Abstract
Background
Obstruction of the third part of the duodenum (D3) is a very rare cause of gastric outflow obstruction. Rapid weight loss is the biggest risk factor. Patients seen in acute rehabilitation settings, not uncommonly, have a period of rapid weight loss. We report two cases of superior mesenteric artery (SMA) syndrome and review the literature.
Clinical details
The patients presented differently, one with repeated, refractory autonomic dysreflexia and severe spasticity and one with nausea, abdominal discomfort, and vomiting. CT abdomen with contrast identified dynamic duodenal (D3) obstruction against the posterior structures by narrow angled SMA, gastric distension and, in one case, dilation of the left renal vein. Both patients responded well to optimizing nutrition in different ways. Surgery was successfully avoided.
Discussion
SMA syndrome is an atypical cause of high intestinal obstruction, frequently occurring in patients who have had rapid weight loss during spinal cord injury (SCI) rehabilitation. It may co-exist with left renal vein dilation “nutcracker phenomena”. The associated neurogenic bowel dysfunction due to the nature of SCI could possibly contribute to delay in diagnosis.
Conclusion
Clinicians should consider the risk of SMA syndrome in patients with SCI with rapid weight loss. Early diagnosis is possible by doing a CT abdomen with contrast and angiography if there is a high index of suspicion. SMA syndrome can be successfully treated by aggressive nutritional management. This may include total parenteral nutrition or feeding by a nasojejunal tube. Duodenojejunostomy could be required in refractory cases.
Keywords: Spinal cord injury, Superior mesenteric artery, Duodenum obstruction, Nutcracker, Weight loss
Background
Autonomic dysreflexia (AD), spasms, and spasticity are not uncommon features after spinal cord injury (SCI) above the T6 level. The most common triggering factors are bladder stone/obstruction including a blocked catheter, faecal impaction, infections, pressure sores, post-traumatic syrinx, severe anxiety, and severe neuropathic pain. Obstruction of the third part of the duodenum (D3) by a narrow angled superior mesenteric artery (SMA) (superior mesenteric artery syndrome) following rapid weight loss is very rare following SCI, but may contribute to these symptoms, and poses a diagnostic challenge in these patients.
Clinical details
We identified two cases (one delayed diagnosis) of obstruction of D3 by the SMA, initially causing repeated, refractory episodes of AD and generalized spasticity, followed by classical symptoms and signs of upper gastrointestinal obstruction in traumatic C4 AIS A patients admitted for specialist spinal rehabilitation at a regional spinal cord injury centre.
Case 1
Mr. P., a 19-year-old male, was admitted 4 months postinjury with postoperative C5 fracture dislocation (C4 AIS A), following removal of percutaneous endoscopic gastrostomy (PEG). He started having repeated episodes of AD, and increasing spasticity, which interfered with rehabilitation. This was followed by recurrent abdominal distension, nausea and reduced appetite, a significant weight loss of 13 kg over 6 weeks, and voluminous bilious vomiting (3.5 l/day), eructation, and gastric distension.
Thorough assessment over 6 weeks for AD, spasticity, and abdominal symptoms was carried out with normal blood parameters and normal MRI of the whole spine. Fecal impaction in the transverse and distal colon was seen on X-ray and CT scan. This was thought to be contributing to his symptoms but was refractory to treatment. A subsequent CT scan of the abdomen with contrast revealed classical radiological signs of SMA syndrome with dilation of the left renal vein (“Nutcracker” phenomenon) consistent with the clinical picture.
The patient had free gastric drainage and high calorie supplements (3000 kcal/day) with a triluminal nasojejunal (NJ) feeding tube (to bypass the obstruction and to build up the mesenteric retroperitoneal fat), placed endoscopically after a difficult procedure due to the tight D3 obstruction. Symptoms were resolved successfully after 3 weeks with adequate weight gain. Oral intake improved and the NJ tube was then removed. All symptoms of AD and spasms resolved in 3 weeks and anti-spasticity medications were reduced. The patient was then able to successfully participate in his rehabilitation programme.
