Abstract
Objective
To investigate the anatomical and histological features of spinal nerve roots and provide base data for neuroanastomosis therapy for paraplegia.
Methods
Spinal nerve roots from C1 to S5 were exposed on six adult cadavers. The diameter and the number of nerve fibers of each nerve root were measured, respectively, with a caliper and image analysis software.
Results
As for ventral roots, the diameter of C5 (2.50 ± 0.55 mm) was the largest in cervical segments. In thoracic and lumbosacral segments, the diameter gradually increased from T11 to S1 and then decreased from S1 to S5 except L3. S1 (1.43 ± 0.16 mm) was the thickest root and S5 (0.14 ± 0.02 mm) was the thinnest one. As for dorsal roots, the diameter of C7 (4.61 ± 0.87 mm) was the largest in cervical segments. From T11 to S1, the diameter increased and then decreased gradually from S1 to S5. The diameter of dorsal roots from T1 to S5 was largest at S1 (2.95 ± 0.57 mm) and smallest at S5 (0.27 ± 0.13 mm), respectively. C7 (8467 ± 1019), T12 (6538 ± 892), L3 (9169 ± 1160), and S1 (8253 ± 1419) ventral roots contained the most nerve fibers in cervical, thoracic, lumbar, and sacral segments, respectively. Similarly, C7 (39 653 ± 8458), T1 (26 507 ± 7617), L5 (34 455 ± 2740), and S1 (41 543 ± 3036) dorsal roots, respectively, contained the most nerve fibers in their corresponding segments.
Conclusion
The findings in the current study provided the imperative data and may be valuable for spinal nerve root microanastomosis surgery in the paraplegic patients.
Keywords: Anastomosis, Nerve transfer, Paraplegia, Spinal cord injuries, Spinal nerve roots
Introduction
Paraplegia caused by spinal cord injury (SCI) is a common disease in neurosurgery; nevertheless, the treatment of paraplegia remains difficult at present. According to a recent epidemiological study, the estimate of annual incidence in traumatic SCI varied from 12.1 to 57.8 cases per million worldwide.1 Furthermore, paraplegia was found to be more common than tetraplegia (paraplegia: 58.7%; tetraplegia: 40.6%).2 Persons with paraplegia usually experience a loss of motor and sensory function in lower extremities and excretion functions causing serious complications such as bedsores and urinary tract infection.
There is no effective method to completely recover the impaired nerve function at present. In recent decades, some scholars have partially reconstructed variable nerve function under the level of SCI by different methods of neuroanastomosis, which provides a new approach for the treatment of paraplegia.3–6 In order to improve the therapeutic effect, the anatomical and histological features of spinal nerve roots have important clinical significance. In this study, we investigate the microstructure, diameter, and nerve fiber quantity of spinal nerve roots from C1 to S5 to provide fundamental data for spinal nerve root microanastomosis treatment.
Methods
Specimen preparation
Spinal cord dissections were performed on 6 (12 sides) human formalin-fixed cadavers donated to the Department of Anatomy in Nanjing Medical University. Two donors were female and four were male, with an average age of 43 years at death. The cadavers were placed in the prone position. Following the removal of all muscles, ligaments, and vertebral laminae, the spinal cord was exposed by a longitudinal midline incision in the posterior dura and arachnoid membrane. Then, all the spinal nerve roots were identified from distal (dural sleeve) to proximal (origin at the spinal cord) in C1–S5 spinal cord segments. At each spinal cord segment, we measured the diameters of ventral and dorsal nerve roots by a caliper with a precision of 0.02 mm. Finally, short segments of these nerve roots were sectioned 3 cm adjacent to the dural sac and then placed in phosphate buffer solution in preparation for immunohistochemical study. Both nerve fibers and diameter were measured at 1 cm adjacent to nerve root outlet inside the spinal dura mater.
