Abstract
Background and study aims: Mallory-Weiss tears (MWTs) are not only a common cause of acute nonvariceal gastrointestinal bleeding but also an iatrogenic adverse event related to endoscopic procedures. However, changes in the clinical characteristics and endoscopic features of MWTs over the past decade have not been reported. The aim of this study was to investigate recent trends in the etiology and endoscopic features of MWTs.
Patients and methods: We retrospectively reviewed the medical records of patients with a diagnosis of MWT at our university hospital between August 2003 and September 2013. The information regarding etiology, clinical parameters, endoscopic findings, therapeutic interventions, and outcome was reviewed.
Results: A total of 190 patients with MWTs were evaluated. More than half (n = 100) of the cases occurred during endoscopic procedures; cases related to alcohol consumption were less frequent (n = 13). MWTs were most frequently located in the lesser curvature of the stomach and right lateral wall (2 – to 4-o’clock position) of the esophagus, irrespective of the cause. The condition of more than 90 % of the patients (n = 179) was improved by conservative or endoscopic treatment, whereas 11 patients (5.8 %) required blood transfusion. Risk factors for blood transfusion were a longer laceration (odds ratio [OR] 2.3) and a location extending from the esophagus to the stomach (OR 5.3).
Conclusions: MWTs were frequently found on the right lateral wall (2 – to 4-o’clock position) of the esophagus aligned with the lesser curvature of the stomach, irrespective of etiology. Longer lacerations extending from the esophagus to the gastric cardia were associated with an elevated risk for bleeding and requirement for blood transfusion.
Introduction
Mallory-Weiss tears (MWTs), characterized by mucosal lacerations in the region of the esophagogastric junction (EGJ), are a common cause of nonvariceal gastrointestinal bleeding. MWTs are usually caused by retching or forceful vomiting, and the reported incidence is as high as 15 % of all cases of acute upper gastrointestinal bleeding 1 2 3. MWTs have also been recognized as an iatrogenic adverse event occurring during upper gastrointestinal endoscopic procedures, initially documented in 1976 4. The incidence has been estimated to range from 0.007 % to 0.49 % of all endoscopic procedures 5 6 7. However, most of these findings were reported in the 1990 s, and no reports have been presented regarding changes in the clinical characteristics and endoscopic features of MWTs during a recent decade, although significant changes in the endoscopic management of upper gastrointestinal diseases have occurred.
Recent technologic advances in endoscopic imaging and treatment procedures, such as image-enhanced endoscopy and endoscopic submucosal dissection (ESD), have dramatically changed the clinical approaches to gastrointestinal tumors 8 9. These techniques are particularly attractive in the management of early cancer because they enable endoscopic resection and accurate staging, although a longer procedure time is generally required for precise evaluation and treatment of the lesions. Accordingly, case reports of MWTs occurring during endoscopic procedures have recently been increasing; these MWTs are likely due to excessive air inflation and/or longer procedure times 7 10.
The pathologic lesions that form in the EGJ show circumferential asymmetry, which is caused by the anatomical and physiologic properties of the gastrointestinal tract 11. In previous studies, we focused on the circumferential distribution of various lesions at the EGJ, including mucosal breaks in reflux esophagitis, ruptured esophageal varices, tongue-like Barrett’s esophagus, and Barrett's esophagus-associated neoplasia, and found that these lesions have a uniquely asymmetric circumferential distribution 12 13 14 15. MWTs have been reported to occur typically on the lesser curvature of the stomach and infrequently in the esophagus 16. However, it remains unclear whether MWTs that differ in pathogenesis occur in the same circumferential spatial locations. The aim of this study was to investigate recent trends in the etiology and endoscopic features of MWTs, with a focus on their circumferential distribution and location according to etiology, in patients treated within a recent decade.
Methods
Patients and data collection
We retrospectively reviewed the medical records of patients given a diagnosis of MWT between August 2003 and September 2013 at our university hospital, a tertiary referral medical center in western Japan. The diagnosis of MWT was based on the detection of mucosal lacerations of the esophagus or stomach adjacent to the EGJ during endoscopic inspection, with or without active bleeding. Patients with MWTs whose location could not be clearly shown by endoscopic imaging were excluded. Information about etiology, clinical parameters, associated co-morbidities, endoscopic findings, therapeutic interventions, and outcome was collected.
The study protocol was approved by the ethics committee of the Shimane University School of Medicine, and the study was carried out in accordance with the principles of the Helsinki Declaration.
