Google Scholar]
- 35.Brinton EA, Nanjee MN, Hopkins PN. Triglyceride-rich lipoprotein remnant levels and metabolismTime to adopt these orphan risk factors? J Am Coll Cardiol 2004;43(12):2233–5. [DOI] [PubMed] [Google Scholar]
- 36.Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, et al. Gender difference in postprandial lipemia importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol 1999;19(10):2448–55. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn P, Lamarche B, Couillard C, Pascot A, Bergeron N, Prud'homme D, et al. Postprandial hyperlipidemia: another correlate of the ‘hypertriglyceridemic waist’ phenotype in men. Atherosclerosis 2003;171(2):327–36. [DOI] [PubMed] [Google Scholar]
- 38.Devaraj S, Wang-Polagruto J, Polagruto J, Keen CL, Jialal I. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metab Clin Exp 2008;57(6):867–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CC, Liu CW, Weng MC, Chen TW, Huang MH. Association of C-reactive protein and insulin resistance in patients with chronic spinal cord injury. J Rehabil Med 2008;40(10):819–22. [DOI] [PubMed] [Google Scholar]
- 40.Lee MY, Myers J, Hayes A, Madan S, Froelicher VF, Perkash I, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med 2005;28(1):20–5. [DOI] [PubMed] [Google Scholar]
- 41.Rhee EJ, Kim YC, Lee WY, Jung CH, Sung KC, Ryu SH, et al. Comparison of insulin resistance and serum high-sensitivity C-reactive protein levels according to the fasting blood glucose subgroups divided by the newly recommended criteria for fasting hyperglycemia in 10059 healthy Koreans. Metabolism 2006;55(2):183–7. [DOI] [PubMed] [Google Scholar]
- 42.Sabanayagam C, Shankar A, Lim SC, Lee J, Tai ES, Wong TY. Serum C-reactive protein level and prediabetes in two Asian populations. Diabetologia 2011;54(4):767–75. [DOI] [PubMed] [Google Scholar]
- 43.Festa A, D'Agostino R, Tracy R, Haffner S. C_reactive protein is more strongly related to post_glucose load glucose than to fasting glucose in non_diabetic subjects; the Insulin Resistance Atherosclerosis Study. Diabetic Med 2002;19(11):939–43. [DOI] [PubMed] [Google Scholar]
- 44.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 45.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol 2007;99(4):15–26. [DOI] [PubMed] [Google Scholar]
- 46.Choi K, Lee K, Kim S, Kim N, Park C, Seo H, et al. Inflammation, insulin resistance, and glucose intolerance in acute myocardial infarction patients without a previous diagnosis of diabetes mellitus. J Clin Endocrinol Metab 2005;90(1):175–80. [DOI] [PubMed] [Google Scholar]
- 47.Gibson AE, Buchholz AC, Martin Ginis KA, SHAPE-SCI Research Group. C-Reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord 2008;46(9):616–21. [DOI] [PubMed] [Google Scholar]
- 48.Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86(2):312–7. [DOI] [PubMed] [Google Scholar]
- 49.Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868–74. [DOI] [PubMed] [Google Scholar]
- 50.Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O'Leary DH, et al. Association Between Obesity, High-Sensitivity C-Reactive Protein≥2 mg/l, and Subclinical Atherosclerosis Implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31(6):1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schols A, Buurman WA, Van den Brekel , Staal AJ, Dentener MA, Wouters E. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996;51(8):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dandona P, Aljada A. A rational approach to pathogenesis and treatment of type 2 diabetes mellitus, insulin resistance, inflammation, and atherosclerosis. Am J Cardiol 2002;90(5):27–33. [DOI] [PubMed] [Google Scholar]
- 53.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25(1):4–7. [DOI] [PubMed] [Google Scholar]
- 54.Festa A, D'Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102(1):42–7. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events an 8-year follow-up of 14 719 initially healthy American women. Circulation 2003;107(3):391–7. [DOI] [PubMed] [Google Scholar]