Case 2
Mr. G., a 23-year-old male, post-C5 fracture with C4 AIS A tetraplegia, was admitted to the Spinal Cord Injury Centre, 4 weeks postinjury, ventilated, and nasogastrically (NG) fed. He was successfully weaned from the ventilator over 3 weeks but still had a tracheostomy. His swallow was not functional, and he was referred for a PEG while the nasogastric feed continued. The PEG was inserted 4 weeks after admission (8 weeks after injury). The following day the patient complained of abdominal pain. The next day, he experienced bilious vomiting. He had an NG inserted overnight on free drainage, which initially drained 400 ml of bile stained fluid. There was no further drainage between 3 a.m. and 10 a.m. the following morning when he was reassessed. At that time his abdomen was distended, he had further episodes of wretching, and a small volume of bilious vomiting.
CT scan of the abdomen with contrast was requested and identified a hugely distended stomach. The NG tube tip was not in the stomach. There was dynamic duodenal obstruction against the posterior structures by narrow angled SMA with gastric distension.
The NG tube was re-sited and aspirated, draining 1.5 l of bilious fluid, and the patient immediately felt better. The management continued with NG tube aspirate decompression, intravenous fluids, and slow introduction of PEG nutrition, increasing as tolerated to a very high-calorie regimen to promote weight gain. He recovered well, continuing on the PEG feed and gradually increased oral intake as his swallow progressed.
Discussion
The SMA syndrome was first described in 1842 in an anatomy text by Rokitansky.1,2 Wilkie3 published the first large series of 75 patients in 1927, and his name is frequently ascribed to this condition. The majority of reports of this condition are limited to case reports and small series. There are 14 cases reported after SCI, with 4 patients having paraplegia.4
The main anatomical feature of this syndrome is the compression and obstruction of the third portion of the duodenum (D3), and potentially the left renal vein between the aorta and SMA, because of narrowing of the angle between the SMA and the aorta due to a rapid change in body fat.5,6 Females and young adults (18–35 years) are more likely to be affected by the condition, though it can occur at any age.3,7,8
This compression of the duodenum causes an extrinsic partial or complete obstruction of the duodenum (D3), resulting in chronic food intolerance, bloating, nausea, eructation, abdominal discomfort, and voluminous bilious vomiting along with secondary features like AD and generalized spasms, as observed in our group of patients.9
However, confusion about the diagnosis develops, because many patients will continue to have good lower intestinal motility with bowel sounds and even bowel movements.10
