Abstract
Objectives.
This study examines the association of daily cortisol with depressive mood and anger.
Method.
Depressive mood, anger and 2 markers of cortisol, area under the curve (AUC), and cortisol awakening response (CAR) were examined for caregivers (N = 164) of individuals with dementia (IWDs) across 8 days, some of which IWDs attended an adult day service (ADS) program. Caregivers were primarily female (86.7%) with a mean age of 61.99. First, multilevel models were run with CAR and AUC each as separate covariates of anger and depressive mood. A second set of models examined contextual factors of caregivers (i.e., care-related stressors and amount of ADS use) were added to the models for anger and depressive mood (Model 2).
Results.
On days where caregivers had AUCs below their average they expressed higher anger scores. However in Model 2, anger was associated with more care-related stressors, but not ADS use or daily cortisol. Caregivers who on average had smaller CARs were more likely to be depressed. In Model 2, depressed mood was associated with more care-related stressors and a low average CAR.
Discussion.
We found that hypocortisol patterns, reflective of chronic stress experienced by caregivers, are associated with negative mood.
Key Words: Adult day services, Anger, Caregiving, Care-related stress, Cortisol, Depression
Family members who care for a relative with dementia find themselves living in an emotionally turbulent atmosphere. Researchers report that caregivers suffer more from emotional difficulties than they do with the financial or physical aspects of their care role (Gallagher, Wrabetz, Lovett, DelMaestro, & Rose, 1989). For example, family caregivers often experience decreased well-being and increased anger and depression (Aneshensel, Pearlin, Mullan, Zarit, & Whitlatch, 1995; Zarit, 2008). Both anger and depressive mood are believed to be common emotions among dementia caregivers and are important constructs of study as they relate to the well-being of the caregiver as well as the care receiver (Cuijpers, 2005; Steffen & Merritt, 2012). Furthermore, anger and depression increase the risk of illness, particularly cardiovascular disease (Miller, Smith, Turner, Guijarro, & Hallet, 1996; Rugulies, 2002). Existing theoretical models regarding caregiver’s strain and mental health, such as Pearlin, Mullan, Semple, and Skaff’s (1990) Stress Process Model, posit different characteristics and categories of risk and protective factors that impact caregiver’s outcomes, such as role strain and overload, and these pathways have been well studied. Our study furthers this understanding by considering the role of stress hormones in association with mood in the context of high daily stress faced by family caregivers.
Cortisol and Mental Health
A number of studies have pointed to the association between hypothalamic–pituitary–adrenal (HPA) axis dysregulation and mental illnesses (Burke, Davis, Otte, & Mohr, 2005; Sternberg & Gold, 2002; Wolkowitz, Burke, Epel, & Reus, 2009). Cortisol, which is a marker of the HPA axis, is an anti-inflammatory hormone which mobilizes energy, communicates with the immune system, and helps the body respond to stressful events (Piazza, Almeida, Dmitrieva, & Klein, 2010; Sternberg & Gold, 2002). Cortisol displays a noticeable diurnal rhythm increasing in level when the individual wakes and peaking around 30min after the awakening time, declining thereafter reaching a floor at night (Heaney, Phillips, & Carroll, 2010; Piazza et al., 2010). When stressors are prolonged or chronic, however, the diurnal pattern may become disrupted. As cortisol helps individuals respond to stress and environmental changes, dysregulation of this system may be associated with mental illness (Burke et al., 2005; Piazza et al., 2010).
Two dysregulated diurnal cortisol patterns are commonly found: diminished (hypo) or elevated (hyper) cortisol responsiveness (Seltzer et al., 2009). Hypercortisolemia is noted by an elevated cortisol awakening response (CAR) (i.e., the difference in awakening level and level at 30min after awakening) with a flattened slope such that there is little decline across the day in cortisol level. Overall output of daily cortisol, which is assessed as area under the curve (AUC), is also greater in hypercortisolemia. Hypocortisolemia, in contrast, is noted by a blunted pattern across the day with a small or no morning peak and consistent low levels (i.e., a small CAR and low total AUC) (Kurina, Schneider, & Waite, 2004).
Depression has been associated with cortisol hypersecretion (Heaney et al., 2010; Jonsdottir, Halford, & Eek, 2012; Kurina et al., 2004). The corticosteroid receptor hypothesis of depression, for example, points to hypercortisolism in the etiology of depression. This theory suggests that impairment of the corticosteroid receptor leads to hypercortisolism after stress exposure (Burke et al., 2005; Holsboer, 2000). On the other hand, a blunted or hypocortisol pattern has been found among individuals with depression and is particularly common among depressed older adults (Bremmer et al., 2007; Burke et al., 2005; Heaney et al., 2010). Hypocortisolism may also be associated with increased allostatic load (i.e., bodily wear and tear) in older adults experiencing chronic stress and depression (Almeida, McGonagle, & King, 2009). Though both hypo- and hypercortisolemic diurnal patterns have been found with depression, we anticipate that in an older sample experiencing chronic stress, the hypocortisol pattern will be associated with increased depressive mood.