Assessment of endoscopic findings
The endoscopic findings were assessed by a single expert endoscopist (M.O.). Based on their location, the MWTs were divided into three groups according to Zeifer’s classification: group I, lacerations only in the esophagus; group II, lacerations limited to the stomach; group III, lacerations extending from the esophagus across the cardia into the stomach (Fig. 1) 17. The number and location of the mucosal lacerations, and the length of the MWTs, were recorded. When multiple lacerations were present in a single patient, the circumferential and longitudinal locations of all the lacerations were counted.
Fig. 1.
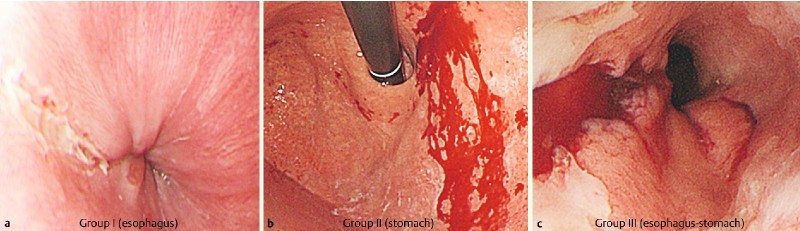
Based on their location, the Mallory-Weiss tears were divided into three groups according to Zeifer’s classification: group I, lacerations only in the esophagus (a); group II, lacerations limited to the stomach (b); group III, lacerations extending from the esophagus across the cardia into the stomach (c).
The circumferential distribution of MWTs in the esophageal wall was evaluated based on the face of a clock, as follows. The anterior wall of the esophagus was always positioned at 12 o’clock, and the 3-o’clock position was defined as the right lateral wall of the esophagus aligned with the lesser curvature of the stomach. MWTs from a cardiac lesion of the stomach were also assessed with the same axial position of the endoscope. Representative endoscopic images for the evaluation of circumferential distribution are shown in Fig. 2. A laceration related to an endoscopic procedure was diagnosed at the end of the procedure. When an MWT with gastric bleeding was suspected during endoscopic retrograde cholangiopancreatography (ERCP) with a side-viewing endoscope, we changed to a forward-viewing endoscope to analyze the entire circumference of the cardia and esophagus.
Fig. 2.
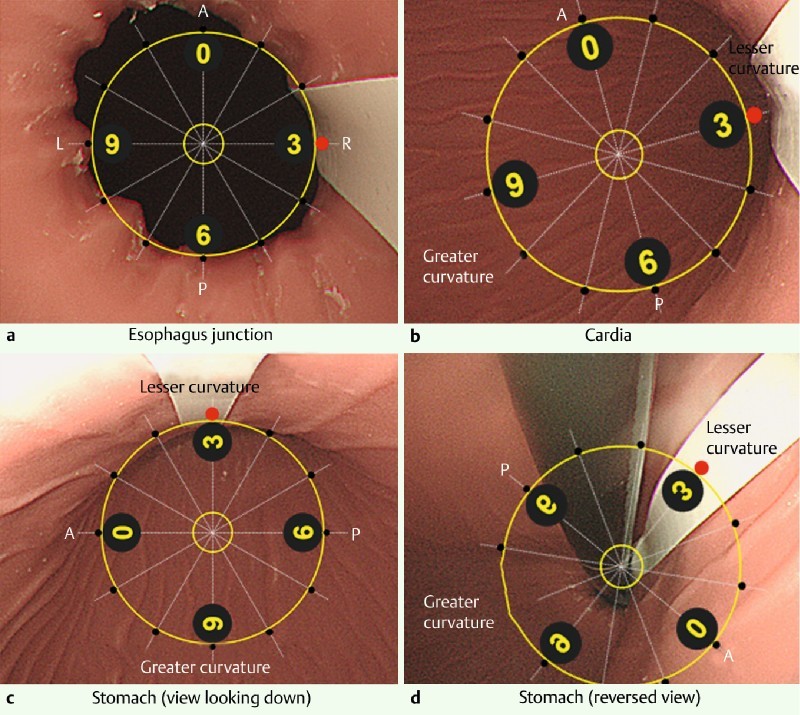
Definition of circumferential distribution at the esophagogastric junction as shown by endoscopy. Representative endoscopic images for the evaluation of circumferential distribution are shown in an upper gastrointestinal model. The 3-o’clock position (red closed circle) was defined as the right lateral wall of the esophagus aligned with the lesser curvature of the stomach, as indicated by the white lines. a Esophagogastric junction. b Cardia. c Stomach (view looking down). d Stomach (reversed view). A, anterior wall; P, posterior wall; R, right lateral wall; L, left lateral wall.
An esophageal hiatal hernia was defined as the presence of a part of the gastric wall above the diaphragmatic hiatus that was unaccompanied by underlying longitudinally arrayed vessels, as previously described 18. The presence of gastric mucosal atrophy was assessed based on endoscopic findings.