A further challenge in diagnosing this condition in patients with SCI arises because they may have neurogenic bowel dysfunction resulting in similar symptoms. Diagnosis has been a problem historically, with a high index of suspicion necessary to proceed with proper workup and prompt identification.
The pain, if some sensation is preserved, is classically described as being relieved by lying prone or in the left lateral decubitus position, manoeuvres which release tension on the small bowel mesentery, and thus release the aortomesenteric angle. The patients are usually significantly underweight at the time of diagnosis.11
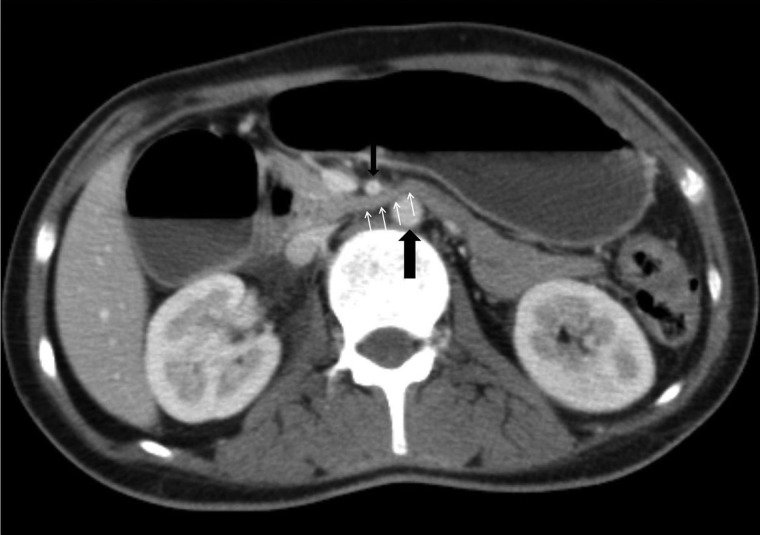
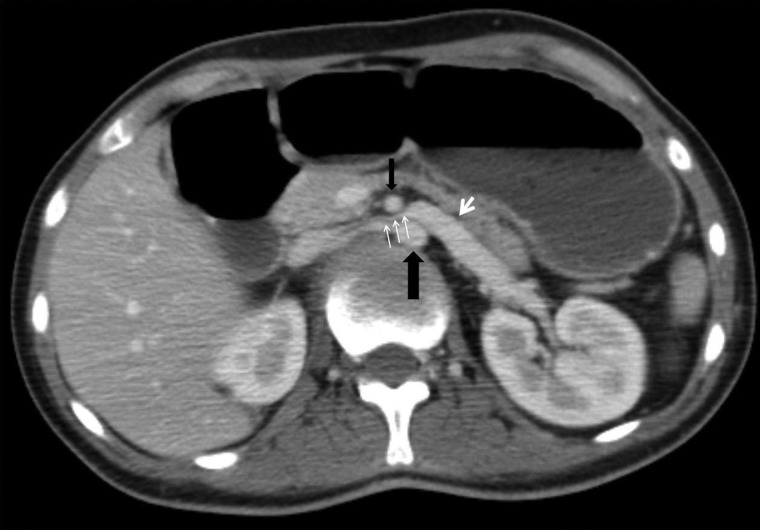
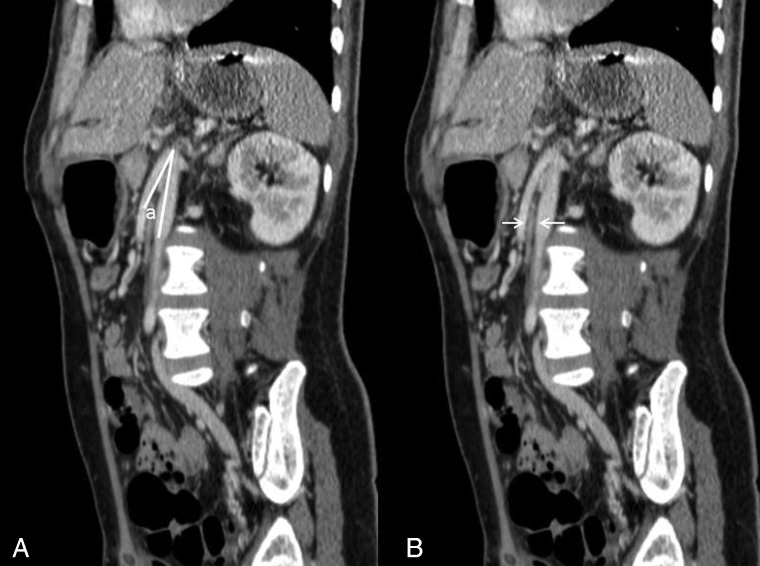
A high index of suspicion in at-risk patients with upper gastrointestinal symptoms will minimize delay in diagnosis and lead to early implementation of therapy.12 CT scan of the abdomen with contrast is the investigation of choice and could be arranged in any institution with standard radiological facilities. The vascular abnormalities are best delineated by the CT angiogram with three-dimensional reconstruction. SMA syndrome is characterized by several findings: compression of the duodenum between the abdominal aorta and SMA, dilation of the left renal vein, and distension of the stomach (Figs. 1 and 2). In normal individuals, the distance between the aorta and SMA (aortomesenteric distance) is 10–34 mm with an aortomesenteric angle of 28–65°.13 An aortomesenteric angle of <25° is regarded as the most sensitive measure of diagnosis, along with the aortomesenteric distance of less than 8 mm (Fig. 3). Endoscopic ultrasound with a miniprobe has also been used to show the pulsatile nature of the duodenal compression and the reduced aortomesenteric distance at the site of stenosis.7 The average aortomesenteric angle for our two patients was 20° and the aortomesenteric distance was 6 mm (Fig. 3).
Figure 1 .
Axial post intravenous contrast enhancement CT image demonstrating distension of the stomach and proximal duodenum leading to compression of the third part of the duodenum (small arrows) between the abdominal aorta (large black arrow) and the SMA (small black arrow).
Figure 2 .
Axial post intravenous contrast enhancement CT image demonstrating left renal vein compression (small white arrows) between the abdominal aorta (large black arrow) and the SMA (small black arrow) with precompression dilation (large white arrow) of the proximal left renal vein.
Figure 3 .
Oblique sagittal post intravenous contrast enhancement CT image demonstrating (A) aortomesenteric angle (a) approximating 18° and (B) aortomesenteric distance (white arrows) approximating 5 mm.
