SUMMARY
The mechanisms underlying human natural killer (NK) cell phenotypic and functional heterogeneity are unknown. Here, we describe the emergence of diverse subsets of human NK cells selectively lacking expression of signaling proteins after human cytomegalovirus (HCMV) infection. The absence of B and myeloid cell-related signaling protein expression in these NK cell subsets correlated with promoter DNA hyperme-thylation. Genome-wide DNA methylation patterns were strikingly similar between HCMV-associated adaptive NK cells and cytotoxic effector T cells but differed from those of canonical NK cells. Functional interrogation demonstrated altered cytokine responsiveness in adaptive NK cells that was linked to reduced expression of the transcription factor PLZF. Furthermore, subsets of adaptive NK cells demonstrated significantly reduced functional responses to activated autologous T cells. The present results uncover a spectrum of epigenetically unique adaptive NK cell subsets that diversify in response to viral infection and have distinct functional capabilities compared to canonical NK cell subsets.
INTRODUCTION
Natural killer (NK) cells are lymphocytes that act at the interface between innate and adaptive immunity (Vivier et al., 2011). Target-cell-mediated activation of NK cells can lead to eradication of virus-infected and neoplastic cells by directed release of cytotoxic granules as well as production of cytokines, including interferon-γ (IFN-γ) and tumor necrosis factor (TNF). Aside from such cytotoxic and pro-inflammatory functions, NK cells can fine-tune adaptive immune responses and maintain immune homeostasis, e.g., through killing of antigen-presenting cells or activated T cells (Crouse et al., 2014; Ferlazzo et al., 2002; Waggoner et al., 2012; Xu et al., 2014). Additionally, NK cells produce IFN-γ in response to combinations of exogenous cytokines such as interleukin-2 (IL-2), IL-12, IL-15, and IL-18 (Caligiuri, 2008).
Unlike the activation of adaptive T and B lymphocytes, which is dictated by somatically recombined, clonally distributed antigen receptors, NK cell activation is controlled by a multitude of activating and inhibitory germline-encoded receptors (Long et al., 2013). Most activating NK cell receptors are expressed on the majority of NK cells. These include NKp30, NKp46, NKp80, signaling lymphocyte activation molecule (SLAM) family receptors such as 2B4, CRACC, and NTB-A, as well as DNAM-1 and NKG2D. These receptors recognize ligands expressed on stressed, transformed, and proliferating cells (Bryceson et al., 2006). In contrast, activating NKG2C and killer cell immunoglobulin-like receptors (KIRs) display variegated expression on NK cell subsets and are encoded by rapidly evolving gene complexes (Khakoo et al., 2000; Valiante et al., 1997). Notably, NK cell responses to receptor engagement are remarkably heterogeneous within a donor population and between individuals.
Developmentally, as well as at the transcriptional level, NK cells are most closely related to cytotoxic T lymphocytes (CTLs) (Bezman et al., 2012). Activation through T and B lymphocyte antigen receptors is instigated upon phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM)-containing cytoplasmic domains and further propagated by two different sets of structurally homologous signaling machineries (Weiss and Littman, 1994). NK cells express not only canonical T but also homologous B and myeloid cell signaling proteins. Hypothetically, modulation of seemingly redundant signaling protein expression could alter signaling properties upon NK cell differentiation, thereby fine tuning activation thresholds and effector responses.
Heterogeneity in NK cell differentiation and function is a topic of growing interest. Among CD3−CD56dim NK cells, loss of CD62L, acquisition of CD57, and expression of inhibitory receptors for self-major histocompatibility complex (MHC) class I correlate with an increased capacity to degranulate and produce cytokines upon target cell engagement (Anfossi et al., 2006; Björkström et al., 2010; Juelke et al., 2010). Subsets of NK cells can also display adaptive immune features including robust recall responses (Sun et al., 2009). In humans, infection with human cytomegalovirus (HCMV) as well as other viruses is associated with lasting expansions of NK cell subsets expressing NKG2C or activating KIRs (Béziat et al., 2013; Gumá et al., 2004). Such expansions occur in response to acute infection or reactivation of latent virus (Foley et al., 2012; Lopez-Vergès et al., 2011) and might, in the case of HCMV, provide protective immunity (Kuijpers et al., 2008; Sun et al., 2009). At the molecular level, however, it is not clear how surface receptor expression and cellular responsiveness is modulated during NK cell differentiation or in response to viral infection. Moreover, specific markers of NK cells responding to infection have not been established.
Here, we identified subsets of human NK cells selectively lacking expression of B-cell- and myeloid-cell-related signaling proteins along with reduced expression of the transcription factor promyelocytic leukemia zinc finger (PLZF). Such subsets arose in response to HCMV infection and displayed an adaptive NK cell surface receptor phenotype. These cells exhibited altered functional responses through activating receptors in response to exogenous cytokine stimulation and upon co-culture with activated autologous T cells. Comparative analysis of NK cell and CD8+ T cell subsets uncovered genome-wide epigenetic differences among NK cell subsets, with adaptive NK cell differentiation paralleling that of CTLs. Given the critical importance of NK cells in controlling herpesvirus infections (Biron et al., 1989) and reports demonstrating an association between HCMV and reduced leukemia relapse risk (Green et al., 2013; Ito et al., 2013) that correlates with the expansion of adaptive NK cells (Foley et al., 2012), the findings presented here advance our understanding of the role of NK cells in viral immunity as well as cancer control.
RESULTS
Subsets of CD56dim NK Cells Devoid of FcɛRγ, SYK, and EAT-2 Are Induced by HCMV Infection
To gain insight into mechanisms underlying heterogeneity in NK cell responsiveness, we examined expression of homologous pairs of the transmembrane adaptor proteins CD3ζ and FcɛRγ, the tyrosine kinases ZAP-70 and SYK, and the intracellular adaptors SAP and EAT-2 in peripheral blood mononuclear cells (PBMCs). As expected, T cells expressed CD3ζ, ZAP-70, and SAP, B cells expressed SYK, myeloid cells expressed FcɛRγ, SYK, and EAT-2, and NK cells expressed all six proteins (Figure 1A). In a cohort of 196 healthy adults, subsets of CD3−CD56dim NK cells from many individuals lacked FcɛRγ, SYK, and EAT-2 expression, correlating with HCMV seropositivity but not with age or sex (Figures 1B–1D and S1). By comparison, CD3γ, ZAP-70, and SAP expression was unchanged (Figures 1B–1D). Within the total CD3−CD56dim NK cell population, the size of the CD3−CD56dim FcɛRγ− NK cell subset was generally greater than the SYK− or EAT-2− subsets (Figure 1E). In total, the lack of at least one signaling protein was observed in 10.1% and 50.4% of HCMV-seronegative (HCMV−) and HCMV-seropositive donors (HCMV+), respectively (Figure 1F), suggesting HCMV-induced modulation of NK cell signaling.
Figure 1. The Presence of NK Cell Subsets Devoid of FcɛRγ, SYK, and EAT-2 Expression Is Associated with Cytomegalovirus Infection.
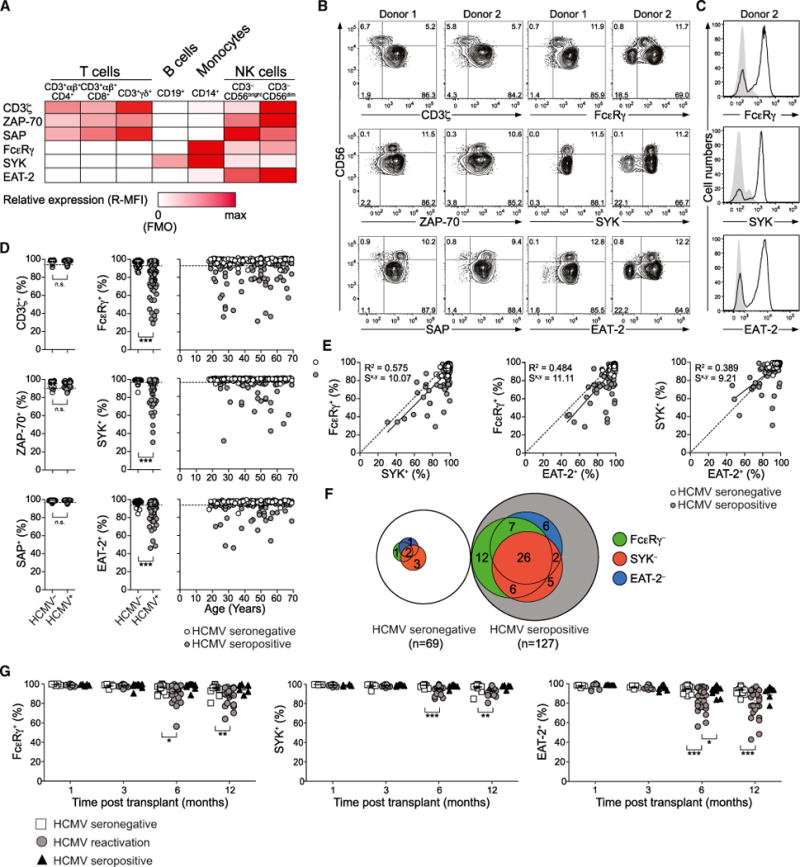
(A–F) PBMCs from healthy human blood donors were analyzed by flow cytometry.