Risk factors for a complicated course in Mallory-Weiss tear
A complicated clinical course was defined as one in which a blood transfusion was required. The decision whether to treat with blood transfusion was based on the judgment of the clinician and depended on the patient's underlying condition and clinical presentation. A blood transfusion was generally administered once the hemoglobin level dropped below 7 g/dL. In patients with active bleeding or exposed vessels, endoscopic hemostasis was performed. Risk factors predicting a complicated course in MWT were evaluated with multivariate analysis.
Statistical analysis
Chi-squared and Mann-Whitney U tests were used to examine significant differences. Comparisons among the three groups were performed with a Kruskal-Wallis test. After the identification of significant predictors by univariate analysis, multivariate logistic regression analysis was performed to evaluate independent predictors of a complicated course that required a blood transfusion; then, we determined relative risks as an OR with a 95 % confidence interval (CI). A P value below 0.05 was considered to indicate statistical significance. All statistical analyses were performed with SPSS Statistics Desktop Version 22.0 (IBM, Armonk, New York, USA).
Results
Patient profiles
In the period between August 2003 and September 2013, 217 patients received a diagnosis of MWT; of these, 190 were finally enrolled in this study and 27 were excluded because their mucosal lacerations could not be assessed on endoscopic images. Of the 190 patients, 135 were male and 65 were female, for a male-to-female ratio of 2.1:1, and their age (mean ± SD) was 66.7 ± 16.1 years (range 7 – 94). Co-morbidities in the enrolled patients included malignant disease (n = 46), peptic ulcer (n = 30), liver disease (n = 17), heart disease (n = 8), and cerebrovascular disease (n = 6). Oral antiplatelet drugs and/or anticoagulants were administered to 32 patients (16.8 %). Of the 140 patients noted to have an alcohol drinking habit, 55 (39.3 %) were regular drinkers and 4 had a history of chronic alcoholism.
Etiology
In 100 of the patients (52.6 %), lacerations developed during endoscopic procedures as an iatrogenic adverse event. During the study period, 25,549 endoscopic examinations were performed at our institution. Of the 100 cases of iatrogenic MWT, most (n = 82) occurred during diagnostic endoscopy rather than therapeutic endoscopy (n = 18) (Table 1). The incidence of MWT for each endoscopic procedure is shown in Table 2. The incidence of MWT during therapeutic endoscopy, especially ESD, was much higher than the incidence during diagnostic endoscopy, as previously reported 5 6 7. There were no significant associations between the incidence of MWT and endoscopic expertise (data not shown). As for patient position, ERCP and double-balloon enteroscopy (DBE) were performed with the patient in a prone position, whereas others, including esophagogastroduodenoscopy (EGD) and ESD, were performed with the patient in a left lateral decubitus position (Table 3). In all patients undergoing ERCP, endoscopic ultrasonography (EUS), DBE, ESD, endoscopic mucosal resection (EMR), or endoscopic variceal ligation (EVL), sedation was administered with intravenous midazolam. Most of the patients undergoing EGD did not receive sedation (n = 69), but 5 patients did request and receive sedation. Because the number of patients in a prone position was small (n = 10), the relationship between patient position and risk for MWT could not be evaluated.
Table 1. Etiology of Mallory-Weiss tears.
| n | % | |
| Related to endoscopic procedure | 100 | 52.6 |
| Diagnostic endoscopy | ||
| EGD | 74 | 38.9 |
| ERCP | 4 | 2.1 |
| EUS | 3 | 1.6 |
| Upper DBE | 1 | 0.5 |
| Therapeutic endoscopy | ||
| ESD | 11 | 5.8 |
| ERCP | 5 | 2.6 |
| EMR | 1 | 0.5 |
| EVL | 1 | 0.5 |
| Other causes | 90 | 47.4 |
| Vomiting | 44 | 23.2 |
| Coughing | 1 | 0.5 |
| No preceding history of vomiting | 45 | 23.7 |
| Total | 190 | 100 |
EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; DBE, double-balloon enteroscopy; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection; EVL, endoscopic variceal ligation.
Table 2. Incidence of Mallory-Weiss tears (MWTs) during endoscopic procedures.
| Endoscopic procedure | Total number of cases | Cases with MWTs, n | Incidence, % |
| EGD | 21 060 | 74 | 0.35 |
| ERCP | 1 873 | 9 | 0.48 |
| EUS | 1 717 | 3 | 0.17 |
| Upper DBE | 40 | 1 | 2.50 |
| ESD | 424 | 11 | 2.59 |
| EMR | 303 | 1 | 0.33 |
| EVL | 132 | 1 | 0.76 |
| Total | 25 549 | 100 | 0.39 |
EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; DBE, double-balloon enteroscopy; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection; EVL, endoscopic variceal ligation.
