Abstract
Introduction:
Understanding the mechanisms by which bupropion promotes smoking cessation may lead to more effective treatment. To the extent that reduced smoking reinforcement is one such mechanism, a longer duration of pre quit bupropion treatment should promote extinction of smoking behavior. We evaluated whether 4 weeks of pre quit bupropion (extended run-in) results in greater pre quit reductions in smoking rate and cotinine and, secondarily, greater short-term abstinence, than standard 1 week of pre quit bupropion (standard run-in).
Methods:
Adult smokers (n = 95; 48 females) were randomized to a standard run-in group (n = 48; 3-week placebo, then 1-week bupropion pre quit) or an extended run-in group (4-week pre quit bupropion; n = 47). Both groups received group behavioral counseling and 7 weeks of post quit bupropion. Smoking rate (and craving, withdrawal, and subjective effects) was collected daily during the pre quit period; biochemical data (cotinine and carbon monoxide) were collected at study visits.
Results:
During the pre quit period, the extended run-in group exhibited a greater decrease in smoking rate, compared to the standard run-in group, interaction p = .03. Cigarette craving and salivary cotinine followed a similar pattern, though the latter was evident only among women. Biochemically verified 4-week continuous abstinence rates were higher in the extended run-in group (53%) than the standard run-in group (31%), p = .033.
Conclusions:
The extended use of bupropion prior to a quit attempt reduces smoking behavior during the pre quit period and improved short-term abstinence rates. The data are consistent with an extinction-of-reinforcement model and support further investigation of extended run-in bupropion therapy for smoking cessation.
Introduction
In 1997, bupropion became the first non-nicotine pharmacotherapy approved by the U.S. Food and Drug Administration for smoking cessation.1,2 While bupropion approximately doubles the odds of cessation relative to placebo, cessation rates at 6 months are modest with an average of 25% of participants in randomized clinical trials remaining smoke-free.3,4 These results are comparable to quit rates with nicotine replacement therapy and somewhat lower than quit rates with varenicline.5
An improved understanding of the mechanisms by which bupropion works may lead to more targeted and effective use of bupropion for smoking cessation.6–8 Most clinical studies to date have focused on bupropion’s ability to attenuate post cessation increases in withdrawal, craving, or general negative affect. Although each of these effects is robust, evidence that they actually mediate bupropion’s efficacy for smoking cessation is mixed.9,10
The neurobiological actions of bupropion suggest that smoking reinforcement should be evaluated as a treatment mechanism. Bupropion is a stimulator and weak reuptake inhibitor of both norepinephrine and dopamine, as well as a nicotinic acetylcholine receptor antagonist.11 These effects may reduce positive reinforcement from smoking.12,13 Indeed, preclinical data suggest that chronic bupropion reduces nicotine self-administration in rats14–16 and responding for stimuli associated with nicotine,17 but see Paterson and colleagues.18 As noted by Cryan and colleagues,19 these findings “give neurobiological credence to the clinical practice of initiating bupropion therapy prior to nicotine cessation…bupropion may act…to attenuate the rewarding effects of nicotine, thus increasing the likelihood of cessation” (p.355).
In humans, there is surprisingly little research on the effect of bupropion on smoking reinforcement.20 Post quit data from clinical trials demonstrate that bupropion reduces subjective reward and satisfaction during smoking lapses.21 However, to more fully determine the effect of bupropion on smoking reinforcement, it is important to examine the pre quit period, paralleling the animal literature on self-administration. This also makes sense clinically, as bupropion is typically administered for a week prior to quitting,1 offering a window for bupropion to attenuate reinforcement during typical smoking.22
From a learning perspective, the blockade of reinforcement should result in extinction, a decrease in the frequency of smoking. Although acute bupropion increased the ad libitum smoking among non-treatment-seeking smokers,23 this increase could reflect an extinction burst, a temporary increase in behavior in the context of reinforcement blockade, that is followed by a reduction in behavior over time.24,25 For extinction to occur, participants must continue smoking in order to learn that the reinforcing effects are attenuated.