Immunohistochemistry
After routine dehydration, clarification, paraffin embedding, and sectioning (5 µm, cross section), all cross sections were stained in the laboratory. Neurofilament 200 (NF200), a specific marker of neurofilament, was used to determine the number of nerve fibers. Immunohistochemical expression of NF200 in spinal nerve roots was examined as follows: paraffin-embedded cross sections were dewaxed, rehydrated, and immersed in phosphate buffered saline for 5 minutes. After antigen retrieval by microwave, the cross sections were blocked for 20 minutes in normal goat serum, and then incubated over-night with the primary antibody against NF200 (Abcam, Cambridge, MA, USA) at 4°C. After washing, the cross sections were further incubated with secondary antibody (KIT5010, MaxVision™, Fuzhou, Fujian Province, China) for 15 minutes at room temperature, and then the reactivity was visualized with 3,3′-diaminobenzidine tetrahydrochloride hydrate (Boster, Wuhan, China). Finally, all slices were mounted for light microscopic observation. In addition, the histological structure of nerve roots was observed by HE staining in some cross sections.
Photography and measurement
All samples were photographed using a light microscope (Olympus BX50, Tokyo, Japan) connected with a CCD camera (Olympus DP70, Tokyo, Japan) which has a high resolution of 4080 × 3072 pixels. Then, systematic nerve fiber counts of stained cross sections were performed by the Image Pro Plus 6.0 image analysis software.
Data analysis
All data were collected and inputted into Excel 2007 (Microsoft, Seattle, WA, USA) to save for further statistical analysis conducted with SPSS 13.0 software (SPSS, Chicago, IL, USA). Statistical analysis was used to determine the average, standard deviation (SD), and differences between the left and right sides. Linear correlation analysis was used to calculate the relationship between the diameter and the number of nerve fibers of spinal nerve roots. A P value of <0.05 was considered to be statistically significant.
Results
Histological microstructure of spinal nerve roots
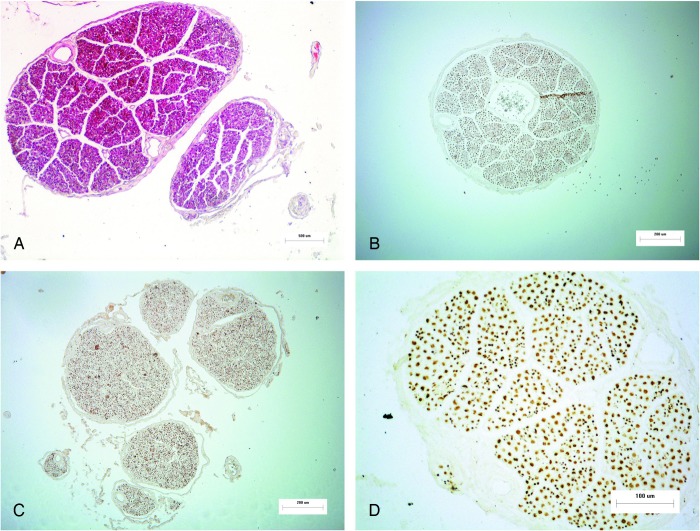
Spinal nerves are composed of ventral and dorsal roots in their corresponding spinal cord segments. Typically, a number of rootlets originate from the corresponding level of spinal cord, and then they are aggregated gradually to form nerve sub-bundles, bundles, and finally a nerve root.7 As shown in Fig. 1 (Parts A, B, and C), the ventral roots adjacent to the dura usually consisted of only one nerve bundle while most dorsal roots normally comprised several nerve bundles. With varying sizes and shapes, the nerve bundles were loosely organized by small amounts of connective tissues. Each nerve bundle was wrapped by a membrane tissue with a mean thickness of 18.92 µm. Within the bundle, blood vessels were enclosed in its connective tissues.
Figure 1 .
Overviews of ventral and dorsal roots. A: Cross-section of C3 dorsal root (HE stain; Magnification 100×); B: Cross-section of T12 ventral root (NF200 stain; Magnification 100×); C: Cross-section of T2 dorsal root (NF200 stain; Magnification 100×); D: Cross-section of C2 ventral root (NF200 stain; Magnification 200×).