(A) Relative median fluorescence intensity (MFI) of CD3ζ, ZAP-70, SAP, FcɛRγ, SYK, and EAT-2 expression in immune cell subsets. Data represent mean MFI values from three donors.
(B) Expression of CD3ζ, ZAP-70, SAP, FcɛRγ, SYK, and EAT-2 in CD3−CD56dim NK cells from two representative donors.
(C) Expression of FcɛRγ, SYK, and EAT-2 in CD4+ T cells (filled histograms) and CD3−CD56dim NK cells (black lines) in a representative donor lacking signaling proteins.
(D) Frequency of CD3−CD56dim NK cells expressing CD3ζ, ZAP-70, SAP, FcɛRγ, SYK, and EAT-2 from 196 healthy donors versus age of the individuals. HCMV− (open circles) and HCMV+ (filled circles) individuals are indicated. Dotted lines indicate a threshold for outliers calculated as the mean of HCMV− individuals plus 3 SD. ***p < 0.001 (Mann-Whitney test).
(E) Relationship between the frequencies of CD3−CD56dim NK cells expressing FcɛRγ, SYK, or EAT-2. Solid lines represent the correlation for HCMV+ individuals only. The coefficient of determination (R2) and standard error of estimate (Sx,y) values for HCMV+ individuals are indicated.
(F) Relationship between donors with CD3−CD56dim NK cells lacking FcɛRγ (green), SYK (red), or EAT-2 (blue) expression among HCMV+ and HCMV− donors. See also Figure S1.
(G) PBMCs from 78 allogeneic hematopoietic cell transplant patients with a total of 37 HCMV reactivation events were analyzed by flow cytometry for intracellular FcɛRγ, SYK, and EAT-2 expression within the CD3−CD56dim NK cell subset at 1, 3, 6, and 12 months after transplant. Transplant patients were monitored weekly for HCMV reactivation, which occurs between 30 and 90 days after transplant, by PCR for HCMV DNA copy number. *p < 0.05, **p < 0.01, and ***p < 0.001 (Student’s t test).
HCMV infection can trigger emergence and persistence of “adaptive” or “memory-like” NK cells (Foley et al., 2012; Sun et al., 2009). Therefore, we examined expression of FcɛRγ, SYK, and EAT-2 in a cohort of hematopoietic cell transplant (HCT) recipients of allogeneic umbilical cord blood grafts whom either reactivated latent HCMV after transplant, were HCMV+ but did not reactivate, or were HCMV−. HCMV reactivation, if it occurred, was typically detected between 1 and 3 months after transplantation. CD3−CD56dim NK cells from HCMV− recipients and HCMV+ individuals who did not experience HCMV reactivation were uniformly positive for FcɛRγ, SYK, and EAT-2 up to a year after transplantation. However, in HCT recipients who reactivated HCMV, significant populations of CD3−CD56dim NK cells lacking each signaling protein were observed 6 months and 1 year after transplant (Figure 1G). Thus, numerical expansion of NK cell subsets selectively lacking B-cell- and myeloid-cell-related signaling protein expression is driven by acute HCMV infection.
Lack of FcɛRγ, SYK, and EAT-2 Expression Correlates with an Adaptive NK Cell Phenotype
Latent HCMV infection is associated with imprints on NK cell surface receptor expression (Béziat et al., 2013; Bezman et al., 2012; Gumá et al., 2004; Sun et al., 2009). Lack of FcɛRγ, SYK, and EAT-2 in CD3−CD56dim NK cells correlated with expression of NKG2C (Figure 2A), and the lack of FcɛRγ correlated with significantly reduced NKp30 expression, reflecting a requirement for FcɛRγ in NKp30 surface expression at the single-cell level (Figures 2B and S2; Hwang et al., 2012). Of note, increased NKG2C expression on CD3−CD56dim NK cell subsets correlated strictly with HCMV+, whereas FcɛRγ, SYK, or EAT-2 deficiency was also observed in a small subset of CD3−CD56dim NK cells from HCMV− individuals (Figure 2C).
Figure 2. FcɛRγ−, SYK−, and EAT-2− NK Cells Display an Adaptive NK Cell Phenotype.
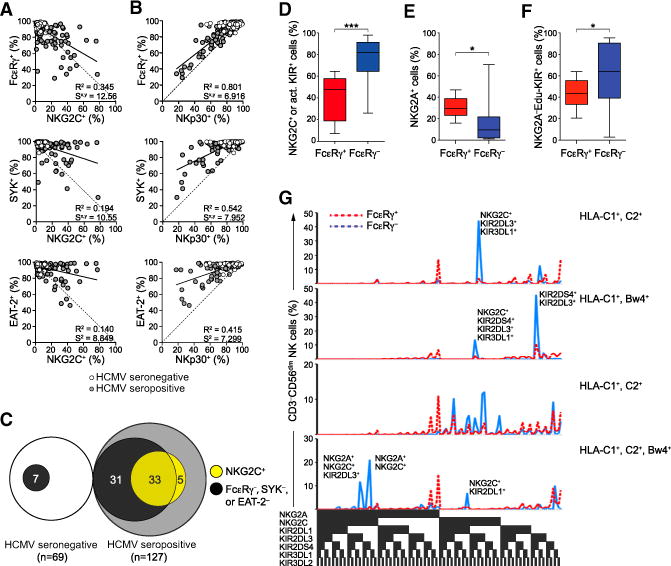
(A–C) PBMCs from 196 healthy human blood donors were analyzed by flow cytometry. The relationship between the frequencies of CD3−CD56dim NK cells expressing NKG2C (A) or NKp30 (B) versus FcɛRγ, SYK, and EAT-2. Solid lines represent the correlation for HCMV+ individuals only. The coefficient of determination (R2) and standard error of estimate (Sx,y) values for HCMV+ individuals are indicated. Relationship (C) between donors with CD3−CD56dim NK cells lacking FcɛRγ, SYK, and/or EAT-2 expression (black) versus displaying elevated NKG2C expression (light gray) among HCMV+ and HCMV− donors.
(D–G) PBMCs from 15 healthy KIR haplotype A donors were analyzed by flow cytometry
(D–F) Percentages of CD3−CD56dim NK cells expressing DAP12-coupled receptors NKG2C and/ or KIR2DS4 (D), inhibitory NKG2A (E), or educating KIRs without NKG2A (F). Bars indicate SD.
(G) The distributions of CD3−CD56dim NK cells expressing specific KIRs, NKG2A, and NKG2C in four HLA-typed individuals are shown. *p < 0.05 and ***p < 0.001 (Student’s t test).
See also Figure S2.
Deviations in inhibitory KIR expression have been used to define adaptive human NK cells (Béziat et al., 2013). To analyze the expression of inhibitory receptors for self-MHC and DAP12-coupled receptors on CD3−CD56dim FcɛRγ− NK cells, we stained for all KIRs on FcɛRγ− NK cells from MHC-genotyped donors. The majority of CD3−CD56dimFcɛRγ− NK cells expressed DAP12-coupled receptors (76 ± 23, mean ± SD; Figure 2D). With respect to inhibitory receptors specific for MHC class I, NKG2A expression was reduced and inhibitory self-KIR expression was increased on CD3−CD56dimFcɛRγ− relative to CD3−CD56dim FcɛRγ+ NK cells (15 ± 19 versus 31 ± 9 and 61 ± 32 versus 43 ± 13, respectively; Figures 2E and 2F). Within individual donors, multiple FcɛRγ− subsets with distinct expression patterns of activating NKG2C and KIRs, as well as inhibitory NKG2A and self-KIR, were evident (Figure 2G), suggesting distinct expansions. In summary, FcɛRγ−, SYK−, and EAT-2− NK cells were heterogeneous, yet phenotypically resembled adaptive NK cells with respect to surface receptor expression. Thus, FcɛRγ, SYK, and/or EAT-2 silencing represent markers that broadly encompass adaptive NK cells.