In this study, we extend the typical 1 week of pre quit bupropion for two reasons. First, extinction is greatest when numerous “trials” are conducted over a prolonged period of time.26–30 Given that bupropion and the metabolite hydroxybupropion do not reach steady-state concentrations until 5–8 days, a longer duration of bupropion pre quit treatment is likely necessary to adequately test the extinction mechanism. Second, and relatedly, extinction is context-specific. Simply changing the physical environment renews previously extinguished behavior26,28 and craving.31 It may be critical to extinguish smoking across a range of physical, social, and affective contexts.29 Because learning theory predicts that extinction in smokers does not generalize from one trigger situation (e.g., while driving or on the phone) to another (e.g., while drinking coffee or alcohol or under stress), it is important that smokers take bupropion and continue smoking long enough to allow repeated exposures to a variety of contexts.
Given the target population, we recruited smokers motivated to quit32,33 and randomized them to the standard pre quit run-in of 1 week or an extended pre quit run-in of 4 weeks (following prior extinction work).24,34–37 Based on an extinction-of-reinforcement framework, we predicted that the extended bupropion run-in group would exhibit greater pre quit reductions in smoking rate and salivary cotinine compared with the standard bupropion run-in group. We also examined pre quit changes in overnight withdrawal and craving and in the subjective effects of smoking the first cigarette of the day. Though the study was under-powered to detect group differences in cessation, we provide preliminary data on short-term (4-week) continuous abstinence from smoking.
Methods
Participants
Radio, television, newspaper ads, and flyers in the community were used to recruit 95 adult (18–65 years old) treatment-seeking heavy cigarette smokers (at least 15 cigarettes per day) motivated to quit in the next 3 months. Exclusion criteria included self reported bupropion allergy, chronic renal or hepatic disease, history of head trauma or seizures, central nervous system tumor, insulin-treated diabetes, or uncontrolled hypertension; active cancer treatment; current use of St. John’s Wart, antipsychotics, antidepressants, theophylline, systemic steroids, over-the-counter stimulants and anorectics, L-Dopa, or recent discontinuation of a benzodiazepine; currently pregnant (confirmed with urine screen) or lactating; history of bulimia or anorexia nervosa; and current substance dependence or abuse, psychosis, or depression per MINI International Neuropsychiatric Interview (MINI).38 Participants received remuneration for attending study visits and adherence to study procedures, as detailed below.
Study Design and Medication
All procedures were approved by the Roswell Park Cancer Institute IRB. This experiment was designed and powered to evaluate the effects of extended pre quit bupropion treatment on changes in smoking behavior before participants tried to cut down or quit smoking. Thus, participants were randomized (double-blind) to either 4 weeks of bupropion SR (purchased from GlaxoSmithKline) prior to the target quit day (TQD) (extended run-in group) or to 3 weeks of placebo (visually identical to bupropion, purchased from the University of Pennsylvania), followed by 1 week of bupropion SR prior to TQD (standard run-in group). The initial week of treatment followed GSK guidelines and Phase III clinical trials1,2,39: 1 tablet (150mg by mouth) once daily for 3 days, then 1 tablet (150mg by mouth) twice daily. Both groups received a standard 7-week course of post-TQD bupropion. Prior quit attempts using bupropion were uncommon (n = 3 and 2 in the standard and extended run-in groups, respectively).
Procedures
During an orientation/baseline session (Visit 1) participants were given a study overview and provided informed consent, after which they completed assessments of smoking behavior (e.g., nicotine dependence40, smoking history), demographics, personality, psychiatric disorders (MINI), and met with the study physician. Eligible participants received instruction regarding daily smoking diary procedures and a visit schedule.
At Visit 2 (Day 8), participants were randomized to either the standard run-in group (n = 48) or the extended run-in group (n = 47). During Visit 2 and subsequent visits (Visit 3 [Day 15], Visit 4 [Day 29], Visit 5 [Day 36; target quit date], Visit 6 [Day 50], Visit 7 [Day 64]), participants received medication and group counseling39; assessments included collection and review of daily smoking diary data, vital signs, side effects, carbon monoxide (CO), and saliva for assessment of cotinine.
Counseling groups included participants in both run-in conditions. Thus, counseling focused on standard smoking cessation topics,39 including honing motivation and social support for quitting, identifying smoking triggers and developing coping strategies, and relapse prevention. Participants were not informed of the extinction rationale or hypothesis. Therefore, participants were not advised to smoke in a manner that might facilitate extinction, except that during the pre quit period, participants were asked to continue smoking at least 25% of their baseline rate, following their urges to smoke, to allow their bodies time to adjust to the medication.24,35
Measures
Primary outcomes were self-reported cigarettes smoked per day and salivary cotinine during the pre quit period. Secondary outcomes were pre quit craving, withdrawal, and subjective effects, as well as short-term (4-week) continuous abstinence.