As shown in Fig. 1 (Part D), the immunohistochemistry showed positive staining (brown granules) in the axons, but negative in the myelin sheaths of spinal nerve root. Thus, the nerve fiber counts were available by the immunohistochemical staining.
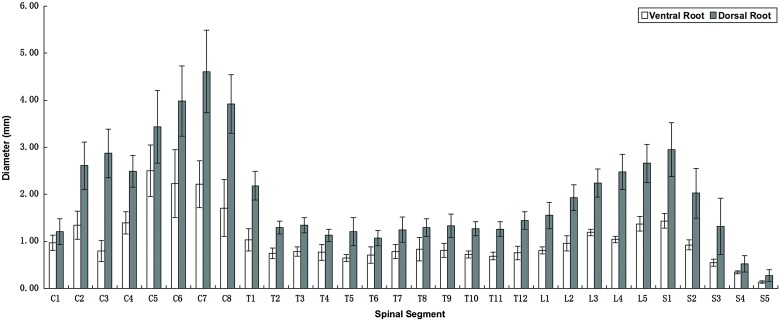
Diameters of spinal nerve roots
As shown in Table 1 and Fig. 2, different segments of spinal nerve roots had various sizes. The diameter of dorsal root was always larger than the corresponding ventral root, the diameter ratio being about 1.961.
Table 1 .
The diameters and number of nerve fibers in spinal nerve roots
| Segment | Diameter (mm) |
Number of nerve fibers |
||
|---|---|---|---|---|
| Ventral root | Dorsal root | Ventral root | Dorsal root | |
| C1 | 0.97 ± 0.16 | 1.21 ± 0.28 | 2751 ± 639 | 6430 ± 1606 |
| C2 | 1.34 ± 0.30 | 2.61 ± 0.51 | 3116 ± 724 | 11 947 ± 2977 |
| C3 | 0.80 ± 0.23 | 2.87 ± 0.52 | 2460 ± 471 | 21 876 ± 1916 |
| C4 | 1.39 ± 0.24 | 2.49 ± 0.34 | 3833 ± 408 | 10 647 ± 887 |
| C5 | 2.50 ± 0.55 | 3.43 ± 0.77 | 7841 ± 1020 | 23 300 ± 2856 |
| C6 | 2.23 ± 0.73 | 3.99 ± 0.75 | 7048 ± 1157 | 36 353 ± 7451 |
| C7 | 2.22 ± 0.50 | 4.61 ± 0.87 | 8467 ± 1019 | 39 653 ± 8458 |
| C8 | 1.71 ± 0.60 | 3.92 ± 0.62 | 5883 ± 1000 | 31 156 ± 8273 |
| T1 | 1.03 ± 0.23 | 2.18 ± 0.31 | 5788 ± 1186 | 26 507 ± 7617 |
| T2 | 0.75 ± 0.11 | 1.30 ± 0.14 | 3576 ± 398 | 10 234 ± 1728 |
| T3 | 0.78 ± 0.10 | 1.35 ± 0.16 | 5499 ± 1126 | 14 888 ± 2514 |
| T4 | 0.77 ± 0.17 | 1.13 ± 0.13 | 5485 ± 973 | 10 849 ± 1832 |
| T5 | 0.64 ± 0.08 | 1.21 ± 0.30 | 5326 ± 1314 | 8355 ± 1390 |
| T6 | 0.71 ± 0.18 | 1.07 ± 0.16 | 3666 ± 1407 | 10 015 ± 1666 |
| T7 | 0.78 ± 0.15 | 1.25 ± 0.27 | 4297 ± 1130 | 9123 ± 1178 |
| T8 | 0.83 ± 0.25 | 1.29 ± 0.18 | 3643 ± 1340 | 7619 ± 903 |
| T9 | 0.81 ± 0.15 | 1.33 ± 0.25 | 5209 ± 704 | 8369 ± 967 |
| T10 | 0.72 ± 0.08 | 1.27 ± 0.15 | 5269 ± 963 | 11 329 ± 2724 |
| T11 | 0.69 ± 0.08 | 1.26 ± 0.16 | 4870 ± 895 | 9713 ± 1824 |
| T12 | 0.76 ± 0.14 | 1.45 ± 0.19 | 6538 ± 892 | 10 420 ± 802 |
| L1 | 0.81 ± 0.07 | 1.55 ± 0.28 | 5384 ± 833 | 16 820 ± 3456 |