In comparison with depression, few studies have examined the association between anger and cortisol. Feelings of anger and hostility have been most commonly associated with elevated levels of cortisol. Anger may be exacerbated with elevated cortisol or the “metabolic fuel” stemming from the HPA axis (Kemeny & Shestyuk, 2008). Elevated cortisol levels have been found in adults reporting high anger following their participation in the Trier Social Stress Test (Moons, Eisenberger, & Taylor, 2010) and among teachers reporting high job strain and high anger (in comparison with teachers who reported low job strain and high anger) (Steptoe et al., 2000). In a population-based sample of older adults, feelings of anger were associated with a same-day flattened, elevated cortisol slope (Adam, Hawkley, Kudielka, & Cacioppo, 2006). Among patients with atherosclerosis, individuals with high cynical hostility showed less steep decline in cortisol across the day in comparison with individuals who scored low on cynical hostility (Ranjit et al., 2009). Based on existing research, it is expected that hypercortisol levels will be associated with daily anger.
While cortisol–mood associations have been observed and hypotheses may be drawn, more research is needed in the context of high levels of naturally occurring daily stress, rather than experimentally manipulated stress in laboratory settings. Given that both depressive mood and anger are negative emotions that may be experienced conjointly and will be examined in a caregiving sample experiencing high levels of care-related stress, we will compare these associations and determine whether they might have similar blunted cortisol patterns or different patterns of association.
A Context of Care-Related Stress and Service Use
We examine the association of diurnal cortisol with both depressive mood and anger in a context of daily stress, which has also been found to impact the mental health of caregivers. For example, the frequency of daily care-related stressors, particularly behavior problems, faced by dementia caregivers, has been found to be associated with caregivers’ mental health and well-being (Aneshensel et al., 1995; Pearlin, Mullan, Semple, & Skaff, 1990; Schulz, O’Brien, Bookwala, & Fleissner, 1995). Further, caregivers in this study used adult day services (ADS) for their relative on some days of observation. ADS use has the effect of lowering caregiver’s exposure to care-related stressors, as it provides a block of time when they are not providing care (Zarit et al., 2011).
Our conceptual model and the primary hypothesized pathways are shown in Figure 1. Using a two-level, multilevel model with depressive mood and anger as outcomes in separate analyses, between- and within-person measures of CAR and AUC cortisol are examined as covariates (Model 1 in Figure 1). This model also allows us to consider whether depressive mood and anger share similar associations with cortisol in the context of care-related stress. Our primary research question posits “how are two dynamic measures of cortisol, the CAR and AUC, associated with depressive mood and anger in a sample of caregivers for individuals with dementia (IWDs)?” Prior studies have tested these associations, but mainly through experimental manipulations of stressor reactivity tasks and/or in individuals with low daily stress. Our study also uniquely considers both between- and within-person associations of cortisol with mood over 8 days.
Figure 1.
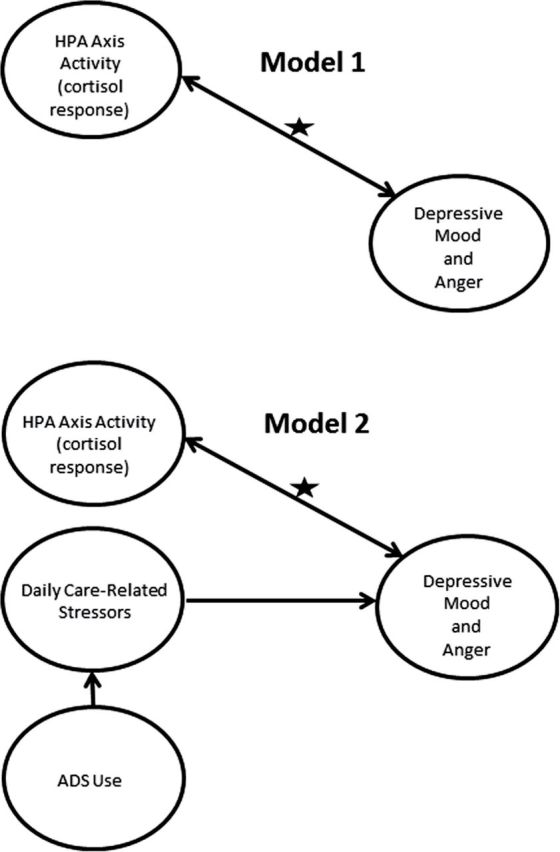
Conceptual model for associations between caregiver’s depressive mood and anger and cortisol response. The star indicates the hypothesized association of interest. We consider the association of cortisol with depressive mood and anger in a high stress context of family caregiving for individuals with dementia. Model 1 considers just the hypothesized association, whereas model 2 considers this association adjusting for sources of daily variability in the care process (i.e., care-related stressors and adult day services [ADS] use).
Next, as care-related stressors have considerable between- and within-person variability among caregivers and this variability is influenced by ADS use, we add a second multilevel model that considers both care-related stressors and ADS use, in addition to demographic characteristics (Model 2 in Figure 1). We control for these factors to see if the cortisol–mood association is due to fluctuating levels of care-related stressors. The association between perceived stress and HPA axis activation is complex, and researchers have suggested that the strength of covariation between the two may be small (Hellhammer, Wüst, & Kudielka, 2009). Therefore, while cortisol and care-related stressors are likely related, we expect these factors to have independent associations with daily mood.