All pre quit measures except cotinine were collected via a daily diary, which began 1 week prior to the randomization visit and continued throughout the 4-week pre-TQD phase (daily data for the first 5 participants in the study were lost due to multiple data collection, upload, and integrity issues with the SmokeSignals Pro [MedSignals Inc.] electronic cigarette case; thereafter we moved to diary format). Timing of daily assessments was based on the first cigarette of the day. Prior to smoking, participants recorded the number of cigarettes smoked the previous day, indicated the time of waking and time of report, and completed a 5-item craving measure41 and the Minnesota Nicotine Withdrawal Scale.42 Participants then smoked the first cigarette of the day and completed the Subjective Effects of Smoking scale43 (e.g., satisfying, good taste; scale = 0 “not at all” to 4 “extreme”).
To prevent many of the issues inherent in diary data,44 participants were required to document completion of the paper diary via voicemail each day within 1hr of completing the assessments (participants dictated their responses, which were transcribed by a research assistant and later compared to physical diaries returned at each visit). To enhance compliance with daily assessments, a bonus was offered for reporting on more than the required minimum of 3 days per week (i.e., $5 for 4 days… $20 for all 7 days).
During the pre quit period, salivary cotinine obtained at the end of the baseline week (Visit 2) and the end of the 3-week drug manipulation phase (Visit 4) provided an additional measure of cigarette smoking.34 (Budgetary constraints prohibited assaying cotinine at Visits 1, 3, and 6.) Saliva samples were stored at −80 °C until shipped to Salimetrics for duplicate enzyme immunoassay. Cotinine analyses were conducted on n = 78, after excluding participants for whom Visit 2 samples were not stored properly (n = 13) and participants with inadequate or contaminated samples (n = 4). Pre quit CO was not a reasonable alternative because logistical issues within the clinical research setting led to marked and variable delays between arrival and assessment of CO, particularly at Visits 1 and 2.
Continuous abstinence after the target quit day was a secondary outcome, as in other studies of extended pre quit medication.45,46 Timeline follow-back interviews were conducted at each post quit follow-up and bio-verified with CO samples obtained at each visit, using a cut-off of 8 ppm.47–49 Although we had planned to focus on 3-month continuous abstinence rates, compliance with study visits decreased markedly after the 4-week follow-up (Visit 7; the last visit in which counseling and study medication were provided). Given that the majority of relapse occurs within the first few weeks after quitting6,50–52 and the exploratory nature of the abstinence data in this study, we focus on rates of 4-week continuous abstinence (not even a single puff, per self-report, and negative CO at all three in-person visits).53
Analyses
To evaluate pre quit changes in smoking rate (and secondary outcomes of craving, withdrawal, and subjective effects), piecewise linear mixed models were estimated (SPSS MIXED) for the baseline week (days 1–7; base), the 3-week pre quit intervention period (days 8–28; drug manipulation), and the final week prior to the TQD (days 29–35; final pre-TQD).35 Random intercept and slopes were included in the models and a first-order auto-regressive covariance structure was employed.54 For all models, run-in group (standard vs. extended) was included as between-subjects factors. In addition, given that participant sex often moderates the behavioral pharmacology of nicotine and smoking,55,56 as well as cessation,57,58 we explored the moderating role of sex in the effects of pre quit duration.
To evaluate pre quit changes in salivary cotinine, a run-in group × sex × time repeated measures ANOVA examined change in cotinine from the end of the baseline week (day 8, Visit 2) to the end of the drug manipulation phase (day 29, Visit 4).
For 4-week continuous abstinence, logistic regression analyses were used to test the effect of treatment condition on cessation outcome. Run-in group (standard vs. extended) and sex were included as between-subjects factors and the 2-way interaction was tested. Odds ratios and 95% confidence intervals (CIs) are reported and all significance tests were 2-tailed and set at α = .05. Participants lost to follow-up were assumed to be smoking (n = 9; 4 extended run-in).
Results
Participant Characteristics
As shown in Table 1, the run-in groups did not significantly differ on a range of demographic and smoking variables. Compliance with the daily diaries was excellent (approximately 33 out of 35 days, on average) and the number of days of daily data completed did not differ between groups, F < 1.