| L2 | 0.96 ± 0.16 | 1.93 ± 0.27 | 7374 ± 720 | 18 615 ± ±3284 |
| L3 | 1.19 ± 0.07 | 2.24 ± 0.30 | 9169 ± 1160 | 26 191 ± 2772 |
| L4 | 1.04 ± 0.07 | 2.48 ± 0.38 | 7878 ± 1386 | 31 175 ± 2686 |
| L5 | 1.37 ± 0.16 | 2.66 ± 0.40 | 8657 ± 1396 | 34 455 ± 2740 |
| S1 | 1.43 ± 0.16 | 2.95 ± 0.57 | 8253 ± 1419 | 41 543 ± 3036 |
| S2 | 0.93 ± 0.11 | 2.02 ± 0.53 | 4766 ± 1035 | 18 642 ± 1716 |
| S3 | 0.55 ± 0.07 | 1.32 ± 0.60 | 2233 ± 299 | 11 971 ± 964 |
| S4 | 0.34 ± 0.03 | 0.52 ± 0.17 | 1356 ± 193 | 3402 ± 304 |
| S5 | 0.14 ± 0.02 | 0.27 ± 0.13 | 906 ± 111 | 2206 ± 197 |
Values represent mean ± SD.
Figure 2 .
Diameters of spinal nerve roots.
As for ventral roots, the diameter of C5 (2.50 ± 0.55 mm) was the largest in cervical segments. In thoracic and lumbosacral segments, the diameter gradually increased from T11 to S1 and then decreased from S1 to S5 except L3. S1 (1.43 ± 0.16 mm) was the thickest root and S5 (0.14 ± 0.02 mm) was the thinnest one. Different segments of the dorsal roots also had a similar trend. As for dorsal roots, the diameter of cervical nerve roots was largest at C7 (4.61 ± 0.87 mm). From T11 to S1, the diameter increased and then decreased gradually from S1 to S5. The diameter of dorsal roots from T1 to S5 was largest at S1 (2.95 ± 0.57 mm) and smallest at S5 (0.27 ± 0.13 mm), respectively.
No meaningful differences between the left and right sides were observed (P > 0.05).
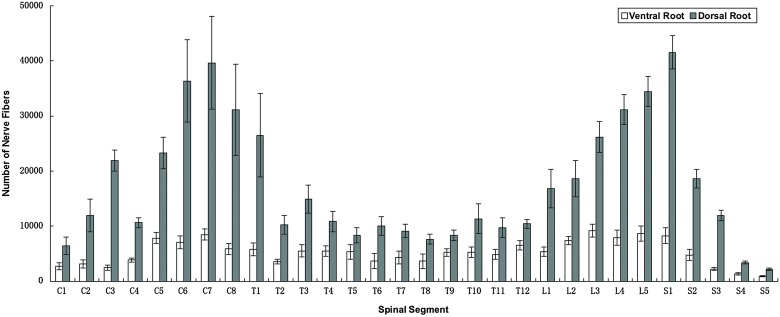
Nerve fiber counts
As shown in Table 1 and Fig. 3, C7 (8467 ± 1019), T12 (6538 ± 892), L3 (9169 ± 1160), and S1 (8253 ± 1419) ventral roots contained the most nerve fibers in cervical, thoracic, lumbar, and sacral segments, respectively. S5 (906 ± 111) ventral root contained the least nerve fibers in sacral segments. As for dorsal roots, similarly, C7 (39 653 ± 8458) also contained the most nerve fibers among the cervical nerve roots. T1 (26 507 ± 7617), L5 (34 455 ± 2740), and S1 (41 543 ± 3036) contained the most nerve fibers in thoracic, lumbar, and sacral segments, respectively. From T1 to S5, S5 (2206 ± 197) contained the least nerve fibers.