Method
Sample
Participants were drawn from the Daily Stress and Health Study (DaSH) which examines the stresses and daily experiences of primary, family caregivers of a relative with diagnosed dementia who attends ADS at least twice a week. Participants were recruited from ADS programs in Colorado, New Jersey, Pennsylvania, and Virginia. Participants were eligible for the study if they were an informal caregiver with primary responsibility for the individuals with dementia (IWDs), lived with the IWD, did not have an endocrine disorder or other problem that could affect saliva production, if the IWD had received a dementia diagnosis not including mild cognitive impairment, and the IWD had attended ADS at least 2 days a week for at least 1 month. A total of 200 caregivers were eligible for the study. Sixteen (8%) did not complete an initial interview and another 11 (6%) either completed no daily interviews or their interview days did not include both ADS and non-ADS days. An additional nine (5%) caregivers were excluded for missing or invalid saliva samples (e.g., mixed up salivettes). The resulting analytic sample was 164 caregivers. See Table 1 for caregiver and care receiver demographic information.
Table 1.
Sample Characteristics of Caregivers and Individuals with Dementia
| M | SD | Range | |
|---|---|---|---|
| CG’s characteristics | |||
| Female, % | 87.2 | ||
| Age | 61.79 | 10.47 | 39–87 |
| Race, % | |||
| White | 72.6 | ||
| African American | 25.6 | ||
| Other | 1.8 | ||
| Married, % | 68.9 | ||
| College graduate, % | 53.1 | ||
| Employed, % | 41.5 | ||
| Relation to IWD, % | |||
| Spouse | 38.4 | ||
| Child | 57.3 | ||
| Others | 4.2 | ||
| Number of ADS days | 4.15 | 1.45 | 1–6 |
| Length of care (in months) | 61.56 | 46.20 | 3–264 |
| IWD’s characteristics | |||
| Age | 81.83 | 8.49 | 57–100 |
| Female, % | 59.1 | ||
| ADL impairmenta | 3.05 | 0.49 | 2–4 |
Notes. Participant N = 164. ADS = adult day services; CG = caregiver; IWD = individual with dementia.
aMean scores of 13 items rated on a 4-point scale ranging from 1 (does not need help) to 4 (cannot do without help).
Procedure
Participating ADS programs made flyers available to caregivers and also posted announcements of the study in their newsletters. After an initial telephone interview to determine eligibility, participants were given a baseline interview in their home or another place of their choosing (i.e., the ADS or public restaurant). The interviewer obtained informed consent, obtained sociodemographic and other measures, and provided training on saliva collection.
After the baseline interview, caregivers filled out daily diaries on 8 consecutive days, some of which the IWD was attending day care and some of which the caregiver was actively caring for the IWD, and relayed their responses to a telephone interviewer from the Penn State Survey Research Center each evening. They provided saliva samples five times each day: before getting out of bed, half an hour after getting out of bed, before lunch, late afternoon, and before bed. Participants had a home saliva collection worksheet to keep track of their collections and salivettes were numbered and color-coded to indicate the day and time they were to be used. Participants were instructed to take samples before eating, drinking, or brushing teeth and to use no tobacco or caffeinated products within 30min of providing a sample. Participants chewed on a cotton swab for 2min until saturated, placed the swab in the salivette, and kept it in the refrigerator until the end of the 8 days. Salivettes were then picked up by an express mail service and delivered overnight to the lab at the Pennsylvania State University where they were assayed.
Participants recorded the times of their saliva collections, any medications they took over the past 48hr (including steroids which may impact salivary steroid hormone assessment; Granger, Hibel, Fortunato, & Kapelewski, 2009), and their tobacco smoking status. These procedures are consistent with prior studies (Almeida, Piazza, & Stawski, 2009). Flags, or indications that a saliva sample may not be valid, were created for samples that had extreme values or where the caregivers sleep-waking pattern did not allow the normal diurnal cortisol curve to take place (i.e., awake <12hr or >20hr or if >60min elapsed between the first and second morning samples). A valid day for CAR depended on the first two cortisol samples. For AUC, all five samples needed to be valid. In analyses including CAR, we used 1,142 days (87.04% of the total 1,312 days) from 164 participants. In analyses including AUC, we analyzed 1,005 days (76.60% of the total 1,312 days) from 164 participants.
Measures
Outcomes
Depressive mood.—
Depressive symptoms were assessed from an inventory of emotions from the Non-Specific Psychological Distress Scale developed for the original Midlife in the United States (MIDUS) survey and adapted for daily use in the National Study of Daily Experiences (NSDE) (Kessler et al., 2002). MIDUS is a nationally representative study of middle-aged adults from which the NSDE, an intensive study of daily experiences, sample was drawn. The scale included seven items (α = .78). Participants were asked how frequently they felt each emotion on a 5-point scale from 1 (none of the day) to 5 (all day) with higher scores indicating greater depressive mood. Sample items include feeling worthless, hopeless, and so sad that nothing could cheer you up.
Anger/hostility.—
Anger/hostility items were also drawn from the Non-Specific Psychological Distress Scale (Kessler et al., 2002) with the same 5-point scaling as the depressive items such that a higher score indicates greater anger. The scale consisted of three items: angry, frustrated, and irritable (α = .78).
Covariates
Cortisol: CAR and AUC with respect to ground.—
The CAR is calculated by subtracting the nmol/L waking score from the 30min after waking nmol/L value and then dividing by the difference in time between the two samples (i.e., [Cort B − Cort A]/[Time B − Time A]) (Seltzer et al., 2010). The AUC with respect to ground is assessed by summing the mean of adjacent samples across the day weighted by the amount of time between the samples given that they were not equal (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). AUC was thus calculated as:
The daily levels of AUC and CAR cortisol were centered at the person-means to represent within-person effects (i.e., daily deviations from an individual’s own mean; Hoffman & Stawski, 2009). We also include the person-means of AUC and CAR cortisol as a between-person covariate (i.e., individual’s average across 8 days).