Table 1.
Demographic and Tobacco Use Characteristics at Baseline
| Run-in group | |||
|---|---|---|---|
| Standard (n = 48) | Extended (n = 47) | p value | |
| Age, years | 46.7 (9.3) | 45.8 (10.1) | .68 |
| Sex, female | 54% | 48% | .76 |
| Racial/ethnic minority | 10% | 8% | .75 |
| Education beyond high school | 45% | 43% | .84 |
| Married, n (%) | 27 (55%) | 22 (45%) | .36 |
| Income | 40K–55K | 25K–39K | .06 |
| Cigarettes per day | 22.0 (6.0) | 23.4 (7.9) | .33 |
| FTND | 5.8 (1.7) | 6.0 (1.9) | .50 |
| Years smoking | 28.8 (9.0) | 26.7 (11) | .34 |
| Daily assessment compliancea | 96% | 94% | .46 |
FTND = Fagerström Test of Nicotine Dependence. Values are mean (SD) unless otherwise noted.
a n = 90 (46 standard run-in group) for daily assessments.
Pre quit Cigarettes Smoked Per Day
During the baseline week, cigarette smoking rate was comparable between run-in groups and across days, Fs < 1. During the drug manipulation phase, the critical run-in group × time interaction was significant, F(1,86) = 4.7, p = .03; follow-up tests were consistent with a greater average decrease in cigarettes smoked per day (CPD) over time in the extended run-in group, b = −0.26, p = 1.1×10−9 compared to the standard run-in group, b = −0.16, p = 1.4×10−6 (Figure 1). CPD continued to decrease across the final pre-TQD week when all participants were taking bupropion, time F(1,86) = 10.3, p = .002, but the rate of decline did not vary by run-in group or sex, Fs < 1. None of the run-in group × sex × time interactions were significant, Fs < 1.
Figure 1.
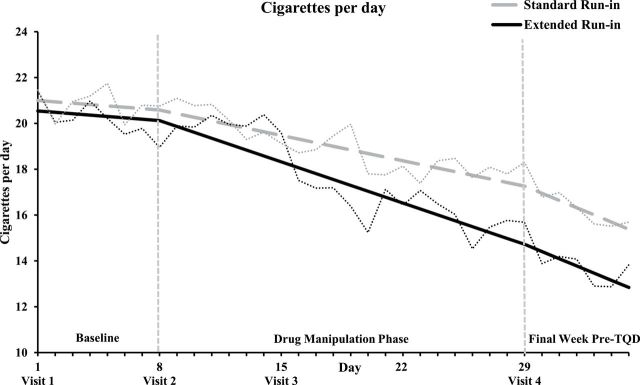
Cigarettes smoked per day across the 35-day pre quit period for each run-in group. Note. Solid lines represent predicted values based on parameter estimates from mixed models. Dotted lines represent raw values. Vertical dashed lines denote the three phases of the pre quit period: baseline (days 1–7), drug manipulation phase (days 8–28), final week pre target quit day (days 29–35).
Pre quit Salivary Cotinine
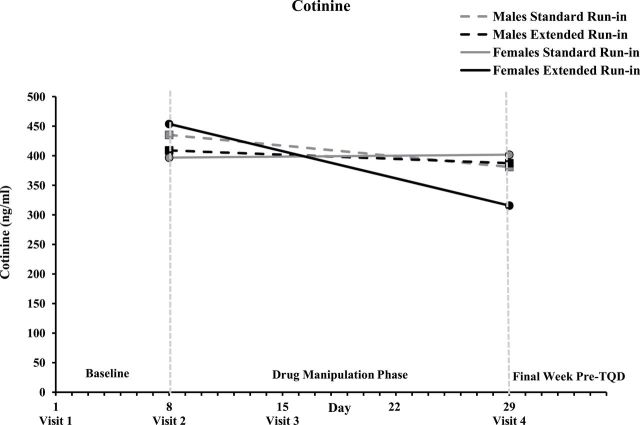
Cotinine levels did not differ by group and/or sex at the end of the baseline week, Fs < 1. As can be seen in Figure 2, the predicted pattern of a greater reduction in cotinine from the end of the baseline week to the end of the 3-week drug manipulation phase was observed among women, run-in group × time F(1,41) = 5.3, p = .027, but not men, F < 1, run-in group × sex × time F(1,74) = 4.4, p = .039. Women in the extended run-in group exhibited a significant reduction across the drug manipulation phase, p = .001; ps > .21 in all other run-in group × sex cells.