Adaptive FcɛRγ− and EAT-2− NK Cells Display Promoter DNA Methylation
The molecular mechanisms underlying differentiation of adaptive NK cell subsets are not well understood (Cichocki et al., 2013). In CD8+ T cells responding to acute viral infection, genome-wide DNA methylation remodeling accompanies acquisition of the effector phenotype and repression of the naive cell state (Scharer et al., 2013). In our analysis, flow cytometry plots of CD3−CD56dim NK cells revealed bi-modal downregulations of FcɛRγ, SYK, and EAT-2 (Figure 3A). FcɛRγ, SYK, and EAT-2 expression patterns were variegated and stable over time (Figures 3B and S3). Together, tri-modal expression patterns and non-synchronized deficiencies in signaling protein expression suggested regulation by DNA methylation-dependent processes.
Figure 3. Lack of Signaling Protein Expression in Adaptive NK Cells Is Associated with Promoter DNA Hypermethylation.
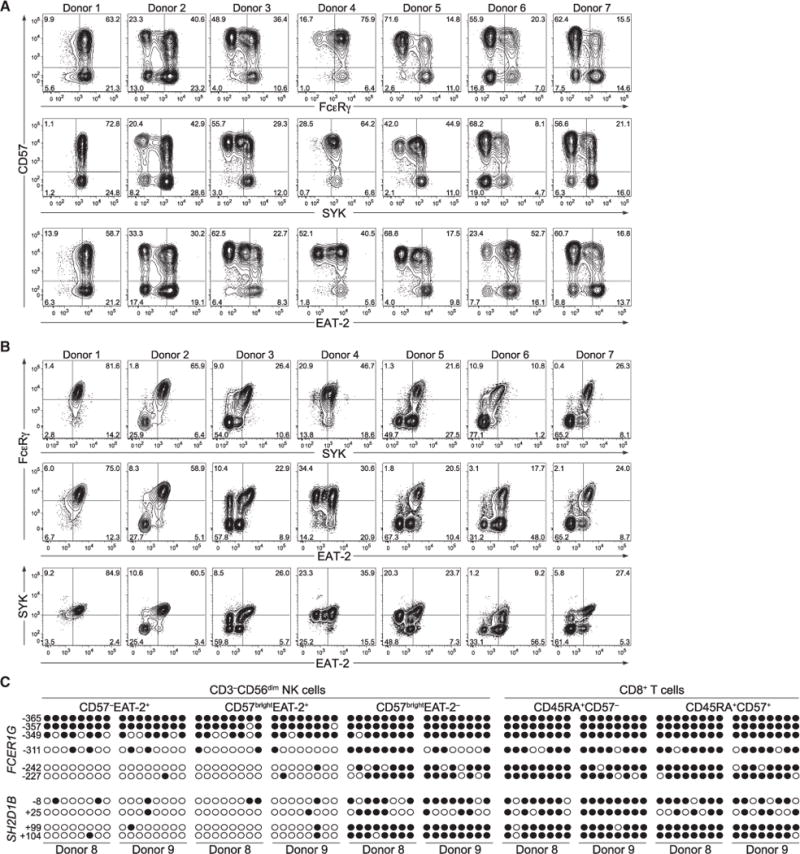
(A and B) PMBCs from healthy donors were analyzed by flow cytometry. Plots depict CD57 versus signaling protein expression (A) or combinations of signaling proteins in CD3−CD56dim NK cells (B) from representative HCMV− (donor 1) and HCMV+ (donors 2–7) donors.
(C) NK cell and T cell subsets were sorted from two HCMV+ donors. Genomic DNA from each cell subset was treated with sodium bisulfite, purified and amplified by PCR using primers specific for the promoter regions of FCER1G and SH2D1B. PCR fragments were cloned, sequenced, and analyzed for CpG methylation content. Each row represents one sequenced PCR clone. Open circles represent unmethylated and closed circles methylated cytosines.
See also Figure S3.
To determine whether DNA methylation regulates signaling protein expression upon differentiation of adaptive NK cells, we sorted NK cell subsets as well as naive CD3+CD8+CD45RA+CD57− and effector CD3+CD8+CD45RA+CD57bright T cell subsets (i.e., CTLs) from two HCMV+ donors. Genomic DNA from each cell subset was analyzed by bisulfite sequencing. Relative to early mature CD3−CD56dimCD57− and late mature CD3−CD56dim CD57brightEAT-2+ NK cells, adaptive CD3−CD56dimCD57bright−EAT-2− NK cells displayed hypermethylation of the FCER1G and SH2D1B (encoding EAT-2) promoters (Figure 3C). The methylation patterns of the FCER1G and SH2D1B promoters in the adaptive NK cell subset were similar to those of naive and effector CD8+ T cell subsets that do not express FcɛRγ or EAT-2. Thus, promoter hypermethylation probably explains the lack of signaling protein expression in subsets of adaptive NK cells and, hypothetically, might reflect large-scale epigenetic changes associated with adaptive NK cell differentiation.
Genome-wide Analyses of DNA Methylation Reveal Parallels between Differentiation of Adaptive NK Cells and Cytotoxic Effector T Cells
To test our hypothesis of widespread epigenetic modifications during adaptive NK cell differentiation, we examined genome-wide DNA methylation in NK cell and CD8+ T cell subsets from HCMV+ individuals. As expected, the global methylation profiles of early and late mature EAT-2+ “canonical” NK cells strongly resembled each other. Adaptive EAT-2− NK cells diverged from both early and late mature NK cells in their methylation profile, with widespread hyper- as well as hypomethylation (Figure 4A). By contrast, late mature NK cells had a methylation profile dissimilar to that of CD8+ T cell subsets, particularly effector T cells (Figure 4A). The methylation profile of adaptive NK cells correlated strongly with that of effector T cells (Figure 4B). These relationships were also evident upon multidimensional scaling analysis of probes with a high variability of methylation across the different populations (SD/mean > 0.1) (Figure 4C), confirming that the variation in our data was mainly derived from the different cell types.
Figure 4. Changes in DNA Methylation Patterns in Adaptive NK Cells Are Similar to Those in Cytotoxic T Cells.
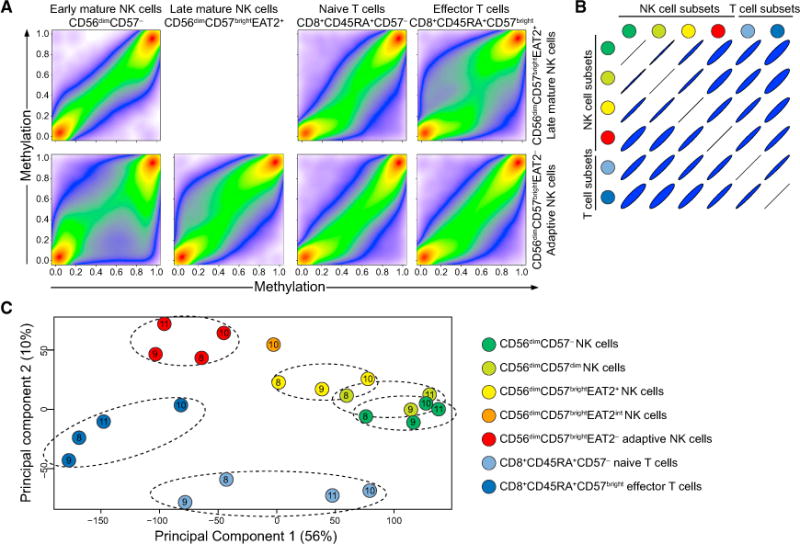
(A–C) NK and T cell subsets were sorted from four HCMV+ donors. Genomic DNA from each subset was treated with sodium bisulfite and subjected to genome-wide DNA methylation profiling.
(A) Pseudo-color scatterplots illustrating multiple pairwise comparisons of methylation (β-value) over all probes from specific subsets in donor 10 after data filtering and probe normalization. A β-value close to zero corresponds with no methylation, and a β-value close to 1 corresponds with full methylation.
(B) Correlation matrix of all pairwise subset comparisons.