Care-related stressors.—
We assessed daily care-related stressors using the Daily Record of Behavior (DRB). Caregivers reported on the occurrence of behavior problems of the IWD during the previous 24hr from the time of the interview call (Fauth, Zarit, Femia, Hofer, & Stephens, 2006). The DRB includes 19 items from six categories: depressive behaviors, disruptive behaviors, memory-related behaviors, reality problems, restless behaviors, and resistance to help with activities of daily living, and up to three additional items caregivers identified that were not included in the list. Caregivers reported the occurrence of behavior problems for four periods of the day: waking to 9:00 a.m., 9:00 a.m. to 4:00 p.m., 4:00 p.m. to bedtime, and overnight. A frequency count was computed by summing the total number of behaviors reported from each time frame for a maximum count of 88 (α = .92). Scores were constructed by summing last night’s overnight behavior problems through today’s bedtime problems, in other words the four periods that would influence today’s mood. We include the person-mean centered score representing the daily deviations or within-person effects and the person-mean score as a between-person covariate of care-related stressors.
ADS use.—
Day care attendance was confirmed during the daily telephone interviews. Individuals’ total number of days using ADS was summed across the 8 study days to indicate the total amount of intervention received and included as a person-specific score.
Demographics.—
Caregivers’ age, gender (1 = female, 0 = male), and the duration of time providing care were all considered as controls. Current smoking status (1 = currently smoking) and steroidal medication use (1 = took a steroid medication) were considered as controls in analyses but were not significant and thus excluded for the parsimony of the final model.
Analysis
Before running models to test the research question regarding cortisol and daily mood, descriptive analyses were run to test the associations of the demographic variables with the level and variability in daily depressive mood and anger. Heterogeneous variance models were utilized which assume there are group differences in between- and within-person variance and examine the association of variables with variability in the outcome (Raudenbush & Bryk, 2002). We examined the association between age, gender, and duration of care with level and variability in mood. In this way, we can better determine the importance of controlling for our demographic variables in the multilevel models.
Next, multilevel modeling was used to examine variability in depressive mood and anger among caregivers (Raudenbush & Bryk, 2002). We utilized two-level multilevel models to examine the daily data which was nested within individuals thus consisting of two levels: day level and person level (SAS PROC MIXED; Littell, Miliken, Stroup, & Wolfinger, 1996). By partitioning the variability into two levels, the association between depressive mood/anger and daily cortisol can be estimated at each level such that the variation across days and between individuals is estimated. Separate multilevel models were run for depressive mood and anger (Model 1). To examine our research question regarding the effect of daily cortisol on depressed mood and anger, we modeled depressive mood and anger for the dth day in the ith person as:
Level 1 (within-person):
Level 2 (between-person):
| (Model 1) |
At Level 1 (within-person), daily mood (i.e., depressive mood or anger) is a function of an intercept (β0i, the mean level of mood for each individual averaged across days), CARdi (β1i, slope parameter for the within-person variability of the CAR), and the person-specific deviations from the intercept (e di). CARdi is a person-mean centered score to show daily fluctuations around one’s mean or within-person effects. At Level 2 (between-person), person-mean scores of CAR were entered as a between-person covariate (γ01, slope parameter for an individual’s average level of CAR across days). γ00 reflects the group mean level of individual mood levels and u 0i reflects individual deviations from that mean. Also, a γ10 represents the average level of (individual) within-person CAR slopes. This model was also run with AUC as a covariate of both anger and depressive mood.
We next examined whether the cortisol–mood association is accounted for by fluctuations in care-related stressors and service use (i.e., ADS) (Model 2).
Level 1 (within-person):
Level 2 (between-person):
| (Model 2) |
where the intercept (β0i) represents the mean level of depressive mood/anger for each individual (averaged across days). The first slope (β1i) represents the effect of today’s CAR on the caregiver’s daily depressive mood or anger. The second slope parameter (β2i) represents the effect of today’s care-related stressors on today’s mood. These covariates reflect deviations from an individual’s own mean, by centering around the person-mean.
At Level 2, person-mean levels of CAR cortisol (γ01), care-related stressors (γ02), and total amount of ADS use (γ03) are entered as between-person covariates of the level of depressive mood/anger at the intercept (β0i), each covariate indicating individuals’ average level of the covariate across days. Age (γ04), gender (γ05), and duration of care (γ06) were also entered as between-person level controls. γ10 and γ20 represent the average effects of within-person slopes for CAR and care-related stressors, respectively. The same model was repeated with AUC as a covariate of both anger and depressive mood.
Results
Demographic characteristics of caregivers and IWDs are given in Table 1. Across days, caregivers reported IWDs displaying a mean of 5.67 behavioral and psychological problems (i.e., care-related stressors; SD = 6.59; range: 0–63). On average, the IWDs spent 4.15 days in ADS (SD = 1.45; range: 1–6). Expression of anger and depressive mood symptoms was low but with moderate daily variation, with an average reported daily anger score at 2.09 (SD = 2.11; range: 0–10) and depressive mood at 2.51 (SD = 3.39; range: 0–24). Intraclass correlations of the two outcomes revealed that for depression, 63% of the variance is between-person and 37% is within-person, and for anger, 51% of the variance is between-person and 49% is within-person.