Figure 2.
Mean salivary cotinine at the end of the baseline week (Visit 2) and end of the drug manipulation phase (Visit 4) for all run-in group × sex conditions.
Secondary Pre quit Subjective Measures
At the beginning of the baseline week (Day 1), mean morning craving was just above the midpoint of the 0–4 scale (mean[SD] = 2.4[1.0]), satisfaction with the first cigarette of the day was just below the scale midpoint (mean[SD] = 1.7[0.7]), and morning withdrawal was minimal (mean[SD] = 0.3[0.4]). During the baseline week, there were significant decreases in morning craving and satisfaction with the first cigarette of the day, Fs(1,84 and 1,86) = 28.8 and 129.6, bs = −0.05 and −0.07, ps < .001, but not morning withdrawal symptoms, p = .41. Changes during the baseline week were unrelated to run-in group and sex, Fs < 1.
Across the critical 3-week drug manipulation phase, the modest further decreases in craving tended to be greater in the extended run-in group, t(42) = −6.5, b = −0.033, p = .3×10−7, compared to the standard run-in group, t(45) = −4.5, b = −0.02, p = .0001, but the run-in group × time interaction was marginal F(1,85) = 3.6, p = .06. Smoking satisfaction and withdrawal symptoms declined across the drug manipulation phase, Fs = 48.6 and 11.4, bs = −0.01 and −0.004, ps < .001 (with the decrease in withdrawal driven primarily by men, sex × time F(1,84) = 4.4, p = .04), but these effects were not moderated by run-in group, Fs < 1.
During the final week pre-TQD, when all participants were taking bupropion, there were no further declines in smoking satisfaction or craving, Fs < 1. Withdrawal symptoms increased across the week leading up to the TQD, but remained near the floor of the scale, Day 34 (mean[SD] = 0.5[0.6]), F(1,81) = 32.1, b = 0.06, p < .001, independent of run-in group and sex, Fs < 1.
Secondary Clinical Endpoint—Short-Term Abstinence
Biochemically verified 4-week continuous abstinence rates were higher in the extended run-in group (53%) than the standard run-in group (31%), p = .033, OR = 2.5, 95% CI = 1.1–6.0). Although females were less likely to be abstinent compared to males (32% and 53%, respectively, p = .039, OR = 0.40, 95% CI = 0.17–0.95), there was no indication of a meaningful run-in group × sex interaction, p = .57. At the request of an anonymous reviewer, we also examined CO-verified 7-day point prevalence of abstinence at 4 weeks post-TQD: point prevalence abstinence rates were higher in the extended run-in group (72%) than the standard run-in group (44%), p = .006, and tended to be lower among females (50%) than males (67%), p = .10; interaction p = .53.
Discussion
The present study evaluated the hypothesis that extending the pre quit run-in period for bupropion from 1 to 4 weeks would alter smoking behavior in a manner consistent with an extinction-of-reinforcement mechanism. Consistent with our primary hypothesis, the extended run-in group exhibited greater pre quit reductions in self-reported smoking rate than did the standard run-in group. Importantly, self-report was captured daily, minimizing retrospective biases.59
Craving followed a pattern similar to CPD; the decrease in craving across the 3-week drug manipulation phase tended to be greater among the extended run-in group compared to the standard run-in group. Neither satisfaction with the first cigarette of the day nor morning withdrawal exhibited the predicted group differences across the pre quit period. Although these null findings may appear inconsistent with the extinction hypothesis, two aspects of the data mitigate this concern. First, smoking satisfaction and withdrawal were low, on average, prior to the drug manipulation phase. Thus, there was restricted range in which to observe further decreases on these measures. Second, as Rose and others have noted,35,60 run-in group differences in smoking satisfaction would be most evident if smoking rate remained comparable between the two groups. However, when pre quit treatment results in a greater decrease in smoking rate (CPD)—as in the present study—such decreases in smoking behavior likely attenuate differences in self-reported smoking satisfaction.