(C) MDS analysis of DNA methylation in NK cell and T cell subsets after data filtering and normalization. Samples are color coded based on the cell type and labeled according to the donor they were derived from (donors 8, 9, 10, and 11). Only probes with a high variability in β-values across the different cell subsets are shown (arbitrarily set as a ratio of standard deviation over a mean greater than 0.1, n = 271,769). Dimensions 1 and 2 represent the largest source of variation (totaling 66%).
See also Figure S4.
As expected, probed regions in close proximity to the SH2D1B and FCER1G transcriptional start sites (TSSs) were hyperme-thylated in adaptive relative to canonical NK cells (Figure S4A). Comparisons of early mature and adaptive NK cells revealed 2,372 differentially methylated regions (DMRs), with an enrichment of hypomethylated regions in adaptive NK cells (Figure S4B). In contrast, comparisons of adaptive NK cells versus effector CD8+ T cells identified only 61 DMRs, supporting the notion of convergent differentiation of adaptive NK cells and effector CD8+ T cells. The methylation patterns of CD3D, CD8A, BCL11B, NCR1, NCR3, FCGR3A, and TYROBP confirmed the disparate identity of adaptive NK cells and effector CD8+ T cells (Figures S4C and S4D).
Epigenetic Changes in DNA Methylation Regulate Adaptive NK Cell Gene Expression
Prompted by the profound epigenetic differences in NK cell subsets, we performed gene expression arrays comparing canonical, late mature CD3−CD56dimCD57brightNKG2A−NKG2C− and adaptive CD3−CD56dimCD57brightNKG2A−NKG2C+ NK cells from four selected donors where surface expression of NKG2C marked FcɛRγ− cells (Figures S5A and S5B). With 2-fold modulation as a cut-off, 89 upregulated and 102 downregulated genes were identified (Figure 5A). KLRC2, KLRC3, and KLRC4 transcripts encoding NKG2C isoforms were upregulated up to 52-fold. Conversely, transcripts for FCER1G, IRS2, and PLXDC2, which encode signaling and transmembrane proteins, were downregulated by 92% or more. Cytokine receptor-encoding transcripts for IL12RB2, IL18RAP, and IL2RB were also signifi-cantly downregulated. In general, hypomethylated or hyperme-thylated DMRs within TSS regions correlated with upregulated and downregulated genes, respectively (Figure 5B). In this context, probes in the vicinity of the TSSs of CD7, KLRB1 (encoding CD161), and KLRC1 (encoding NKG2A) were hyperme-thylated in adaptive NK cells, whereas probes in the vicinity of the TSSs of CD2, ITGAL (encoding CD11a), and LILRB1 (encoding CD85j) were hypomethylated (Figures 5C and 5D). Thus, both the methylation and transcriptional data reflected the reduced expression of CD7 and CD161 and increased expression of CD2, CD11a, and CD85j, which was confirmed at the protein level by flow cytometry (data not shown). This expression profile is consistent with reported surface phenotypes of HCMV-induced adaptive NK cells (Béziat et al., 2013; Gumá et al., 2004).
Figure 5. Transcript Expression Analyses of Canonical and Adaptive NK Cells.
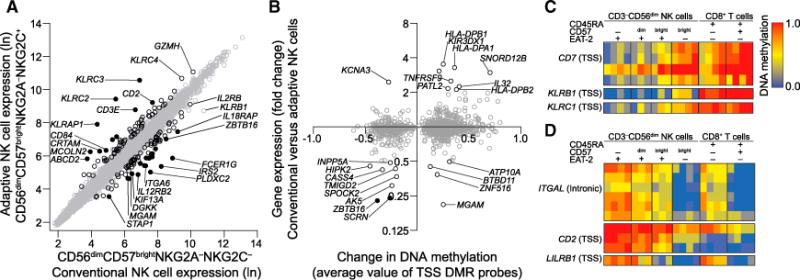
(A) Microarray analyses of gene expression in sorted CD3−CD56dimCD57brightNKG2A−NKG2C− versus CD3−CD56dimCD57brightNKG2A−NKG2C+ (corresponding to FcɛRγdim/−) NK cell subsets from four HCMV+ donors. Each circle represents the mean expression value of 20,364 individual genes. Genes regulated more than 2-fold (black circles) and more than 4-fold (filled circles) consistently among donors (p < 0.05, Student’s t test) are highlighted.
(B) Relationship between the mean change in DNA methylation values of DMRs located in the vicinity of gene TSS (comparison of CD3−CD56dimCD57− versus CD3−CD56dimCD57brightEAT-2− NK cells) versus the corresponding mean change in gene expression (CD3−CD56dimCD57brightNKG2C− versus CD3−CD56dimCD57brightNKG2C+ NK cells).
(C and D) DNA methylation in the vicinity of TSSs of receptors that are downregulated (C) or upregulated (D) on the surface of adaptive NK cells.
See also Figure S5.
Reduced Expression of PLZF Marks Adaptive NK Cells
The BTB-zinc finger (BTB-ZF) family of transcription factors can orchestrate changes in DNA methylation (Mathew et al., 2012; Puszyk et al., 2013). ZBTB16, encoding PLZF, was the most highly differentially regulated of all transcription factor transcripts, being downregulated by 77% in adaptive NK cells (Figure 6A). In addition, ZBTB38 transcripts were 1.8-fold upregulated (Figure 6A). Accordingly, an intronic sequence in ZBTB16 was hypermethylated, and the TSS of ZBTB38 was hypomethylated in adaptive NK cells (Figure 6B). Moreover, the TSSs of ZBTB20 and ZBTB32 were hypermethylated and hypomethylated, respectively. Bisulfite sequencing confirmed hypermethylation of the intronic ZBTB16 sequence in adaptive NK cells (Figure S6A). Chromatin immunoprecipitation experiments with NK cells from HCMV donors revealed that PLZF bound the promoters of the genes encoding FcɛRγ, SYK, and EAT-2 but not those encoding CD3z, ZAP-70, and SAP (Figure 6C). Intracellular flow cytometry revealed strong downregulation of PLZF expression in HCMV+ individuals (Figure 6D). FcɛRγ, SYK, and EAT-2 NK cell subsets were encompassed within the PLZF NK cell population, and PLZF NK cell subsets were consistently larger than those individually lacking expression of FcɛRγ, SYK, or EAT-2 (Figures 6E–6H). Intermediate PLZF expression was also evident in one donor, corresponding to NK cell subsets displaying reduced expression of FcɛRγ (Figure 6E, donor 3). To visualize heterogeneity in NK cell populations, we optimized algorithms for nonlinear dimensionality reduction with stochastic neighbor embedding capable of handling large amounts of data from multiple individuals. These multivariate analyses of flow cytometry data illustrated how all individuals, irrespective of HCMV serostatus, contained a range of PLZF-expressing canonical NK cells (Figures 6I–6K). In contrast, adaptive NK cells in HCMV+ individuals distinctively displayed uniformly reduced PLZF expression along with unique, donor-specific signaling protein expression patterns (Figure 6K). Of note, expression of the transcription factors T-bet and EOMES was not altered in adaptive NK cell subsets, whereas Helios expression could be reduced or increased (Figures S6B–S6E). Thus, PLZF binds the promoters of the genes encoding FcɛRγ, SYK, or EAT-2, and reduced PLZF expression reliably marks adaptive NK cells displaying a probabilistic, epigenetically regulated lack of FcɛRγ, SYK, and EAT-2 expression.
Figure 6. Reduced PLZF Expression Specifically Marks Adaptive NK Cells.