Next, heterogeneous variance models were run to determine the association of the demographic control variables with the level and variation in daily depressive mood and anger (Raudenbush & Bryk, 2002). Older individuals were found to have greater variability in daily depressive mood (B = 0.01, p < .01), however, age was not significantly associated with level of depressive mood. Similarly, duration of care was not associated with the level of depressive mood, yet individuals who had cared for their relative with dementia for less time had greater variability in depressive mood (B = −0.01, p < .001). Gender was not associated with level or variability in depressive mood. Neither age nor gender was associated with the level or variability in anger. Duration of care, however, was associated with both variability (B = −0.004, p < .001) and level (B = −0.01, p < .05) in caregiver anger such that individuals who had cared for less time had higher levels and greater variability in anger.
Model 1
In examining the base model regarding the association of cortisol and daily mood, multilevel models indicated that the between-person, mean level of CAR (γ01) was significantly associated with depressive mood (B = −0.09, p < .01) such that a reduced or blunted CAR on average was associated with more depressive mood (Table 2). The person-mean centered, within-person AUC (γ10) was significantly associated with anger (B = −0.003, p < .05) such that individuals had higher anger on a day their AUC was smaller than their personal average (Table 3). Depressive mood was not significantly associated with the within-person fluctuations in CAR or between- or within-person levels of AUC. Anger was not significantly associated with between-person levels of AUC or between- or within- person levels of CAR.
Table 2.
The Effects of CAR Cortisol, ADS Use, and Daily Stressors on the Depressive Mood and Anger of Family Caregivers
| Depressive mood | Anger | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| B (SE) | B (SE) | B (SE) | B (SE) | |
| Fixed effects | ||||
| Intercept, γ00 | 2.56*** (0.22) | 2.57*** (0.21) | 2.12*** (0.13) | 2.11*** (0.11) |
| Within-person covariatesa | ||||
| CAR, γ10 | −0.00 (0.01) | −0.00 (0.01) | −0.00 (0.00) | −0.00 (0.00) |
| Care-related stressors, γ20 | 0.14*** (0.02) | 0.10*** (0.01) | ||
| Between-person covariatesb | ||||
| CAR, γ01 | −0.09** (0.03) | −0.09** (0.03) | −0.02 (0.02) | −0.02 (0.02) |
| Care-related stressors, γ02 | 0.15*** (0.03) | 0.11*** (0.02) | ||
| Number of ADS use, γ03 | −0.20 (0.14) | −0.10 (0.08) | ||
| Between-person controls | ||||
| Age, γ04 | 0.04 (0.02) | −0.01 (0.01) | ||
| Female (yes = 1), γ05 | −0.06 (0.64) | −0.15 (0.34) | ||
| Duration of care, γ06 | −0.00 (0.00) | −0.00 (0.00) | ||
| Random effects | ||||
| Intercept, σ2 u0 | 7.63*** (0.92) | 6.55*** (0.81) | 2.25*** (0.29) | 1.72*** (0.23) |
| Residual, σ2 e1 | 4.13*** (0.19) | 3.86*** (0.17) | 2.28*** (0.10) | 2.14*** (0.10) |
| −2 Log likelihood | 5298.3 | 5229.2 | 4532.5 | 4460.9 |
Notes. Participant N = 164; observation N = 1,142. ADS = adult day services; CAR = cortisol awakening response.
aPerson-mean centered scores (i.e., time-varying).
bPerson-mean scores across days (i.e., time-invariant).
**p < .01. *** p < .001.
Table 3.
The Effects of AUC Cortisol, ADS Use, and Daily Stressors on the Depressive Mood and Anger of Family Caregivers
| Depressive mood | Anger | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 2 | Model 2 | |
| B (SE) | B (SE) | B (SE) | B (SE) | |
| Fixed effects | ||||
| Intercept, γ00 | 2.57*** (0.25) | 2.58*** (0.22) | 2.10*** (0.13) | 2.09*** (0.11) |
| Within-person covariatesa | ||||
| AUC, γ10 | −0.00 (0.00) | 0.00 (0.00) | −0.00* (0.00) | −0.00 (0.00) |
| Care-related stressors, γ20 | 0.14*** (0.02) | 0.10*** (0.01) | ||
| Between-person covariatesb | ||||
| AUC, γ01 | −0.01 (0.01) | −0.01 (0.01) | −0.00 (0.00) | 0.00 (0.00) |
| Care-related stressors, γ02 | 0.15*** (0.03) | 0.10*** (0.02) | ||
| Number of ADS use, γ03 | −0.15 (0.15) | −0.08 (0.08) | ||
| Between-person controls | ||||
| Age, γ04 | 0.04* (0.02) | −0.01 (0.01) | ||
| Female (yes = 1), γ05 | −0.53 (0.68) | −0.39 (0.35) | ||
| Duration of care, γ06 | −0.01 (0.00) | −0.01* (0.00) | ||
| Random effects | ||||
| Intercept, σ2 u0 | 8.42*** (1.04) | 7.35*** (0.93) | 2.24*** (0.30) | 1.73*** (0.24) |
| Residual, σ2 e1 | 4.22*** (0.21) | 3.95*** (0.19) | 2.31*** (0.11) | 2.17*** (0.11) |
| −2 Log likelihood | 4729.6 | 4673.0 | 4022.6 | 3959.9 |
Notes. Participant N = 164; observation N = 1,005. ADS = adult day services; AUC = area under the curve.
aPerson-mean centered scores (i.e., time-varying).
bPerson-mean scores across days (i.e., time-invariant).