The “Nutcracker” phenomenon describes compression of the left renal vein between the aorta and the SMA at its origin. Affected patients may be asymptomatic or experience haematuria and pain, and a left-sided varicocoele may also be present.14 The differential diagnosis depends on the presentation as gastrointestinal complications are common.15 Motility disorders such as intestinal neuronal dysplasia type B and familial mega duodenum may have similar clinical features of SMA syndrome.11
Conservative treatment of SMA syndrome is usually successful and includes free gastric aspiration and drainage, feeding via a NJ tube, bypassing the obstruction, or total parenteral nutrition (TPN) in some cases aiming for high-calorie supplementation and rebuilding the mesenteric fat pad to relieve the obstruction.
Refractory cases may be treated surgically with mobilization of the duodenum by division of the ligament of Trietz, allowing the duodenum to fall away from the aorta (Strong's procedure),16 duodenojejunostomy,17 with or without division of the fourth part of the duodenum, and gastrojejunostomy.18
Duodenojejunostomy as a treatment for this condition was first described by Stavely in 190817 and is generally accepted as having superior results to both Strong's procedure and gastroenterostomy.
Conservative management for the “Nutcracker” phenomenon is often sufficient, but endovascular stenting is the treatment of choice if required.
Conclusion
SMA syndrome is a rare but serious complication after SCI. Risk factors include cervical spine injury, asthenic body habitus, and rapid weight loss. Clinical presentation may be non-specific initially with refractory AD and worsening spasticity or nausea but will eventually include symptoms and signs of upper gastrointestinal obstruction.
The key to management is the initial decompressive aspiration and a strategy for optimizing nutrition, which may require jejunal placement of the feeding tube distal to the obstruction and very rarely surgery.
Disclaimer statements
Contributors All three authors gave a substantial and sufficient contribution to the paper.
Funding None.
Conflicts of interest None.
Ethics approval Not required.
References
- 1.Geer MA. Superior mesenteric artery syndrome. Mil Med 1990;155(7):321–3. [PubMed] [Google Scholar]
- 2.Yon Rokitanski C. Lehrbuch der Pathologischen Anatomie. Vienna: Braumttller & Seidel; 1861. p. 187. [Google Scholar]
- 3.Wilkie DP. Chronic duodenal ileus. Am J Med Sci 1927;173:643–9. [Google Scholar]
- 4.Laffont I, Bensmail D, Rech C, Prigent G, Loubert G, Dizien O. Late superior mesenteric artery syndrome in paraplegia: case report and review. Spinal Cord 2002;40(2):88–91. [DOI] [PubMed] [Google Scholar]
- 5.Pedoto MJ, O'Dell MW, Thrun M, Hollifield D. Superior mesenteric artery syndrome in traumatic brain injury: two cases. Arch Phys Med Rehabil 1995;76(9):871–5. [DOI] [PubMed] [Google Scholar]
- 6.Barnes JB, Lee M. Superior mesenteric artery syndrome in an intravenous drug abuser after rapid weight loss. South Med J 1996;89(3):331–4. [DOI] [PubMed] [Google Scholar]
- 7.Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002;37(8):640–3. [DOI] [PubMed] [Google Scholar]
- 8.Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20-year experience. J Pediatr Gastroenterol Nutr 2006;42(5):522–5. [DOI] [PubMed] [Google Scholar]
- 9.Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am 2006;88(10):2252–7. [DOI] [PubMed] [Google Scholar]
- 10.Bunch W, Delaney J. Scoliosis and acute vascular compression of the duodenum. Surgery 1970;67(6):901–6. [PubMed] [Google Scholar]
- 11.Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg 2009;13(2):287–92. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery 2013;153(4):601–2. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal GA, Johnson PT, Fishman EK. Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol 2007;41(1):62–5. [DOI] [PubMed] [Google Scholar]
- 14.Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010;85(6):552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore RM, Mintzer RA, Calenoff L. Gastrointestinal complications of spinal cord injury. Spine (Phila PA 1976) 1981;6(6):538–44. [DOI] [PubMed] [Google Scholar]
- 16.Strong EK. Mechanics of arteriomesentric duodenal obstruction and direct surgical attack upon etiology. Ann Surg 1958;148(5):725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stavely AL. Acute and chronic gastromesenteric ileus with cure in a chronic case by duodenojejeunostomy. Bull Johns Hopkins Hosp 1908;19:252. [Google Scholar]
- 18.Lee CS, Mangla JC. Superior mesenteric artery compression syndrome. Am J Gastroenterol 1978;70(2):141–50. [PubMed] [Google Scholar]