Figure 3 .
The number of nerve fibers in spinal nerve roots.
In addition, compared with the corresponding ventral roots, the dorsal roots always contained more nerve fibers. There were no significant differences between right and left sides (P > 0.05).
Relationship between the diameter and number of nerve fibers
The results of Spearman's rank correlation analysis indicated a significantly positive correlation between the number of nerve fibers and diameters of roots (r = 0.573, P < 0.01 for ventral roots; r = 0.803, P < 0.01 for dorsal roots).
Discussion
Treatment of paraplegia caused by SCI is always a difficult challenge. With the unceasing study of the regeneration capacity of the spinal nerve root axon,8–12 spinal nerve root microanastomosis has been explored to recover the impaired nerve function in paraplegia patients. Furthermore, some researchers have partially restored muscle and pelvic organs function in paraplegia patients by various surgical procedures,13–15 providing a new therapeutic approach for paraplegia. However, the optimum choice of donor nerve for anastomosis remains uncertain because of the lack of anatomical and histological data of related spinal nerve roots. So, we conducted the anatomical observations, measurements, and statistical analysis of six adult spine specimens.
Nerve fiber counts
Previous studies by Arnell,16 Davenport and Bothe,17 Ingbert,18 Schalow,19 and Hauck et al.20 have provided some information regarding the number of nerve fibers in only a few spinal segments using silver or osmium staining. In our study, the detailed data regarding the number of nerve fibers of both the ventral and dorsal root in entire C1–S5 segments were investigated. Moreover, as a specific marker of neurofilament, NF200 staining used in our study is also a relatively current method for the staining of nerve tissue. With a better staining of small diameter axon as shown in Fig. 1 (Part D), this method ensured an accurate and convenient quantitative analysis of nerve fibers.
Clinical significance of our research on spinal nerve roots
The curative effect of nerve transfer procedure greatly depends on the choice of the donor nerve. The following key factors should be considered: the nerve diameter, the number of nerve fibers, relatively secondary function, and the level of SCI.
Nerve diameter
Variable diameters of nerve roots may cause difficulties for anastomosis between them and affect nerve function recovery, so it is necessary to choose a donor nerve with similar diameter of receptor nerve. The measurements of diameters shown in Table 1 could provide the correlative data for surgeons.
Worthy of note, as we mentioned in Fig. 1, the dorsal roots adjacent to the dural sac usually contained several nerve bundles loosely organized by little connective tissues, so we could easily separate or combine these nerve bundles to anastomose the nerve roots with different diameters.
In addition, owing to the limited operative visual field and the dense arrangement in the narrow subdural space, especially in lumbosacral segments, it is difficult to identify the spinal nerve roots. The detailed measurements shown in Table 1 could provide some morphological bases for the identification during neuroanastomosis surgery. Our results in all cadavers indicate that, both S1 ventral root (1.43 ± 0.16 mm) and S1 dorsal root (2.95 ± 0.57 mm) were the thickest in lumbosacral level, which could be a reliable landmark for the location of other nerve roots. While in cervical segments, C5 ventral root (2.50 ± 0.55 mm) and C7 dorsal root (4.61 ± 0.87 mm) could also be anatomical landmarks.
Number of nerve fibers
The number of donor nerve fibers is one of the key factors determining the efficacy of nerve transfer.21 However, clinical and animal experiments showed that it is not required to restore all the original nerve fibers of receptor nerve to recover the normal function of the target muscle or organ in neuroanastomosis.22 That is to say, in nerve transfer, just a certain proportion of nerve fibers could restore the impaired function of the muscle or organ instead of 100% of the receptor nerve fibers. Further studies conducted by Kalantarian et al.23 showed that the smallest proportion of nerve fibers to maintain the target muscle function is about 40%. By animal experiments, Wei24 found that 37% may be the smallest proportion of nerve fibers to maintain the basic function of muscle. Based on the abovementioned facts, it can be concluded that the donor nerve fibers must outnumber at least about 40% of the receptor nerve fibers. As we mentioned above, C7, T12, L3, and S1 ventral roots relatively consisted of the most motor fibers in their corresponding segments. While C7, T1, L5, and S1 dorsal roots, respectively, contained the most sensory fibers. These spinal roots should be given prior consideration in the neuroanastomosis as donor nerves.