*p < .05. *** p < .001.
Model 2
Next, we examined whether cortisol would be associated with daily mood when taking into account care-related stressors and ADS use. As shown in Table 2, the between-person, person-mean score CAR was significantly associated with depressive mood, reflecting an association between a hypocortisol pattern and depression, after entering stressors and ADS use. Both between- and within-person levels of care-related stressors were also associated with depressive mood. Total ADS use did not have a significant association with depressive mood or anger. As shown in Table 3, the within-person association of AUC cortisol with anger (γ10) was not significant after entering stressors and ADS use (B = −0.0053, p < .05 in Model 1; B = −0.0028, not significant in Model 2). However, both between- and within-person levels of care-related stressors were associated with anger.
Discussion
Our findings suggest that hypocortisolemic or blunted cortisol patterns are associated with negative mood under conditions of chronic stress. We found that the between-person level, person-mean CAR was associated with depressive mood such that individuals with smaller CARs had more depressive symptoms. This association was found in Model 1 and in Model 2 including stressors, ADS use, and demographics. Anger was significantly associated with the within-person daily fluctuations in AUC in Model 1 such that a day with a below average AUC output of cortisol for an individual was associated with greater anger. This association did not hold in Model 2.
Though prior research has found both hypo- and hypercortisol to be associated with depression and anger, our findings lend further support to studies finding hypocortisol patterns in populations experiencing chronic stress. For example, the hypocortisol pattern has been found in mothers caring for adolescent or adult children with autism spectrum disorder or other serious mental disorders, women caregivers for IWDs, nonelderly caregivers, parents of cancer patients, employees experiencing burnout, combat veterans, Holocaust survivors, and victims of domestic violence (Barker, Greenberg, Seltzer, & Almeida, 2012; Holland et al., 2011; Miller, Cohen, & Ritchey, 2002; Seedat, Stein, Kennedy, & Hauger, 2003; Seltzer et al., 2009, 2010; Sonnenschein et al., 2007; Yehuda et al., 1995; Yehuda, Boisonuae, Lowy, & Giller, 1995). Furthermore, a recent meta-analysis supports the finding that a blunted awakening response is common among people experiencing chronic stress (Miller, Chen, & Zhou, 2007).
Likewise a blunted cortisol pattern has been found in prior work examining individuals with depression, and in particular among older adult samples (Bremmer et al., 2007; Burke et al., 2005; Heaney et al., 2010). Our sample of family caregivers, who are 61.79 years old on average and are experiencing the chronic stress associated with caring for a loved one with dementia, may face a heightened, accumulated allostatic load, or bodily wear and tear, that is associated with hypocortisolism (Almeida, McGonagle, et al., 2009). Though anger’s association with cortisol has been examined less frequently than depression, a hypercortisol pattern is also commonly found (Adam et al., 2006; Kemeny & Shestyuk, 2008; Moons et al., 2010; Ranjit et al., 2009; Steptoe et al., 2000). Our findings differ from these prior studies in that greater anger was associated with less cortisol output, the hypocortisol pattern. It may be that a blunted cortisol pattern leaves caregivers with fewer cognitive and emotional resources for controlling angry outbursts. Our study suggests that negative mood states such as anger and depression are similarly associated with a dysregulated cortisol pattern of hypocortisolemia under conditions of chronic stress. This is a unique contribution given that prior work has predominantly associated feelings of anger with hypercortisolemia patterns.
Consistent with prior studies (Alspaugh, Stephens, Townsend, Zarit, & Greene, 1999; Schulz et al., 1995), we found that care-related stressors were significantly associated with both depressive mood and anger. In a post hoc analysis, we tested for an association between care-related stressors and cortisol and did not find a significant association. Daily care-related stressors are only one important facet of the stress that may be experienced by family caregivers and a lack of covariation with HPA activity has been cited (Hellhammer et al., 2009). Amount of ADS use across the 8 days did not have a significant association with depressive mood or anger. Prior research has found that ADS use significantly reduces exposure to stressors (Zarit et al., 2011), and therefore future research may consider whether stressors mediate the association between day care use and mental health.
Limitations
There are several limitations to the study. First, we do not have information on past history of mental health problems in our caregivers. Additionally, while our within-person approach allows us to compare caregivers to themselves by examining day-to-day fluctuations in mood, cortisol, and stressors, we were not able to compare the association of diurnal cortisol with daily mood in our sample with caregivers not using ADS. Our use of a daily diary design offered a rare opportunity to examine what impacts caregiver’s mental health daily, but limited the measures included so as not to over-burden the caregivers. Therefore, other constructs such as control, cultural issues, or participation in other interventions which may impact daily mood were not included. Furthermore, our depressive mood and anger scales indicate only expression of symptoms, not a diagnosable depression or anger/hostility disorder. Though variability was moderate, symptom levels were low which may reflect a sample of caregivers who were using a respite intervention and willing to participate in our study. The caregivers self-selected into our study by volunteering and thus there may be an element of selection bias as caregivers who had time to participate may have lower levels of stress. The lack of gender difference may be due to the small sample of male caregivers who have above average levels of depressive symptoms. However, the observable sample characteristics of our caregivers compare similarly to other studies of dementia caregivers.