Table 3. Conditions associated with Mallory-Weiss tears (MWTs) during endoscopic procedures.
| Endoscopic procedure | Patients with MWT | Patient position | Sedation with midazolam, n | Median time (range), min |
| EGD | 74 | Left | ||
| Screening | 57 | Left | 0 | 10 (3 – 24) |
| Further examination | 17 | Left | 5 | 32.5 (9 – 75) |
| ERCP | 9 | Prone | 9 | 59 (25 – 90) |
| EUS | 3 | Left | 3 | 55 (26 – 63) |
| Upper DBE | 1 | Prone | 1 | 49 |
| ESD | 11 | Left | 11 | 112 (33 – 368) |
| EMR | 1 | Left | 1 | 60 |
| EVL | 1 | Left | 1 | 6 |
| Total | 100 |
EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; DBE, double-balloon enteroscopy; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection; EVL, endoscopic variceal ligation
The remaining 90 patients presented to our emergency unit with hematemesis, and MWT was diagnosed based on endoscopic inspection. The incidence of MWT was 9.3 % in all patients with acute upper gastrointestinal bleeding during this period. Half of the patients (n = 45) did not have a preceding history of retching or vomiting, and there were 13 with alcohol-induced vomiting.
The clinical and endoscopic characteristics of the patients with and without iatrogenic MWT are shown in Table 4. The mean age was significantly higher in the patients with iatrogenic MWT. Most patients had a single laceration, and multiple lacerations were rare among the patients with iatrogenic MWT (P = 0.02). Localized gastric lesions were more frequent in the patients with endoscopic procedure-related MWT, whereas esophagogastric lesions were more frequent in the patients with other types of MWT (P < 0.001). Lacerations tended to be located in the right lateral wall (2 – to 4-o’clock position) in both the patients with iatrogenic MWT and those with MWT of other causes (Fig. 3).
Table 4. Characteristics of patients with Mallory-Weiss tears.
| Patients with tears related to endoscopic procedure (n = 100) | Patients with tears with other causes (n = 90) | P value | |||
| Age, mean ± SD, y | 69.2 ± 14.7 | 64.0 ± 16.8 | 0.02 | ||
| Male, n (%) | 65 (65.0) | 70 (77.8) | 0.05 | ||
| Regular drinker, n (%) | 26 (26.0) | 29 (32.2) | 0.07 | ||
| Antithrombotic drug, n (%) | 13 (13.0) | 19 (21.1) | 0.65 | ||
| Atrophic gastritis, n (%) | 58 (58.0) | 47 (52.2) | 0.54 | ||
| Hiatal hernia, n (%) | 52 (52.0) | 55 (61.0) | 0.18 | ||
| Multiple lacerations, n (%) | 15 (15.0) | 26 (28.9) | 0.02 | ||
| Length, mean ± SD, cm | 0.9 ± 0.6 | 0.9 ± 0.6 | 0.99 | ||
| Location (esophagus/stomach/both) | 16/67/17 | 13/36/41 | < 0.001 | ||
SD, standard deviation.
Fig. 3.
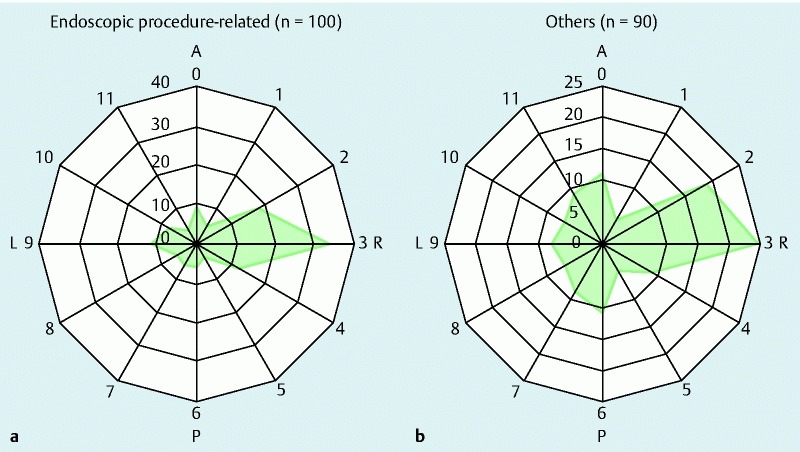
Circumferential distribution of Mallory-Weiss tears in patients (a) with and (b) without endoscopic procedures. Mallory-Weiss tears were frequently located in the right lateral wall (2- to 4-o’clock position) at the esophagogastric junction in both groups. A, anterior wall; P, posterior wall; R, right lateral wall; L, left lateral wall.