On the other hand, the transfer of donor nerve normally causes the loss of motor and sensory function in original regions innervated by it, which should be minimized as far as possible. Especially in the dorsal roots, selective nerve bundle anastomosis instead of entire nerve root transfer could also be an option to preserve a part of donor nerve.
In a word, understanding about the number of nerve fibers will contribute to the optimal selection of donor nerve.
Linear relationship between the diameter and the number of nerve fibers
The significantly positive correlation between the diameter of nerve roots and the number of nerve fibers indicates that a thicker root normally contains more nerve fibers, and vice versa. Knowledge of this anatomical relationship may be helpful to roughly estimate the number of nerve root fibers during neuroanastomosis surgery.
Due to the different methods of neuroanastomosis, the selection of donor nerve for each specific spinal segment was not explained in detail in this paper. In clinical practice, similar diameter with the receptor nerve, sufficient nerve fibers (at least 40% of the receptor nerve fibers), and relatively subordinate nerve function (such as T7–T12 innervating abdominal muscles and skins) should be satisfied simultaneously in the selection of donor nerve. It is noteworthy that this paper mainly described the selection principle of donor nerve from the diameter and the number of nerve fibers perspectives. Some other factors such as the level of SCI and the distance between donor and receptor nerve should also be considered in practical application.
Conclusion
In this study, we investigated in detail the anatomical and histological information of intradural spinal nerve roots from C1 to S5. The data regarding the diameter of nerve roots and the number of nerve fibers were obtained. Correlation analysis indicated a significantly positive correlation between them. All these findings in our study may be valuable for spinal nerve root microanastomosis surgery in paraplegic patients.
Disclaimer statements
Contributors There are no other contributors in the study.
Funding This work was supported by the National Natural Science Foundation of China (Grant Nos. 30973058 and 81171694), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Jiangsu Province Nature Science Foundation (BE2010743 and BK2012718).
Conflicts of interest None.
Ethics approval Ethical approval was not required in our study.
References
- 1.van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 2010;34(3):184–92; discussion 192. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi-Movaghar V, Sayyah MK, Akbari H, Khorramirouz R, Rasouli MR, Moradi-Lakeh M, et al. Epidemiology of traumatic spinal cord injury in developing countries: a systematic review. Neuroepidemiology 2013;41(2):5–85. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson CA, Sundin T. Reconstruction of afferent and efferent nervous pathways to the urinary bladder in two paraplegic patients. Spine (Phila Pa 1976) 1980;5(1):37–41. [DOI] [PubMed] [Google Scholar]
- 4.Dai KR, Yu CT, Wu RS, Zhang XF, Yuan JX, Sun YH. Intercostal-lumbar-spinal nerve anastomoses for cord transection. A preliminary investigation. J Reconstr Microsurg 1985;1(3):223–6. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Johnston L, Zhang Z, Ma Y, Hu Y, Wang J, et al. Restoration of stepping-forward and ambulatory function in patients with paraplegia: rerouting of vascularized intercostal nerves to lumbar nerve roots using selected interfascicular anastomosis. Surg Technol Int 2003;11:244–8. [PubMed] [Google Scholar]
- 6.Livshits A, Catz A, Folman Y, Witz M, Livshits V, Baskov A, et al. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord 2004;42(4):211–7. [DOI] [PubMed] [Google Scholar]