Implications and future directions
This study provides support for an association of hypocortisolemia with negative mood in a sample of dementia caregivers, adding to the existing body of work on diurnal cortisol patterns in other populations experiencing chronic stress. We are uniquely able to consider cortisol associations with both depressive mood and anger, an infrequently considered though common emotion, as well as within-person variability in cortisol patterns over 8 days. Our findings further substantiate the known association between care-related stressors with depressive mood and anger. Future work should not ignore the importance of cortisol, as a biomarker of the stress process, in association with daily mood in family caregivers and other individuals experiencing prolonged stress. Determining how to prevent a blunted diurnal pattern may help caregivers more adaptively respond to stress, provide better care for the IWD, and reduce their risk for a number of mental and physical health concerns.
Funding
This work was supported by grant RO1 AG031758 from the National Institute on Aging . Dr. Leggett is funded by a National Institute of Mental Health (T32 MH073553) Geriatric Mental Health Services Fellowship.
References
- Adam E. K., Hawkley L. C., Kudielka B. M., Cacioppo J. T. (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, 103, 17058–17063. 10.1073/pnas.0605053103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida D. M., McGonagle K., King H. (2009). Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology, 55, 219–237. 10.1080/19485560903382338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida D. M., Piazza J. R., Stawski R. S. (2009). Inter-individual differences and intra individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging, 24, 819–827. 10.1037/a0017910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. E. L., Stephens M. A. P., Townsend A. L., Zarit S. H., Greene R. (1999). Longitudinal patterns of risk for depression in dementia caregivers: Objective and subjective primary stress as predictors. Psychology and Aging, 14, 34–43. 10.1037/0882-7974.14.1.34 [DOI] [PubMed] [Google Scholar]
- Aneshensel C. S., Pearlin L. I., Mullan J. T., Zarit S. H., Whitlatch C. J. (1995). Profiles in caregiving: The unexpected career. New York: Academic Press. 10.1016/B978-012059540-2/50003-X [Google Scholar]
- Barker E. T., Greenberg J. S., Seltzer M. M., Almeida D. M. (2012). Daily stress and cortisol patterns in parents of adult children with a serious mental illness. Health Psychology, 31, 130–134. 10.1037/a0025325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer M. A., Deeg D. J. H., Beekman A. T. F., Pennix B. W. J. H., Lips P., Hoogendijk W. J. G. (2007). Major depression in late life is associated with both hypo- and hypercortisolemia. Biological Psychiatry, 62, 479–486. 10.1016/j.biopsych.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Burke H. M., Davis M. C., Otte C., Mohr D. C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30, 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Cuijpers P. (2005). Depressive disorders in caregivers of dementia patients: A systematic review. Aging and Mental Health, 9, 325–330. 10.1080/13607860500090078 [DOI] [PubMed] [Google Scholar]
- Fauth E. B., Zarit S. H., Femia E. E., Hofer S. M., Stephens M. A. P. (2006). Behavioral and psychological symptoms of dementia and caregivers’ stress appraisals: Intra-individual stability and change over short-term observations. Aging and Mental Health, 10, 563–573. 10.1080/13607860600638107 [DOI] [PubMed] [Google Scholar]
- Gallagher D., Wrabetz A., Lovett S., DelMaestro S., Rose J. (1989). Depression and other negative effects in family caregivers. In Light E., Lebowitz B. (Eds.), Alzheimer’s disease treatment and family stress: Directions for future research (pp. 218–244). Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Granger D. A., Hibel L. C., Fortunato C. K., Kapelewski C. H. (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34, 1437–1448. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Heaney J. L. J., Phillips A. C., Carroll D. (2010). Ageing, depression, anxiety, social support and the diurnal rhythm and awakening response of salivary cortisol. International Journal of Psychophysiology, 78, 201–208. 10.1016/j.ijpsycho.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Hellhammer D. H., Wüst S., Kudielka B. M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34, 163–171. 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Hoffman L., Stawski R. S. (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–120. 10.1080/15427600902911189 [Google Scholar]
- Holland J. M., Thompson L. W., Cucciare M. A., Tsuda A., Okamura H., Spiegel D, … Gallagher-Thompson D. (2011). Cortisol outcomes among Caucasian and Latina/Hispanic women caring for a family member with dementia: A preliminary examination of psychosocial predictors and effects of a psychoeducational intervention. Stress and Health, 27, 334–346. 10.1002/smi.1375 [Google Scholar]
- Holsboer F. (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology, 23, 477–501. 10.1016/S0893-133X(00)00159-7 [DOI] [PubMed] [Google Scholar]
- Jonsdottir I. H., Halford C., Eek F. (2012). Mental health and salivary cortisol. In Kristenson M., Garvin P., Lundberg U. (Eds.), The role of saliva cortisol measurement in health and disease (pp. 132–172). Bentham eBooks. 10.2174/97816080534211120101 [Google Scholar]
- Kemeny M. E., Shestyuk A. (2008). Emotions, the neuroendocrine and immune systems, and health. In Lewis M., Haviland-Jones J. M., Barrett L. F. (Eds.), Handbook of emotions (pp. 661–675). New York: Guilford Press. [Google Scholar]