Characteristics of Mallory-Weiss tears
MWTs were most frequently located in the stomach (84.7 %), with only 15.3 % (n = 29) located in the esophagus (Table 5). The presence of atrophic gastritis or a hiatal hernia was not associated with MWT location.
Table 5. Characteristics of Mallory-Weiss tears.
| Location of Mallory-Weiss tear | P value | ||||||
| I, esophagus (n = 29) | II, stomach (n = 103) | III, esophagus-stomach (n = 58) | |||||
| Age, mean ± SD, y | 64.7 ± 16.1 | 69.4 ± 15.5 | 62.9 ± 16.7 | 0.02 | |||
| Male, n (%) | 20 (69.0) | 67 (65.0) | 48 (82.8) | 0.06 | |||
| Atrophic gastritis, n (%) | 13 (44.8) | 58 (56.3) | 34 (58.6) | 0.27 | |||
| Hiatal hernia, n (%) | 14 (48.3) | 57 (55.3) | 36 (62.0) | 0.39 | |||
| Multiple lacerations, n (%) | 4 (13.8) | 15 (14.6) | 22 (37.9) | < 0.01 | |||
| Laceration length, mean ± SD, cm | 0.77 ± 0.53 | 1.0 ± 0.71 | 0.91 ± 0.53 | 0.11 | |||
SD, standard deviation.
The circumferential distribution of mucosal lacerations in each group was assessed (Fig. 4). A total of 243 lacerations were found in 190 patients. MWTs were frequently located in the right lateral wall (2 – to 4-o’clock position) in group I (esophagus). Likewise, the location of most tears in group II (stomach) and group III (esophagus-stomach) was the lesser curvature (3-o’clock position), indicating that mucosal lacerations occurred mainly in the right side of the esophagus aligned with the lesser curvature of the stomach. Next, we focused on the relationship between the MWT location and the presence of hiatal hernia. The frequency of hiatal hernia was not associated with the MWT location (Table 5). In addition, the circumferential distribution of MWT did not differ significantly between the group with and the group without hiatal hernia (data not shown).
Fig. 4.
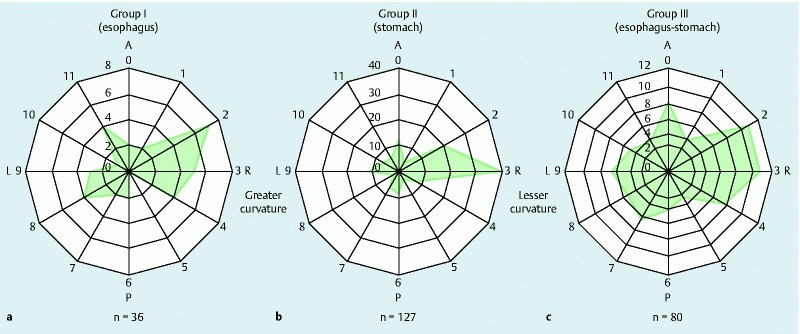
Circumferential distribution by location of mucosal lacerations at the esophagogastric junction. a Group I (esophagus). b Group II (stomach). c Group III (esophagus-stomach). Mucosal lacerations were frequently located in the right lateral wall (2- o 4-o’clock position) at the esophagogastric junction in all groups. A, anterior wall; P, posterior wall; R, right lateral wall; L, left lateral wall.
Clinical course
Approximately 70 % of the patients (n = 130) were managed conservatively without undergoing any endoscopic hemostatic procedures, whereas endoscopic hemostasis was performed in the other 60 patients. The modalities used to achieve endoscopic hemostasis included hemoclip placement, saline plus epinephrine injection, argon plasma coagulation, thrombin spray, and placement of a Sengstaken – Blakemore tube (Table 6). Only 11 patients (5.8 %) required a blood transfusion, 3 of whom had iatrogenic WMT and 8 of whom had WMT with other causes. Recurrent bleeding during the same hospital stay was observed in 6 patients (3.2 %). No patient required surgical treatment, and none had a fatal outcome.
Table 6. Treatment of Mallory-Weiss tears.
| Treatment | n (%) |
| Conservative management | 130 (68.4) |
| Endoscopic hemostasis | 60 (31.6) |
| Hemoclip placement | 41 (21.6) |
| Hemoclip placement + thrombin spray | 9 (4.7) |
| Thrombin spray | 8 (4.2) |
| Argon plasma coagulation | 1 (0.5) |
| Hemoclip placement + SB tube + saline + epinephrine injection | 1 (0.5) |
| Total | 190 (100) |
SB tube, Sengstaken – Blakemore tube.