- 7.Zhou MW, Wang WT, Huang HS, Zhu GY, Chen YP, Zhou CM. Microsurgical anatomy of lumbosacral nerve rootlets for highly selective rhizotomy in chronic spinal cord injury. Anat Rec (Hoboken) 2010;293(12):2123–8. [DOI] [PubMed] [Google Scholar]
- 8.Hoang TX, Nieto JH, Dobkin BH, Tillakaratne NJ, Havton LA. Acute implantation of an avulsed lumbosacral ventral root into the rat conus medullaris promotes neuroprotection and graft reinnervation by autonomic and motor neurons. Neuroscience 2006;138(4):1149–60. [DOI] [PubMed] [Google Scholar]
- 9.Zhong G, Hou C, Wang S. Experimental study on the artificial bladder reflex arc established in therapy of flaccid bladder after spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2006;20(8):812–5. [PubMed] [Google Scholar]
- 10.Carlstedt T, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg 2000;93(2 Suppl):237–47. [DOI] [PubMed] [Google Scholar]
- 11.Huang MC, Chang PT, Tsai MJ, Kuo HS, Kuo WC, Lee MJ, et al. Sensory and motor recovery after repairing transected cervical roots. Surg Neurol 2007;68(Suppl 1):S17–24; discussion S24. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Hou C, Zhen X. Bypassing spinal cord injury: surgical reconstruction of afferent and efferent pathways to the urinary bladder after conus medullaris injury in a rat model. J Reconstr Microsurg 2008;24(8):575–81. [DOI] [PubMed] [Google Scholar]
- 13.Xiao CG, Godec CJ. A possible new reflex pathway for micturition after spinal cord injury. Paraplegia 1994;32(5):300–7. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Beuerman RW, Kline DG. Neurotization of motor nerves innervating the lower extremity by utilizing the lower intercostal nerves. J Reconstr Microsurg 1997;13(1):39–45. [DOI] [PubMed] [Google Scholar]
- 15.Tadie M, Liu S, Robert R, Guiheneuc P, Pereon Y, Perrouin-Verbe B, et al. Partial return of motor function in paralyzed legs after surgical bypass of the lesion site by nerve autografts three years after spinal cord injury. J Neurotrauma 2002;19(8):909–16. [DOI] [PubMed] [Google Scholar]
- 16.Arnell N. About the knowledge of the number of nerve fibers in the roots of spinal nerves in humans. Ups Laekaref Forh 1933;39:97–117. German. [Google Scholar]
- 17.Davenport HA, Bothe RT. Cell and fibres in spinal nerves. II. A study of C2, C6, T4, T9, L3, S2 and S5 in man. J Comp Neurol 1934;59(1):167–74. [Google Scholar]
- 18.Ingbert C. An enumeration of the medullated nerve fibres in the dorsal roots of the spinal nerves of man. J Comp Neurol 1903;13(2):53–120. [Google Scholar]
- 19.Schalow G. Number of fibres and fibre diameter distributions of nerves innervating the urinary bladder in humans. Acceptor nerve analysis. II.(IV). Electromyogr Clin Neurophysiol 1992;32(4–5):187–96. [PubMed] [Google Scholar]
- 20.Hauck EF, Schwefer M, Wittkowski W, Bothe HW. Measurements and mapping of 282,420 nerve fibers in the S1–5 nerve roots. J Neurosurg Spine 2009;11(3):255–63. [DOI] [PubMed] [Google Scholar]
- 21.Gu YD. Diagnosis and treatment of injuries and disorders of the brachial plexus. 2nd ed. Shanghai: Fudan University Press; 2001. p. 287–96. [Google Scholar]
- 22.Chen L, Gu YD, Li DC. An experimental study of the least nerve roots of the brachial plexus for maintaining the normal limb function. Chin J Hand Surg 1998;14(4):234–8. Chinese. [Google Scholar]
- 23.Kalantarian B, Rice DC, Tiangco DA, Terzis JK. Gains and losses of the XII-VII component of the “baby-sitter” procedure: a morphometric analysis. J Reconstr Microsurg 1998;14(7):459–71. [DOI] [PubMed] [Google Scholar]
- 24.Wei L. An experimental study on the functional orientation and micro-structure of the C7 nerve root and its branches [dissertation]. Shanghai: Fudan University; 2003. [Google Scholar]