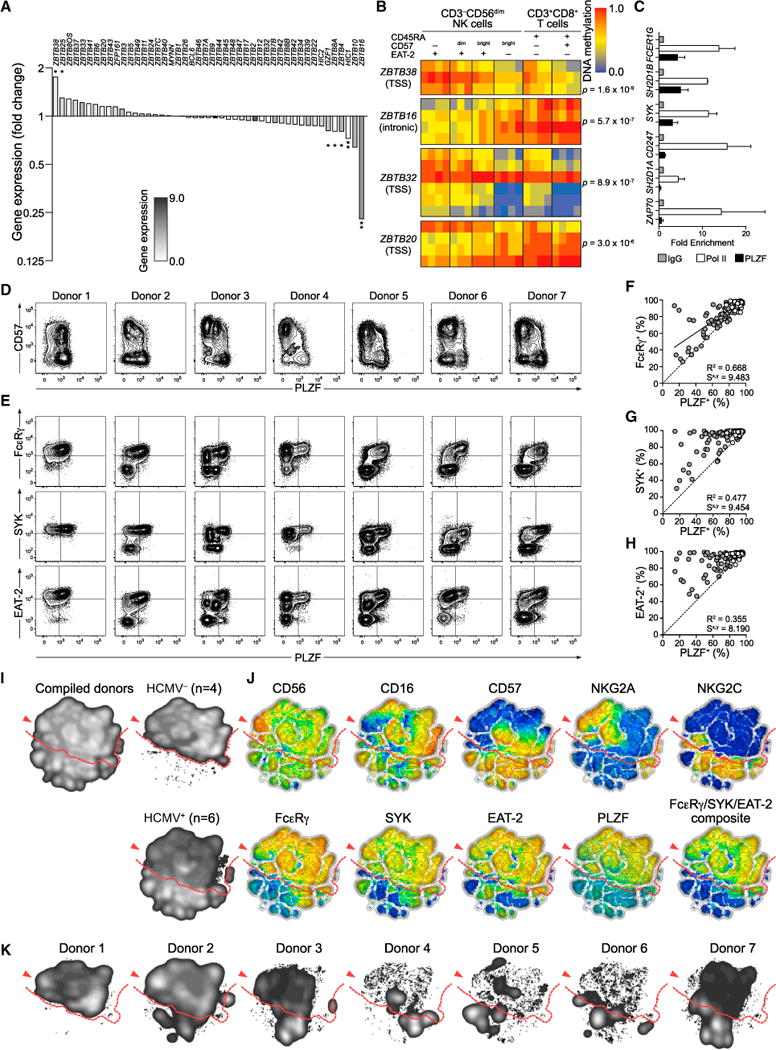
(A) Fold change in gene expression of BTB-ZF transcription factors in NK cell subsets. Bars indicate the mean fold change in expression for four donors. The bar filling reflects the average overall intensity of expression for indicated transcripts. *p < 0.05 and **p < 0.01 (paired Student’s t test).
(B) BTB-ZF transcription factor DMRs. Heat maps show the DNA methylation in different cell subsets for a set of probes within each DMR. A β-value close to zero corresponds with no methylation, and a β-value close to 1 corresponds with full methylation.
(C) ChIP analysis of PLZF and Pol II binding to the promoters of FCER1G, SH2D1B, SYK, CD247, SH2D1A, and ZAP70 using primary NK cell lysates from three donors in two separate experiments. Fold expression values are normalized against input and IgG controls. Bars indicate SD.
(D–J) PMBCs from 196 healthy human blood donors were analyzed by flow cytometry.
(D and E) Plots depict PLZF expression versus expression of CD57 (D) or FcɛRγ, SYK, or EAT-2 (E) in CD3−CD56dim NK cells from representative HCMV− (donor 1) and HCMV+ (donors 2–7) donors.
(F–H) Plots depict the frequency of CD3−CD56dim NK cells expressing PLZF versus FcɛRγ (F), SYK (G), or EAT-2 (H). Each circle represents one individual (n = 196). HCMV− (open circles) and HCMV+ (filled circles) individuals are indicated. Solid lines represent the correlation for HCMV+ individuals only.
(I–K) Barnes-Hut t-distributed stochastic neighbor embedding (t-SNE) analysis of 11-parametric data was performed on CD3−CD56+ NK cells from four HCMV− and six HCMV+ donors (donors 2–7).
(I) Event density in the t-SNE field for all donors compiled together or HCMV− and HCMV+ donors compiled separately.
(J) Protein expression levels for single parameters in t-SNE field. Red represents high expression, whereas blue represents low expression. The composite FcɛRγ/SYK/EAT-2 parameter was constructed post-analysis. CD7 and CD161 stainings were also included in the model but are not shown.
(K) Event density in the t-SNE field for CD3−CD56+ NK cells from individual donors. Red arrows indicate the CD56bright NK cell population, and dashed red lines the border between canonical NK cell populations and adaptive NK cell populations found in HCMV+ individuals.
See also Figure S6.
Distinct Functional Capacities of Adaptive NK Cells
Having uncovered alterations in NK cell signaling and transcription factor expression in adaptive NK cells, we sought to understand the functional consequences of these findings. IFN-γ production was examined in healthy individuals with NKG2C+ adaptive NK cells where the vast majority of PLZF− NK cells lacked FcɛRγ expression. In such individuals, IL-12 and IL-18 co-stimulation induced significantly less IFN-γ production by CD3−CD56dimFcɛRγ− NK cells relative to all other NK cell subsets, including the CD3−CD56dimCD57+FcɛRγ+ NK cell subset (Figures 7A, 7B, and S7A). IL-12 and IL-18 co-stimulation also induced significantly less IFN-γ production by CD3−CD56dimNKG2C+FcɛRγ− NK cells relative to the CD3−CD56dimNKG2C+FcɛRγ+ NK cell subset (Figure 7C). These results demonstrate that adaptive NK cells marked by lack of PLZF expression represent a subset functionally distinct from other NK cells expressing CD57 or NKG2C, but with expression of PLZF. In NK T cells, PLZF is a key regulator of innate effector programs and promotes the expression of IL-12 and IL-18 receptors (Gleimer et al., 2012; Mathew et al., 2012). Transcription of IL-12 (IL12RB2) and IL-18 (IL18RAP) receptor subunits was reduced in adaptive NK cells (Figure 7D), with concomitant IL-12 and IL-18 receptor signaling also greatly reduced (Figures 7E–7G). Adaptive NK cells did not have an intrinsic defect in cytokine production, as shown by the fact that the NK cells lacking FcɛRγ, SYK, EAT-2, or any combination thereof displayed increased IFN-γ and TNF production in response to phorbol-12-myris-tate-13-acetate (PMA) and ionomycin stimulation (Figures 7H and 7I), whereas degranulation was not augmented (Figure 7J). Thus, adaptive NK cells fail to produce IFN-γ in response to innate cytokines, yet might display augmented responses upon target cell recognition. The latter can be explained by observed hypomethylation of IFNG and TNF regulatory regions (Figure S7B).
Figure 7. Adaptive NK Cells Display Altered Functional Responses.
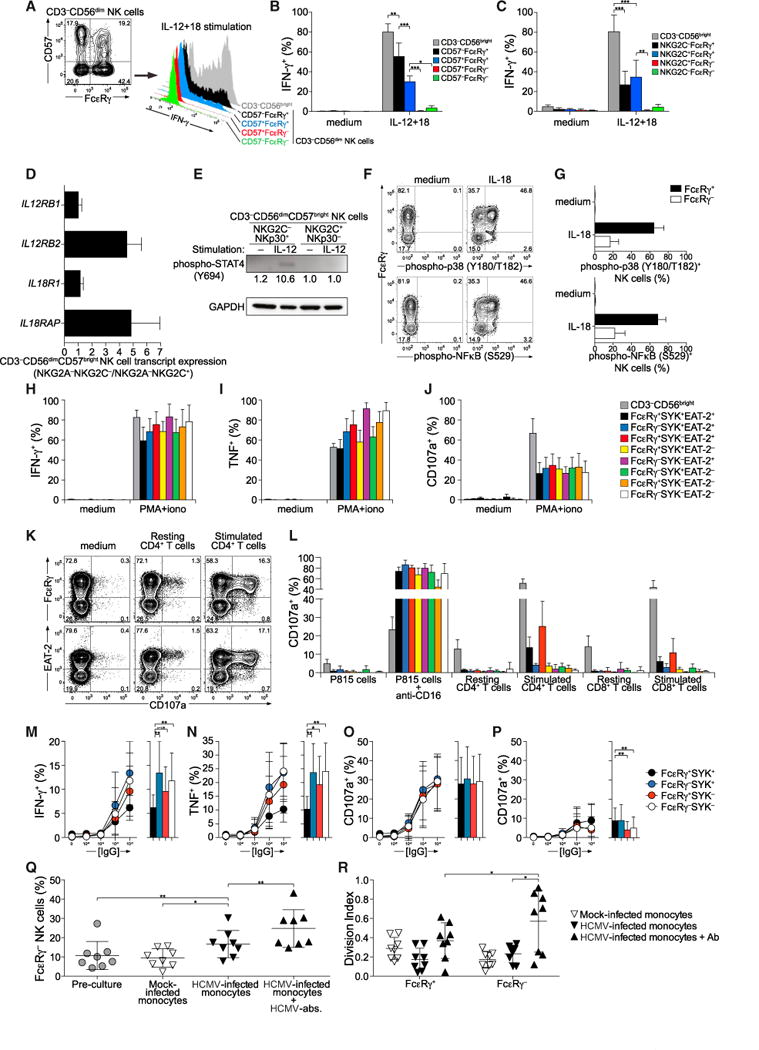
(A–C) PBMCs from HCMV+ individuals with a clear correlation between PLZF and FcɛRγ expression were left untreated or stimulated for 24 hr with combinations of IL-12, IL-15, and IL-18, then stained for intracellular IFN-γ and analyzed by flow cytometry. The frequencies of IFN-γ-expressing NK cells were quantified in CD3−CD56bright or CD3−CD56dim subsets subdivided according to expression of FcɛRγ and CD57.