When the risk factors for requiring a blood transfusion were investigated, the absence of a hiatal hernia, a longer laceration, and a lesion extending from the esophagus to the stomach were found to be statistically significant on univariate analysis. In multivariate analysis, a longer laceration (OR 2.3, 95 %CI 1.1 – 5.1) and a lesion extending from the esophagus to the stomach (OR 5.3, 95 %CI 1.4 – 19.9) remained significant risk factors.
Discussion
The occurrence of MWT during an endoscopic examination as an iatrogenic adverse event has been increasingly recognized over the past decade. Both diagnostic and therapeutic endoscopy examinations are known as possible causes of MWT. In the present study, 11 cases of MWT were found in a total of 424 patients undergoing ESD, for an incidence of 2.59 %, which is approximately 7-fold higher than the incidence during standard diagnostic endoscopy procedures (0.35 %). Kawano et al. reported a similar incidence of MWT (3.4 %) during ESD procedures 19. That study suggested that a low body mass index (BMI) (< 18.5) is a risk factor for endoscopy-related MWT because patients with a low BMI may be more strongly affected by air insufflation and excessive pushing of the scope during the procedure. Recently, insufflation with carbon dioxide (CO2) during gastric ESD has been increasingly used to avoid adverse events, but its use to reduce the frequency of MWT during ESD remains controversial 10 20. Adjustment of the intragastric pressure during ESD may be more useful and effective to avoid this kind of adverse event 21.
Non-iatrogenic MWTs accounted for 9.3 % of the cases of acute upper gastrointestinal bleeding during the study period. These findings are comparable with those of previous reports 1 3, which together suggest that the frequency of MWT in patients with acute upper gastrointestinal bleeding has not changed over the past decade; however, the proportion of cases caused by alcohol abuse in this study was significantly lower than that in previous studies 16 22. According to a Japanese national health and nutrition examination survey, alcohol consumption has not changed over the past decade. On the other hand, the prevalence of Helicobacter pylori infection has decreased significantly over recent decades in Japan as a consequence of improved sanitation and the reduced chance of infection during childhood 23. In addition, H. pylori eradication therapy for atrophic gastritis has been approved by the Japanese health insurance regulating committee. The decrease in H. pylori infection is related to a decrease in peptic ulcer disease and bleeding ulcers. Therefore, MWT still remains a common cause of acute upper gastrointestinal bleeding despite the decrease in alcohol consumption-related MWT.
In the present study, consistent with previous reports, MWTs, irrespective of cause, were most frequently located in the lesser curvature of the stomach and the right lateral wall (2- to 4-o’clock position) of the esophagus, which are coaxial positions. Although the mechanism of MWT that occurs at these circumferential positions has not been clarified, it appears to be closely associated with the anatomical and physiologic properties of the EGJ. The EGJ is a complex valvular structure that controls bolus entry into the stomach and gastric reflux into the esophagus. Among the components of EGJ function, the lower esophageal sphincter (LES) is an important anti-reflux barrier. The LES is not a muscular ring; rather, it is anatomically represented by a combination of gastric sling fibers and semicircular clasp fibers. Muscle thickness at the EGJ is greatest toward the side of the greater curvature, corresponding to the gastric oblique fibers, and least toward the side of the lesser curvature, corresponding to the semicircular fibers 24 25.
External compression by surrounding organs, such as the diaphragm, aorta, and fornix of the stomach, is another important factor related to EGJ function. Intraluminal manometry has helped to reveal EGJ physiology, and previous reports have indicated that circumferential intraesophageal pressure at the LES is asymmetric – highest at the left posterior side and lowest at the right anterior wall 26. Collectively, the asymmetric LES pressure produced by the combination of intrinsic contractions of the circular muscle layer of the LES and extramural compression is thought to result in the asymmetric circumferential distribution of various diseases occurring in the area of the EGJ.
Indeed, we reported that distal esophageal lesions, such as mucosal breaks in low grade reflux esophagitis, tongue-like short-segment Barrett's esophagus, and Barrett's esophagus-associated neoplasia, were most frequently found in the right side of the distal esophagus 12 13 15. Moreover, our initial findings were soon confirmed by studies conducted later in Western countries 27 28. The predominantly right-sided distribution of these diseases may be due to a longer period of acid exposure of the right lateral wall of the esophagus, caused by asymmetric LES pressure generated by the thinner right wall of the distal esophagus 29. Such asymmetric LES pressure may also cause the occurrence of MWT predominantly in the right lateral wall of the EGJ. In addition, mucosal fragility caused by persistent gastric acid reflux at the right lateral wall may decrease resistance to an abrupt rise in intraesophageal pressure 30. Awareness of the tendency of lesions to form in the right lateral wall in the area of the EGJ can help clinicians to detect lesions within a shorter observation time during endoscopic procedures.