As noted in the introduction, the best measure of extinction is the behavior of interest, smoking rate, which was obtained through daily self-report. To assuage concerns about reliance on self-report, cotinine provided a biochemical index of changes in pre quit smoking behavior. For women, cotinine analyses were consistent with the extinction hypothesis, decreasing across the drug manipulation phase in the extended run-in group but not the standard run-in group. However, men in the extended run-in group did not exhibit the predicted decrease in cotinine during the drug manipulation phase. There is some evidence that extended pre quit pharmacotherapy may have more powerful or rapid effects on smoking behavior and abstinence among women compared to men.35,61 However, in the present study, sex did not moderate any other effect of extended run-in bupropion, suggesting the sex effect was specific to cotinine. Interestingly, emerging evidence suggests the cotinine clearance rate is substantially slower among men compared to women (likely due to estrogen-induced increases in CYP2A6 activity in women), resulting in weaker relationships between cotinine and other indicators of tobacco exposure (including urinary total nicotine equivalents) among men.62 In the current study, sex differences in cotinine clearance could have attenuated the degree to which cotinine reflected run-in group differences in smoking rate among men.63 Future large-scale studies of extended pre quit treatment should evaluate moderation by sex and include biomarkers of tobacco exposure that are less sensitive to individual differences in metabolism, such as urinary total nicotine equivalents.62
Although the focus of this study was on evaluating extinction-based predictions during the pre quit period, the participants were treatment-seeking smokers and we examined short-term abstinence rates as a secondary outcome. Consistent with the extinction framework, bio-verified continuous abstinence at 4 weeks post quit was significantly greater among the extended run-in group compared to the standard run-in group. These preliminary outcome data are particularly notable when one considers that the “control” group condition in the present study received standard bupropion therapy (and intensive group behavioral counseling), an evidence-based frontline treatment for smoking cessation.3 Of course, longer-term follow-up in substantially larger samples would be necessary to evaluate the clinical efficacy and cost-effectiveness of extending the duration of pre quit bupropion therapy.
The results of the present study can be integrated within a broader reinforcement and extinction framework.34,37,45,64 For extinction to occur, people must continue smoking in order to learn that the reinforcing effects are attenuated. Extinction is maximized when numerous “trials” are conducted over a long period of time and across a range of contexts.30,65–68 Though there are promising data with as little as 2 weeks of pre quit NRT therapy, the pre quit CPD data (Figure 2) suggests that the effect of pre quit treatment grows over the 3-week drug manipulation phase, as has also been found for varenicline.34,35 Future work might consider whether pre quit therapy might optimally be combined with a flexible quit date69 determined in part by a target reduction in smoking behavior. In the absence of such a reduction, it may be advisable to alter the treatment prior to attempting to quit.70 Alternatively, reinforcing continued smoking near one’s baseline rate during an extended period of pre quit pharmacotherapy, in combination with counseling that facilitates repeated exposure to a range of smoking-associated cues and contexts, would maximize the number of extinction trials. In that case, changes in pre quit craving or smoking satisfaction could guide selecting a quit date or switching therapies.
Extending the duration of pre quit pharmacotherapy is a practical approach to facilitating extinction of smoking behavior across a range of contexts (or “trigger” situations). However, the number of exposures to any particular context is limited, and low base-rate “triggers” or contexts may have relatively few, unsystematic exposures. Preclinical research on extinction of operant behavior has advanced markedly in recent years, demonstrating not only the ease with which extinction can be undone by changes in context (renewal), by subsequent extinction of a behavior that was paired with the original extinction (resurgence), or by rapid reacquisition upon a lapse, but also methods that can attenuate these effects.71 Human behavioral pharmacology studies of these principles and effects are rare,31 but are sorely needed to translate this promising animal literature on extinction and inform large-scale clinical trials.
Pre clinical work also provides clues to the aspects of reinforcement that are altered or extinguished by pre quit treatment. Bupropion increases the bioavailability of dopamine and norepinephrine and is a nicotinic acetylcholine receptor antagonist.11 These effects may block or attenuate positive reinforcement from smoking.12,13,19 Alternatively, the ability of pre quit bupropion to reduce smoking may result from substitution of reinforcement, as bupropion shares many of the effects of nicotine in pre clinical studies.13,17 Interestingly, the ability of nicotine to enhance responding for even weak sensory reinforcers that are not drug-related cues may be critical in understanding the maintenance of smoking.72–74 Bupropion appears to have similar reinforcement-enhancing effects, though perhaps through different neurotransmitter systems.75 Although research extending this work to humans is surprisingly scarce, Perkins and colleagues20 provided initial evidence that bupropion reverses the abstinence-induced decrease in responding for non-drug-related sensory stimuli (music) in human smokers. Further work on understanding the reinforcement-altering mechanisms of pre quit pharmacotherapy may allow tailoring of treatment and provide precise targets for treatment development.