(A) Gating strategy and IFN-γ expression upon IL-12 plus IL-18 stimulation in NK cell subsets from one representative donor.
(B) Frequencies of NK cells expressing IFN-γ within specific subsets upon stimulation with cytokines.
(C) The frequencies of IFN-γ-expressing NK cells were quantified in CD3−CD56bright or CD3−CD56dim subsets subdivided according to expression of FcɛRγ and NKG2C. The frequencies of NK cells expressing IFN-γ within specific subsets upon stimulation with cytokines are presented. Values depict the mean of seven donors, and bars indicate SD.
(D) Quantitative PCR analyses of gene expression in in sorted CD3−CD56dimCD57brightNKG2A–NKG2C– versus CD3−CD56dimCD57brightNKG2A−NKG2C+ (corresponding to FcɛRγdim/−) NK cell subsets from four HCMV+ donors, as indicated. Bars indicate SD.
(E) Immunoblot depicting STAT4 phoshorylation in response to IL-12 stimulation in NK cells from one representative donor. (F and G) PBMCs were stimulated with IL-18 or left untreated, and CD3−CD56dim NK cell subsets were analyzed for phosphorylation of p38 MAPK and NF-kB p65 by phospho-specific flow cytometry.
(F) Contour plots show CD3−CD56dim NK cells from one representative donor.
(G) Bar graphs display frequencies of phospho-p38 MAPK+ and phospho-NF-kB+ cells in CD3−CD56dim NK cell subsets gated according to FcɛRγ expression. Values depict the mean of three donors, and bars indicate SD.
(H–J) PBMCs were left untreated or stimulated for 6 hr with PMA and ionomycin and then analyzed by flow cytometry. Graphs show frequencies of NK cell subsets positive for (H) intracellular IFN-γ, (I) intracellular TNF, or (J) surface CD107a. Values depict the mean of eight donors, and bars indicate SD.
(K and L) Purified CD4+ and CD8+ T cells were left untreated or activated with anti-CD3- and anti-CD28-coated beads for 60 hr, washed, and subsequently co-cultured with resting, autologous NK cells for 6 hr. Co-incubation with P815 target cells with or without anti-CD16 mAb served as the control.
(K) Plots show CD107a surface expression versus FcɛRγ or EAT-2 expression on CD3−CD56dim NK cells.
(L) The graph depicts frequencies of CD107a-expressing NK cells according to stimulation and expression of FcɛRγ, SYK, and EAT-2. Bars indicate SD.
(M–P) PBMCs were incubated for 6 hr with S2 cells pre-coated with increasing concentrations of rabbit serum, as indicated. The cells were stained and analyzed by flow cytometry. Graphs depict the frequencies of intracellular IFN-γ (M), intracellular TNF (N), or surface CD107a (O, P) expressing NK cell subsets. Stimulations were performed in regular medium (M–O) or medium containing 1 μM Src kinase inhibitor PP2 (P). Values depict the mean of eight donors, and bars indicate SD.
(Q and R) Purified monocytes were mock or HCMV infected and cultured with autologous, labeled NK cells added IL-15. After 2 days, anti-HCMV IgG was added where indicated. After a total of 10 days co-culture, NK cells were stained and analyzed by flow cytometry. The frequency of NK cell subsets and their proliferation was determined.
(Q) Frequency of FcɛRγ− cells among total CD3−CD56dim NK cells after co-culture with monocytes.
(R) Proliferation of NK cell subsets after co-culture with monocytes, as indicated. Eight donors were used in the experiment, with mean and SD indicated. *p < 0.05, **p < 0.01, **p < 0.001 (Wilcoxon test). Bars indicate SD. See also Figure S7. Results are representative of at least three independent experiments.
We hypothesized that altered signaling of adaptive NK cells might impede their ability to recognized and eradicate activated T cells. NK cells from individuals with adaptive NK cell subsets were co-cultured with purified autologous CD4+ or CD8+ T cells unstimulated or stimulated with anti-CD3- and anti-CD28-coated beads. Notably, degranulation by adaptive NK cells in response to CD16 stimulation was equal to that of canonical CD3−CD56dim NK cells expressing FcɛRγ, SYK, and EAT-2 (Figure 7L). However, in response to autologous, activated T cells, FcɛRγ− or EAT-2−CD3−CD56dim NK cells manifested significantly diminished degranulation relative to other NK cell subsets (Figures 7K and 7L). These results suggest reduced innate activities of adaptive NK cells subsets maintaining immune homeostasis or relaying signals from innate cytokines such as IL-12 and IL-18.
Individuals with congenital or acquired deficiencies in cellular immune responses are susceptible to severe and prolonged HCMV infections, and heightened antibody-dependent cellular cytotoxcity activity inversely correlates with the severity of infection (Rook, 1988). Thus, given its importance in the control of HCMV, we analyzed Fc receptor-dependent activation of adaptive NK cells. In line with responses to PMA and ionomycin, adaptive NK cells more frequently produced IFN-γ and TNF in response to IgG-coated S2 insect cells (Figures 7M and 7N). In contrast, degranulation was similar between adaptive NK cells and canonical NK cells (Figure 7O). Reflecting the differing capacities of SYK and ZAP-70 to instigate ITAM-dependent signaling, SYK− NK cells displayed weaker degranulation than SYK+ NK cells when treated with the Src kinase inhibitor PP2 (Figure 7P). Importantly, we examined the expansion of adaptive NK cells lacking FcɛRγ in response to autologous HCMV-infected monocytes (Figures 7Q and 7R). A modest increase in the frequency of the FcɛRγ− NK cell subset in response to HCMV-infected monocytes was observed (Figure 7Q). The addition of anti-HCMV antibody accentuated this increase, driving extensive proliferation of the FcɛRγ− NK cell subset (Figure 7R). These results suggest heightened resistance of adaptive NK cells to apoptosis during inflammatory conditions, as well as superior proliferation upon engagement of ITAM-coupled receptors. In summary, our data provide evidence for a functional dichotomy between canonical NK cells and adaptive NK cells in terms of regulatory function and capacity to produce cytokines, survive, and proliferate in response to ITAM-dependent activating receptor signaling.
DISCUSSION
Viral infections can induce numerical expansions of phenotypically distinct NK cell subsets, leading to the establishment of lasting imprints on the receptor repertoire (Béziat et al., 2013; Gumá et al., 2004). Recently, Zhang et al. (2013) reported that FcɛRγ− NK cells are associated with HCMV seropositivity in humans. We found that expression of not only FcɛRγ but also other B and myeloid cell-related signaling proteins (SYK and EAT-2) were stochastically silenced in NK cell populations from HCMV+ individuals and upon HCMV reactivation in HCT recipients. Silencing correlated with promoter DNA methylation. Such NK cells that arose in response to HCMV infection displayed a genome-wide methylation profile that approximated that of CTL, maintained expression of cognate T-cell-related signaling proteins, and had enriched expression of rapidly evolving DAP12-coupled activating receptors.