In addition to the circumferential distribution and location of MWTs, we assessed the risk factors for a complicated course in patients with MWT. Because no patient required surgical treatment and none had a fatal outcome, we defined a complicated clinical course as one in which a blood transfusion was required. Consistently, a longer laceration (OR 2.3) and a lesion extending from the esophagus to the stomach (OR 5.3) remained significant risk factors in multivariate analysis. It is important to note that etiology (iatrogenic or non-iatrogenic cause) was not associated with risk for a complicated course. With all these factors considered together, an endoscopic hemostatic procedure, such as the placement of a hemoclip, should be considered when a longer laceration extending from the esophagus to the gastric cardia is found, irrespective of etiology.
There are some limitations to the present findings. This was a retrospective study conducted at a single tertiary referral center, which is also an educational and training facility. Furthermore, the circumferential directions of the esophageal and gastric lesions were determined in a retrospective manner based on recorded endoscopic photographs. Despite these shortfalls, our results show the recent trend of MWTs and provide useful information regarding their endoscopic features. The risk factors for the development of iatrogenic MWTs in each type of endoscopic procedure should be clarified in more detail in the future.
In summary, MWTs were frequently found in the right lateral wall (2- to-4 o’clock position) of the esophagus aligned with the lesser curvature of the stomach, irrespective of etiology. Longer lacerations extending from the esophagus to the gastric cardia were associated with a higher risk of bleeding and requirement for blood transfusion.
Footnotes
Competing interests: None
References
- 1.Akhtar A J, Padda M S. Natural history of Mallory-Weiss tear in African American and Hispanic patients. J Natl Med Assoc. 2011;103:412–415. doi: 10.1016/s0027-9684(15)30338-2. [DOI] [PubMed] [Google Scholar]
- 2.Ljubicic N, Budimir I, Pavic T. et al. Mortality in high-risk patients with bleeding Mallory-Weiss syndrome is similar to that of peptic ulcer bleeding. Results of a prospective database study. . Scand J Gastroenterol. 2014;49:458–464. doi: 10.3109/00365521.2013.846404. [DOI] [PubMed] [Google Scholar]
- 3.Sugawa C Steffes C P Nakamura R et al. Upper GI bleeding in an urban hospital. Etiology, recurrence, and prognosis Ann Surg 1990212521–526.discussion 526-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts H D. Mallory-Weiss syndrome occurring as a complication of endoscopy. Gastrointest Endosc. 1976;22:171–172. doi: 10.1016/s0016-5107(76)73737-4. [DOI] [PubMed] [Google Scholar]
- 5.Haddad N, Al-Kawas F, Benjamin S. et al. Incidence and natural history of iatrogenic Mallory-Weiss tears during upper gastrointestinal endoscopy. Am J Gastroenterol. 1993;88:1592. [Google Scholar]
- 6.Montalvo R D, Lee M. Retrospective analysis of iatrogenic Mallory-Weiss tears occurring during upper gastrointestinal endoscopy. Hepatogastroenterology. 1996;43:174–177. [PubMed] [Google Scholar]
- 7.Penston J G, Boyd E J, Wormsley K G. Mallory-Weiss tears occurring during endoscopy: a report of seven cases. Endoscopy. 1992;24:262–265. doi: 10.1055/s-2007-1009122. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Esparrach G, Calderon A, de la Pena J. et al. Endoscopic submucosal dissection. Endoscopy. 2014;46:361–370. doi: 10.1055/s-0034-1364921. [DOI] [PubMed] [Google Scholar]
- 9.Hayee B, Inoue H, Sato H. et al. Magnification narrow-band imaging for the diagnosis of early gastric cancer: a review of the Japanese literature for the Western endoscopist. Gastrointest Endosc. 2013;78:452–461. doi: 10.1016/j.gie.2013.03.1333. [DOI] [PubMed] [Google Scholar]
- 10.Hongou H, Fu K, Ueyama H. et al. Mallory-Weiss tear during gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2011;3:151–153. doi: 10.4253/wjge.v3.i7.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita Y, Furuta K, Adachi K. et al. Asymmetrical circumferential distribution of esophagogastric junctional lesions: anatomical and physiological considerations. J Gastroenterol. 2009;44:812–818. doi: 10.1007/s00535-009-0092-0. [DOI] [PubMed] [Google Scholar]