In summary, the present data demonstrate that extended use of bupropion during the weeks leading up to a quit attempt reduces smoking behavior during the pre quit period, without increasing craving, withdrawal or smoking satisfaction. This pattern is consistent with an extinction framework. The outcome data, though exploratory, suggest that extending the duration of pre quit bupropion improves short-term abstinence rates above that obtained with standard bupropion treatment, at least in smokers similar to those studied here. The combination of a strong theoretical foundation, straightforward change in dosing strategy, and encouraging data on both process and outcome support further investigation of extended run-in bupropion therapy for smoking cessation.
Funding
This research was support by grant R21 CA111763 from the National Cancer Institute awarded to LWH.
Declaration of Interests
LWH has served as a consultant on investigator-initiated smoking studies sponsored by Pfizer and the state of Florida. KMC provides expert testimony in litigation against cigarette manufacturers provides consulting advice and has received grants from Pfizer, and previously served as a co-investigator on a multicenter trial evaluating a nicotine vaccine from Nabi Biopharmaceuticals. MCM has served on the Speaker’s Bureau for Pfizer and as the medical director of the New York State Smokers Quit Line. All other authors indicate that they have no competing interests.
References
- 1. Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. [DOI] [PubMed] [Google Scholar]
- 2. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. [DOI] [PubMed] [Google Scholar]
- 3. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Service. Public Health Service; 2008. [Google Scholar]
- 4. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031. [DOI] [PubMed] [Google Scholar]
- 5. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lerman C, LeSage MG, Perkins KA, et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. [DOI] [PubMed] [Google Scholar]
- 8. Group NCITRI. Tobacco Research Implementation Plan. Bethesda, MD: National Cancer Institute, National Institutes of Health; 1998. [Google Scholar]
- 9. Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. [DOI] [PubMed] [Google Scholar]
- 10. Piper ME, Federmen EB, McCarthy DE, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. [DOI] [PubMed] [Google Scholar]
- 11. Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 12. Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10:219–231. [DOI] [PubMed] [Google Scholar]
- 14. Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res. 2005;7:901–907. [DOI] [PubMed] [Google Scholar]
- 15. Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology (Berl). 2003;165:405–412. [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl). 2008;196:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkinson JL, Carroll FI, Bevins RA. An investigation of bupropion substitution for the interoceptive stimulus effects of nicotine. J Psychopharmacol. 2010;24:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008;10:995–1008. [DOI] [PubMed] [Google Scholar]
- 19. Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl). 2003;168:347–358. [DOI] [PubMed] [Google Scholar]
- 20. Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nicotine Tob Res. 2013;15:1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl). 2008;197:371–377. [DOI] [PubMed] [Google Scholar]
- 22. Hatsukami DK, Rennard S, Patel MK, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. Am J Med. 2004;116:151–157. [DOI] [PubMed] [Google Scholar]
- 23. Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl). 2001;157:243–253. [DOI] [PubMed] [Google Scholar]
- 24. Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clin Psychopharm. 1998;6:331–343. [DOI] [PubMed] [Google Scholar]
- 25. Steinberg ML, Williams JM. Extinction burst after varenicline titration: a case report. J Smok Cessat. 2013;8:115–118. [Google Scholar]
- 26. Bouton ME, Nelson JB. The role of context in classical conditioning: Some implications for cognitive behavior therapy. In: O’Donohue WT, ed. Learning and Behavior Therapy. Needham Heights, MA: Allyn & Bacon; 1998:59–84. [Google Scholar]
- 27. Collins BN, Nair US, Komaroff E. Smoking cue reactivity across massed extinction trials: negative affect and gender effects. Addict Behav. 2011;36:308–314. [DOI] [PubMed] [Google Scholar]
- 28. Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Processes. 2012;90:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. 2000;19(suppl 1):57–63. [DOI] [PubMed] [Google Scholar]
- 30. Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. [DOI] [PubMed] [Google Scholar]
- 31. Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–397. [PubMed] [Google Scholar]
- 32. Perkins K, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl). 2006;184:628–636. [DOI] [PubMed] [Google Scholar]