Genome-wide DNA methylation analyses of NK cell and CD8+ T cell subsets revealed that adaptive NK cells epigenetically approximated CD8+ effector T cells. Thus, CD8+ T cells and adaptive NK cells appear to share pathogen-driven differentiation pathways. Highlighting heterogeneity, surface phenotypes and functional responses of virus-specific CD8+ T cells change in a graded and stochastic rather than stepwise manner during the induction of memory to generate a remarkable degree of functional and phenotypic diversity even among cells of the same origin and antigen specificity (Arsenio et al., 2014; Buch-holz et al., 2013; Gerlach et al., 2013; Stemberger et al., 2007). The branching points of and molecular mechanisms that underlie adaptive NK cell differentiation are unclear. Nfil3 is required for normal development of mouse NK cells (Gascoyne et al., 2009). However, recent findings reveal that HCMV infection can nonetheless drive expansions of adaptive NK cells in Nfil3−/− mice, bypassing an early developmental requirement for Nfil3 (Firth et al., 2013). We observed diverse patterns of FcɛRγ, SYK, and EAT-2 expression along with varied expression of maturation markers and surface receptors in adaptive NK cells. Stochastic expression of protocadherin genes in neurons is regulated by the de novo DNA methyltransferase Dnmt3b and is influenced by histone modifications and gene-specific sequence features (Toyoda et al., 2014). Whether a similar process is involved in stochastic regulation of genes encoding signaling molecules in NK cells remains to be determined. Regardless of the precise mechanism, our data suggest stochastic processes, branching, and selection of adaptive NK cells from varying stages of canonical NK cell development along a continuum, similar to what has been reported for the generation of CD8+ T cell diversity (Arsenio et al., 2014).
Decreased expression of the transcription factor PLZF marked adaptive NK cells that lacked FcɛRγ, SYK, and/or EAT-2 expression. PLZF is a member of the BTB-ZF transcription factor family, which can recruit chromatin-remodeling co-factors to regulate gene expression (Lee and Maeda, 2012). PLZF is required for the generation and function of the NK T and γδ T cell lineages and drives innate-like effector differentiation of T cell subsets (Kovalovsky et al., 2008; Kreslavsky et al., 2009; Savage et al., 2008). PLZF can interact with HDAC1, DNMT, and the E3 ubiquitin ligase CUL3, facilitating chromatin modifications (Mathew et al., 2012). Our data indicate that PLZF also maintains innate-like aspects of human NK cells, with absence of PLZF contributing to the adaptive properties of NK cells from HCMV+ individuals. Several known PLZF-regulated target genes, including IL12RB2, IL18RAP, and KLRB1 (Gleimer et al., 2012), were all downregulated in adaptive NK cells, which lack responsiveness to inflammatory cytokines. Despite the well-established role of PLZF as a transcriptional repressor, PLZF directly binds to and induces a subset of IFN-stimulated genes in NK cells (Xu et al., 2009). Our data suggest that PLZF also induces or maintains expression of a select group of genes encoding cytokine receptor components and a subset of genes encoding signaling molecules in canonical NK cells. Notably, fewer than 5% of mouse NK cells express PLZF, and NK cell development appears to be normal in Zbtb16−/− (Constantinides et al., 2014). However, our notion is supported by studies of an individual with biallelic ZBTB16 mutations whose NK cells produced markedly elevated amounts of IFN-γ and TNF upon engagement of activating receptors (Eidson et al., 2011). Comparisons of canonical and adaptive NK cells also revealed regulation of additional BTB-ZF transcription factors. ZBTB38 was hypomethylated and transcriptionally upregulated in adaptive NK cells. ZBTB38 binds methylated CpG sites within DNA and participates in negative regulation of apoptosis (Oikawa et al., 2008). Zbtb32 is critical for proliferation of mouse adaptive NK cells (Beaulieu et al., 2014). Although ZBTB32 transcript levels were similar between adaptive and canonical human NK cells in our array analysis, ZBTB32 appeared poised through TSS hypome-thylation. Thus, these BTB-ZF proteins might orchestrate genome-wide epigenetic responses of NK cells to environmental stimuli.
We have delineated a functional divergence among human NK cell subsets, providing evidence for a critical role of FcɛRγ and EAT-2, linked to NCR- and SLAMFR-mediated recognition, respectively, in regulatory killing of activated immune cells. A lack of FcɛRγ or EAT-2 in NK cells might reduce tonic activating signaling instigated by hematopoietic NCR and SLAMFR ligands. Thus, in adaptive NK cells, IFNG and TNF loci can be relaxed, as evidenced by DNA hypomethylation, reducing the signaling threshold for induction of cytokine expression upon target cell recognition by other activating receptors. NK cell responses to activated T cells were not attenuated by the lack of SYK. In fact, the lack of SYK correlated with an increased capacity of NK cells to produce IFN-γ and TNF. SYK− cells that rely on ZAP-70 for ITAM-coupled receptor activation should receive less tonic NK cell signals (Hesslein et al., 2011; Kolanus et al., 1993). Thus, our data suggest that the lack of B and myeloid cell-related signaling protein expression can reduce tonic signaling and thereby facilitate stronger effector responses by adaptive NK cells specialized for recognition of infected cells. Hypothetically, high frequencies of adaptive NK cells might also bias for enhanced adaptive responses by sparing activated immune cells that express stress ligands, which otherwise can be killed by canonical regulatory NK cells. In addition, PLZF might maintain IL12RB2 and IL18RAP expression (Gleimer et al., 2012; Mathew et al., 2012). Expression of these IL-12 and IL-18 receptor subunits was diminished in adaptive NK cells, resulting in unresponsiveness. From a functional perspective, “canonical” NK cells could be termed “immunoregulatory,” reflecting their capacity to uphold immune homeostasis and contrasting “adaptive” NK cells specialized for immunosurveillance of infected cells and with the capacity to survive and proliferate in inflammatory conditions.
We find long-term persistence of NK cells lacking FcɛRγ, EAT-2, and SYK after HCMV reactivation in HCT recipients. Although reactivation of latent HCMV is associated with an increased risk of non-relapse-related morbidity and mortality, it is also independently associated with substantial reductions in the rates of leu-kemia relapse in patients with acute myelogenous leukemia early after transplantation (Green et al., 2013; Ito et al., 2013). It is possible that adaptive NK cells that arise in response to HCMV reactivation promote a graft-versus-leukemia effect directly through tumor immunosurveillance or indirectly through reduced killing of activated T cells. Therefore, adaptive NK cells have the potential to be therapeutically beneficial in both controlling HCMV infections and treating patients with hematological malignancies.
EXPERIMENTAL PROCEDURES
Cells
This study was approved by The Regional Ethical Review Board in Stockholm. Healthy controls were recruited from the Karolinska University Hospital blood bank. PBMCs were isolated from healthy donors by density gradient centrifu-gation and resuspended in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS], L-glutamine; all Hyclone). The K562 and P815 cell lines (both ATCC) were maintained in complete medium. All mammalian cells were maintained in, and functional assays performed at, 37°C with 5% (v/v) CO2. Insect cells were cultured in Schneider’s Drosophila Medium (Invi-trogen) supplemented with 10% FBS and L-glutamine at 28°C.
Hematopoietic Cell Transplant Patient Samples
Informed consent and approval from the University of Minnesota Institutional Review Board was obtained for sample collection. All patients received he-matopoietic progenitor cells from umbilical cord blood donors. Patients were monitored weekly for HCMV reactivation by quantitative PCR, and HCMV viremia (>100 copies/ml) was treated with an 8-week course of ganci-clovir. PBMCs collected from patients at each time point were isolated by density centrifugation and cryopreserved. The average percentage of donor-derived NK cells across all recipients regardless of HCMV status was 99.3% ± 4.4% at 3 months, 99.9% ± 0.9% at 6 months, and 99.4% ± 2.3% at 1 year.
Flow Cytometry
Fluorochrome-conjugated antibodies were purchased from BD Bioscience, Beckman Coulter, BioLegend, eBioscience, Invitrogen, R&D Systems, or BD Biosciences, as specified in the Supplemental Experimental Procedures. For phenotypic analyses, PBMCs were surface stained with indicated antibodies and a fixable dead cell stain (Invitrogen) in FACS buffer (PBS supplemented with 2% FBS and 2 μM EDTA). Cells were thereafter fixed in 2% formaldehyde, permeabilized in 0.05% Triton X-100, and stained intracellularly. Details on functional flow cytometry assays, including co-incubations with autologous activated T cells or HCMV-infected monocytes, are provided in the Supplemental Experimental Procedures. Flow cytometry data were acquired on an LSR Fortessa instrument (BD Biosciences) and analyzed with FlowJo (v.9.7.4, Tree Star). Multidimensional flow cytometry analysis via Barnes-Hut t-distributed stochastic neighbor embedding is described in the Supplemental Experimental Procedures.