- 12.Katsube T, Adachi K, Furuta K. et al. Difference in localization of esophageal mucosal breaks among grades of esophagitis. J Gastroenterol Hepatol. 2006;21:1656–1659. doi: 10.1111/j.1440-1746.2006.04297.x. [DOI] [PubMed] [Google Scholar]
- 13.Moriyama N, Amano Y, Okita K. et al. Localization of early-stage dysplastic Barrett's lesions in patients with short-segment Barrett's esophagus. Am J Gastroenterol. 2006;101:2666–2667. doi: 10.1111/j.1572-0241.2006.00809_5.x. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto E, Amano Y, Fukuhara H. et al. Does gastroesophageal reflux have an influence on bleeding from esophageal varices? J Gastroenterol. 2008;43:803–808. doi: 10.1007/s00535-008-2232-3. [DOI] [PubMed] [Google Scholar]
- 15.Okita K, Amano Y, Takahashi Y. et al. Barrett's esophagus in Japanese patients: its prevalence, form, and elongation. J Gastroenterol. 2008;43:928–934. doi: 10.1007/s00535-008-2261-y. [DOI] [PubMed] [Google Scholar]
- 16.Sugawa C, Benishek D, Walt A J. Mallory-Weiss syndrome. A study of 224 patients. . Am J Surg. 1983;145:30–33. doi: 10.1016/0002-9610(83)90162-9. [DOI] [PubMed] [Google Scholar]
- 17.Zeifer H D. Mallory-Weiss syndrome. Ann Surg. 1961;154:956–960. [PMC free article] [PubMed] [Google Scholar]
- 18.Amano K, Adachi K, Katsube T. et al. Role of hiatus hernia and gastric mucosal atrophy in the development of reflux esophagitis in the elderly. J Gastroenterol Hepatol. 2001;16:132–136. doi: 10.1046/j.1440-1746.2001.02408.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawano K, Nawata Y, Hamada K. et al. Examination about the Mallory-Weiss tear as the accident of ESD [in Japanese with English abstract] Gastroenterol Endosc. 2012;54:1443–1450. [Google Scholar]
- 20.Maeda Y, Hirasawa D, Fujita N. et al. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy. 2013;45:335–341. doi: 10.1055/s-0032-1326199. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Nakajima K, Yamada T. et al. Esophageal submucosal dissection under steady pressure automatically controlled endoscopy (SPACE): a clinical feasibility study. Endoscopy. 2014;46:680–684. doi: 10.1055/s-0034-1365465. [DOI] [PubMed] [Google Scholar]
- 22.Kortas D Y, Haas L S, Simpson W G. et al. Mallory-Weiss tear: predisposing factors and predictors of a complicated course. Am J Gastroenterol. 2001;96:2863–2865. doi: 10.1111/j.1572-0241.2001.04239.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima S, Nishiyama Y, Yamaoka M. et al. Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J Gastroenterol Hepatol. 2010;25:99–S110. doi: 10.1111/j.1440-1746.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- 24.Jackson A J. The spiral constrictor of the gastroesophageal junction. Am J Anat. 1978;151:265–275. doi: 10.1002/aja.1001510208. [DOI] [PubMed] [Google Scholar]
- 25.Liebermann-Meffert D, Allgower M, Schmid P. et al. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- 26.Kwiatek M A, Pandolfino J E, Kahrilas P J. 3D-high resolution manometry of the esophagogastric junction. Neurogastroenterol Motil. 2011;23:e461–e469. doi: 10.1111/j.1365-2982.2011.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enestvedt B K, Lugo R, Guarner-Argente C. et al. Location, location, location: does early cancer in Barrett's esophagus have a preference? Gastrointest Endosc. 2013;78:462–467. doi: 10.1016/j.gie.2013.03.167. [DOI] [PubMed] [Google Scholar]
- 28.Kariyawasam V C, Bourke M J, Hourigan L F. et al. Circumferential location predicts the risk of high-grade dysplasia and early adenocarcinoma in short-segment Barrett's esophagus. Gastrointest Endosc. 2012;75:938–944. doi: 10.1016/j.gie.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 29.Ohara S, Furuta K, Adachi K. et al. Radially asymmetric gastroesophageal acid reflux in the distal esophagus: examinations with novel pH sensor catheter equipped with 8 pH sensors. J Gastroenterol. 2012;47:1221–1227. doi: 10.1007/s00535-012-0595-y. [DOI] [PubMed] [Google Scholar]
- 30.Edebo A, Vieth M, Tam W. et al. Circumferential and axial distribution of esophageal mucosal damage in reflux disease. Dis Esophagus. 2007;20:232–238. doi: 10.1111/j.1442-2050.2007.00678.x. [DOI] [PubMed] [Google Scholar]