- Kessler R. C., Andrews G., Colpe L. J., Hiripi E., Mroczek D. K., Normand S. -L. T, … Zaslavsky A. M. (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine, 32, 959–976. 10.1017/S0033291702006074 [DOI] [PubMed] [Google Scholar]
- Kurina L. M., Schneider B., Waite L. J. (2004). Stress, symptoms of depression and anxiety, and cortisol patterns in working parents. Stress and Health, 20, 53–63. 10.1002/smi.998 [Google Scholar]
- Littell R. C., Miliken G. A., Stroup W. W., Wolfinger R. D. (1996). SAS systems for mixed models. Cary, NC: SAS Institute. [Google Scholar]
- Miller G., Chen E., Zhou E. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Miller G. E., Cohen S., Ritchey A. K. (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychology, 21, 531–541. 10.1037/0278-6133.21.6.531 [DOI] [PubMed] [Google Scholar]
- Miller T. Q., Smith T. W., Turner C. W., Guijarro M. L., Hallet A. J. (1996). Meta-analytic review of research on hostility and physical health. Psychological Bulletin, 119, 322–348. 10.1037/0033-2909.119.2.322 [DOI] [PubMed] [Google Scholar]
- Moons W. G., Eisenberger N. I., Taylor S. E. (2010). Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity, 24, 215–219. 10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Pearlin L. I., Mullan J. T., Semple S. J., Skaff M. M. (1990). Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist, 30, 583–594. 10.1093/geront/30.5.583 [DOI] [PubMed] [Google Scholar]
- Piazza J. R., Almeida D. M., Dmitrieva N. O., Klein L. C. (2010). Frontiers in the use of biomarkers of health in research on stress and aging. Journal of Gerontology: Psychological Sciences, 65B, 513–525. 10.1093/geronb/gbq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J. C., Kirschbaum C., Meinlschmid G., Hellhammer D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Ranjit N., Diez-Roux A. V., Sanchez B., Seeman T., Shea S., Shrager S, … Watson K. (2009). Association of salivary cortisol circadian pattern with cynical hostility: Multi-ethnic study of atherosclerosis. Psychosomatic Medicine, 71, 748–755. 10.1097/PSY.0b013e3181ad23e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., Bryk A. S. (2002). Hierarchical linear models: Applications and data analysis methods (2nd ed.). London, England: Sage. [Google Scholar]
- Rugulies R. (2002). Depression as a predictor for coronary heart disease: A review and meta-analysis. American Journal of Preventive Medicine, 23, 51–61. 10.1016/S0749-3797(02)00439-7 [DOI] [PubMed] [Google Scholar]
- Schulz R., O’Brien A. T., Bookwala J., Fleissner K. (1995). Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. The Gerontologist, 35, 771–791. 10.1093/geront/35.6.771 [DOI] [PubMed] [Google Scholar]
- Seedat S., Stein M. B., Kennedy C. M., Hauger R. L. (2003). Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology, 28, 796–808. 10.1016/S0306-4530(02)00086-0 [DOI] [PubMed] [Google Scholar]
- Seltzer M. M., Almeida D. M., Greenberg J. S., Savla J., Stawski R. S., Hong J, … Taylor J. L. (2009). Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior, 50, 1–15. 10.1177/002214650905000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer M. M., Greenberg J. S., Hong J., Smith L. E., Almeida D. M., Coe C, … Stawski R. S. (2010). Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders, 40, 457–469. 10.1007/s10803-009-0887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein M., Mommersteeg P. M. C., Houtveen J. H., Sorbi M. J., Schaufeli W. G., van Dooren L. J. P. (2007). Exhaustion and endocrine functioning in clinical burnout: An in-depth study using the experience sampling method. Biological Psychology, 75, 176–184. 10.1016/j.biopsycho.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Steffen A. M., Merritt S. (2012). Idiographic anger ratings by female dementia family caregivers: Generalizability and preliminary validity. Clinical Gerontologist, 35, 205–220. 10.1080/07317115.2012.657821 [Google Scholar]
- Steptoe A., Cropley M., Griffith J., Kirschbaum C., Steptoe A., Cropley M, … Kirschbaum C. (2000). Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosomatic Medicine, 62, 286–292. 10.1097/00006842-200003000-00022 [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Gold P. W. (2002). The mind-body interaction in disease. Scientific American (Updated from The Mysteries of the Mind: Special Issue, 1997, 82–89. [Google Scholar]
- Wolkowitz O. M., Burke H., Epel E. S., Reus V. I. (2009). Glucocorticoids: Mood, memory, and mechanisms. Glucocorticoids and Mood, 1179, 19–40. 10.1111/j/1749-6632.2009.04980.x [DOI] [PubMed] [Google Scholar]
- Yehuda R., Boisonuae D., Lowy M. T., Giller E. (1995). Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without post-traumatic stress disorder. Archives of General Psychiatry, 52, 583–593. 10.1016/S0006-3223(02)01357-4 [DOI] [PubMed] [Google Scholar]
- Yehuda R., Kahana B., Binder-Brynes K., Southwick S. M., Mason J. W., Giller E. L. (1995). Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. American Journal of Psychiatry, 152, 982–986. 10.1016/0006-3223(94)91000–6 [DOI] [PubMed] [Google Scholar]
- Zarit S. H. (2008). Diagnosis and management of caregiver burden in dementia. Handbook of Clinical Neurology, 89, 905–910. 10.1016/S0072-9752(07)01209-2 [DOI] [PubMed] [Google Scholar]
- Zarit S. H., Kim K., Femia E. E., Almeida D. M., Savla J., Molenaar P. C. M. (2011). Effects of adult day care on daily stress of caregivers: A within-person approach. Journal of Gerontology: Psychological Sciences, 66B, 538–546. 10.1093/geronb/gbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]