- 33. Perkins KA, Lerman C. An efficient early phase 2 procedure to screen medications for efficacy in smoking cessation. Psychopharmacology (Berl). 2014;231:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171:770–777. [DOI] [PubMed] [Google Scholar]
- 35. Hawk LW, Jr, Ashare RL, Lohnes SF, et al. The effects of extended pre quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11:1067–1075. [DOI] [PubMed] [Google Scholar]
- 37. Rose JE. Nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology. 2011;217:453–454. [DOI] [PubMed] [Google Scholar]
- 38. Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiat. 1997;12:224–231. [Google Scholar]
- 39. Lerman C, Shields PG, Wileyto EP, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22:541–548. [DOI] [PubMed] [Google Scholar]
- 40. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 41. Shiffman S, Shadel WG, Niaura R, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology (Berl). 2003;166:343–350. [DOI] [PubMed] [Google Scholar]
- 42. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. [DOI] [PubMed] [Google Scholar]
- 43. Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. [DOI] [PubMed] [Google Scholar]
- 44. Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. [DOI] [PubMed] [Google Scholar]
- 46. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 47. Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–763. [DOI] [PubMed] [Google Scholar]
- 48. Bailey SR, Bryson SW, Killen JD. Predicting successful 24-hr quit attempt in a smoking cessation intervention. Nicotine Tob Res. 2011;13:1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. SRNT. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 50. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. [DOI] [PubMed] [Google Scholar]
- 51. Ferguson SG, Gitchell JG, Shiffman S, Sembower MA. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clin Ther. 2009;31:1957–1965. [DOI] [PubMed] [Google Scholar]
- 52. Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med. 2013;7:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glover ED, Laflin MT, Schuh KJ, et al. A randomized, controlled trial to assess the efficacy and safety of a transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102:795–802. [DOI] [PubMed] [Google Scholar]
- 54. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 55. Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addiction Biology. 1998;3:383–404. [DOI] [PubMed] [Google Scholar]
- 56. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 57. Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of Nicotine Replacement Therapy for Smoking Cessation: differences between men and women. J Consult Clin Psychol. 2004;72:712–722. [DOI] [PubMed] [Google Scholar]
- 58. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. [DOI] [PubMed] [Google Scholar]
- 59. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 60. Rose J. New findings on nicotine addiction and treatment. In: Caggiula AR, Bevins RA, eds. The Motivational Impact of Nicotine and its Role in Tobacco Use. Vol 55 New York City: Springer US; 2009:131–141. [DOI] [PubMed] [Google Scholar]
- 61. Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–1148. [DOI] [PubMed] [Google Scholar]
- 62. Zhu AZ, Renner CC, Hatsukami DK, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race and sex. Cancer Epidemiol Biomarkers Prev. 2013;22:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction. 2008;103:557–563. [DOI] [PubMed] [Google Scholar]
- 65. Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. [DOI] [PubMed] [Google Scholar]
- 66. Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. [DOI] [PubMed] [Google Scholar]
- 67. Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. [DOI] [PubMed] [Google Scholar]
- 68. Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–19. [DOI] [PubMed] [Google Scholar]
- 69. Hughes JR, Russ CI, Arteaga CE, Rennard SI. Efficacy of a flexible quit date versus an a priori quit date approach to smoking cessation: a cross-study analysis. Addict Behav. 2011;36:1288–1291. [DOI] [PubMed] [Google Scholar]
- 70. Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. A J Psychiatry. 2013;170:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Winterbauer NE, Lucke S, Bouton ME. Some factors modulating the strength of resurgence after extinction of an instrumental behavior. Learn Motiv. 2013;44:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl). 2006;184:353–366. [DOI] [PubMed] [Google Scholar]
- 73. Lloyd DR, Hausknecht KA, Richards JB. Nicotine and methamphetamine disrupt habituation of sensory reinforcer effectiveness in male rats. Exp Clin Psychopharmacol. 2014;22:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Donny EC, Chaudhri N, Caggiula AR, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl). 2003;169:68–76. [DOI] [PubMed] [Google Scholar]
- 75. Palmatier MI, Levin ME, Mays KL, Donny EC, Caggiula AR, Sved AF. Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms in rats. Psychopharmacology (Berl). 2009;207:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]