Bisulfite Sequencing and Genome-wide DNA Methylation Analyses
Lymphocyte subsets were sorted by flow cytometry (FACSAria II, BD Biosciences), and DNA was extracted with the DNeasy Blood & Tissue Kit (QIAGEN). The EpiTect Bisulfite Kit (QIAGEN) was used to convert unmethylated cytosines to uracils. Primers used for bisulfite sequencing are detailed in the Supplemental Experimental Procedures. PCR products were cloned into the TOPO-XL vector (Invitrogen) and sequenced.
For genome-wide methylation analysis, bisulfite-converted DNA was analyzed with the Infinium HumanMethylation450 BeadChip platform (Illu-mina). A comprehensive description of the analysis pipeline is provided in the Supplemental Experimental Procedures.
Gene Expression Analysis
Donor PBMCs were screened for individuals where FcɛRγ− and PLZF− NK cell subsets correlated with NKG2C surface expression. For the donors used in the expression analysis, 89.3% ± 9.5% (mean ± SD) or 3.6% ± 2.6% expressed FcɛRγ and 89.0% ± 9.8% or 1.7% ± 1.3% expressed PLZF of the canonical CD3−CD56dimCD57+NKG2A−NKG2C− or adaptive CD3−CD56dimCD57+NKG2A−NKG2C+ populations, respectively. Based on CD57, NKG2A, and NKG2C expression, CD3−CD56dim NK cell subsets were sorted from four donors into RLT buffer (QIAGEN) by flow cytometry (FACS Aria III, BD Biosciences). Total RNA was isolated (RNeasy, QIAGEN). At least 100 ng of RNA were used for sample preparation (Ambion whole-transcript expression kit and Affymetrix whole-transcript terminal labeling kit). Samples were hybridized to GeneChip Human Transcriptome Array 2.0 cartridges (Affymetrix). Data conversion and quantile normalization was performed with Affymetrix software.
Statistical Analyses
Statistics were calculated with GraphPad Prism (v.5.0).
Supplementary Material
Highlights.
HCMV infection induces adaptive NK cell subsets with diversified signaling potential
Adaptive NK cells share molecular features of differentiation with CTLs
Adaptive NK cells lose the ability to kill autologous, activated immune cells
Gain of adaptive NK cell functions correlates with silencing of PLZF expression
Acknowledgments
This work was supported by the European Research Council under the Euro-pean Union’s Seventh Framework Programme (FP/2007–2013)/ERC Grant Agreement no. 311335, Swedish Research Council, Swedish Foundation for Strategic Research, Swedish Cancer Foundation, Swedish Children’s Cancer Foundation, Knut and Alice Wallenberg Foundation, the Karolinska Institute Research Foundation, the Frontiers in Biomedical Research Fellowship, and University of Minnesota T32 Haematology Training Grant. We thank Marcus Holm, Uppsala Multidisciplinary Center for Advanced Computational Science, for writing data pre- and post-processing code enabling usage of Barnes-Hut t-SNE, the Karolinska Institutet Bioinformatics and Expression Analysis core facility, supported by the Karolinska Institiutet Board of Research and the Kar-olinska Hospital Research Committee, for assistance with arrays, and Iyadh Douagi for assistance with cell sorting.
Footnotes
ACCESSION NUMBERS
Raw data of resulting chips were uploaded to Gene Expression Omnibus (GEO) database under the accession number GSE66564.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2015.02.008.
AUTHOR CONTRIBUTIONS
H.S. did experiments, analyzed data, and developed and wrote the manuscript; F.C. did experiments, analyzed data, and developed and wrote the manuscript; B.T. performed bioinformatical analyses of DNA methylation and gene expression data and contributed to drafting the manuscript; J.T. developed multivariate flow cytometry data analyses and contributed to drafting the manuscript; V.B. did flow cytometry with staining for KIRs and analyzed data; T.D.H. sorted cells for gene expression analyses, performed phospho-STAT4 experiments, and analyzed data; H.H. analyzed data and provided advice; S.C.C.C. assisted in biobanking and performed experiments; B.F. performed flow cytometry on transplant recipient samples and analyzed data; K.M. assisted in biobanking and performed experiments; S.L. provided access to blood donor samples; M.S. genotyped donors; K.-J.M. supervised research and provided advice; H.-G.L. supervised research and data analysis and provided advice; J.S.M. supervised research and data analysis and provided advice; Y.T.B. coordinated research efforts, supervised research work and data analysis, and prepared the manuscript; and all authors discussed and revised the manuscript.
References
- Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15:365–372. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15:546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Björklund AT, Retière C, Sverremark-Ekström E, Traherne J, Ljungman P, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL, Gautier EL, et al. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Gräf P, Verschoor A, Schiemann M, Höfer T, Busch DH. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki F, Miller JS, Anderson SK, Bryceson YT. Epigenetic regulation of NK cell differentiation and effector functions. Front Immunol. 2013;4:55. doi: 10.3389/fimmu.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Eidson M, Wahlstrom J, Beaulieu AM, Zaidi B, Carsons SE, Crow PK, Yuan J, Wolchok JD, Horsthemke B, Wieczorek D, Sant’Angelo DB. Altered development of NKT cells, gd T cells, CD8 T cells and NK cells in a PLZF deficient patient. PLoS ONE. 2011;6:e24441. doi: 10.1371/journal.pone.0024441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med. 2013;210:2981–2990. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Rohr JC, Perié L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- Gleimer M, von Boehmer H, Kreslavsky T. PLZF controls the expression of a limited number of genes essential for NKT cell function. Front Immunol. 2012;3:374. doi: 10.3389/fimmu.2012.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- Hesslein DG, Palacios EH, Sun JC, Beilke JN, Watson SR, Weiss A, Lanier LL. Differential requirements for CD45 in NK-cell function reveal distinct roles for Syk-family kinases. Blood. 2011;117:3087–3095. doi: 10.1182/blood-2010-06-292219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRg and specialized for antibody-dependent immune functions. Int Immunol. 2012;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, Childs R, Battiwalla M, Barrett AJ. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1313–1316. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- Khakoo SI, Rajalingam R, Shum BP, Weidenbach K, Flodin L, Muir DG, Canavez F, Cooper SL, Valiante NM, Lanier LL, Parham P. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity. 2000;12:687–698. doi: 10.1016/s1074-7613(00)80219-8. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci USA. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- Lee SU, Maeda T. POK/ZBTB proteins: an emerging family of proteins that regulate lymphoid development and function. Immunol Rev. 2012;247:107–119. doi: 10.1111/j.1600-065X.2012.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Seiler MP, Scanlon ST, Mao AP, Constantinides MG, Bertozzi-Villa C, Singer JD, Bendelac A. BTB-ZF factors recruit the E3 ligase cullin 3 to regulate lymphoid effector programs. Nature. 2012;491:618–621. doi: 10.1038/nature11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa Y, Matsuda E, Nishii T, Ishida Y, Kawaichi M. Down-regulation of CIBZ, a novel substrate of caspase-3, induces apoptosis. J Biol Chem. 2008;283:14242–14247. doi: 10.1074/jbc.M802257200. [DOI] [PubMed] [Google Scholar]
- Puszyk W, Down T, Grimwade D, Chomienne C, Oakey RJ, Solomon E, Guidez F. The epigenetic regulator PLZF represses L1 retro-transposition in germ and progenitor cells. EMBO J. 2013;32:1941–1952. doi: 10.1038/emboj.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook AH. Interactions of cytomegalovirus with the human immune system. Rev Infect Dis. 1988;10(3):S460–S467. doi: 10.1093/clinids/10.supplement_3.s460. [DOI] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharer CD, Barwick BG, Youngblood BA, Ahmed R, Boss JM. Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol. 2013;191:3419–3429. doi: 10.4049/jimmunol.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda S, Kawaguchi M, Kobayashi T, Tarusawa E, Toyama T, Okano M, Oda M, Nakauchi H, Yoshimura Y, Sanbo M, et al. Developmental epigenetic modification regulates stochastic expression of clustered protocadherin genes, generating single neuron diversity. Neuron. 2014;82:94–108. doi: 10.1016/j.neuron.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, Phillips JH, Lanier LL, Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Xu D, Holko M, Sadler AJ, Scott B, Higashiyama S, Berkofsky-Fessler W, McConnell MJ, Pandolfi PP, Licht JD, Williams BR. Promyelocytic leukemia zinc finger protein regulates interferon-mediated innate immunity. Immunity. 2009;30:802–816. doi: 10.1016/j.immuni.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Köhrer K, Rahbar R, Diefenbach A, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J Immunol. 2013;190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


