Abstract
BACKGROUND
The American Joint Committee on Cancer’s (AJCC) 7th edition cancer staging manual reflects recent changes in cancer care practices. This report assesses changes from the AJCC 6th to the AJCC 7th edition stage distributions and the quality of site-specific factors (SSFs).
METHODS
Incidence data for renal parenchyma and pelvis and ureter cancers from 18 Surveillance, Epidemiology, and End Results (SEER) registries were examined, including staging trends during 2004–2010, stage distribution changes between the AJCC 6th and 7th editions, and SSF completeness for cases diagnosed in 2010.
RESULTS
From 2004 to 2010, the percentage of stage I renal parenchyma cancers increased from 50% to 58%, whereas stage IV and unknown stage cases decreased (18% to 15%, and 10% to 6%, respectively). During this period, the percentage of stage 0a renal pelvis and ureter cancers increased from 21% to 25%, and stage IV and unknown stage tumors decreased (20% to 18%, and 7% to 5%, respectively). Stage distributions under the AJCC 6th and 7th editions were about the same. For renal parenchymal cancers, 71%–90% of cases had known values for 6 required SSFs. For renal pelvis and ureter cancers, 74% of cases were coded as known for SSF1 (WHO/ISUP grade) and 47% as known for SSF2 (depth of renal parenchymal invasion). SSF values were known for larger proportions of cases with reported resections.
CONCLUSIONS
Stage distributions between the AJCC 6th and 7th editions were similar. SSFs were known for more than two-thirds of cases, providing more detail in the SEER database relevant to prognosis.
Keywords: kidney, renal pelvis, cancer, AJCC collaborative stage, site-specific factors
INTRODUCTION
It has been estimated that there would be 65,150 newly diagnosed kidney parenchyma and renal pelvis cancer cases and 2710 cancer cases of the ureter and other urinary organs (excluding the urinary bladder) in 2013. In addition, 13,680 and 900 people, respectively, were expected to die of these cancers in 2013.1
Incidence rates for kidney and renal pelvis cancers are nearly twice as high among men as women. Among men, higher rates occur among American Indian/Alaska Native men (29.0 per 100,000), with the lowest rates among Asian/Pacific Islander populations (10.1 per 100,000). Incidence rates among Hispanic, white, and black men lie somewhere in-between (19.8, 21.2, and 23.3 per 100,000, respectively).1 Since 1992, incidence rates for these malignancies have increased steadily in both men and women, particularly during the past decade (2.9% increase per year among men, and 3.1% among women).2 Despite the increase in incidence rates, death rates declined during the same period.2 Overall 5-year relative survival was about 73%, ranging from 91% for localized to 64% for regional and 11% for distant-stage cases. Although 62% of cases were diagnosed with localized stage cancers, 17% were diagnosed with regional and distant-stage cancers.2
The American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition, was published in January 20103 and adopted by the Surveillance, Epidemiology, and End Results (SEER) Program registries for cancer cases diagnosed in 2010 and after. Site-specific factors (SSFs) were first introduced into the AJCC 7th edition and Collaborative Stage (CS) System version 2 (CSv2) schemas for renal parenchymal, renal pelvis, and ureter cancers. Understanding these factors has allowed clinicians to better select therapeutic options and facilitates stratifying a patient’s risk of progression and evaluating treatment. The objectives of this report are to: 1) examine staging trends using the AJCC 6th edition4 during 2004–2010, 2) compare stage distribution between the AJCC 6th and 7th editions for 2010 cases, and 3) assess the completeness of SSFs.
MATERIALS AND METHODS
Analytic Cohort
Data from the November 2012 submission of the SEER-18 registries (and not the Arizona Indians or Cherokee Nation registries) were used in this analysis. The SEER-18 registries report cancer incidence data to the National Cancer Institute, including demographic attributes of patients, stage at diagnosis, and survival time from diagnosis. The SEER-18 registries (San Francisco [SF]–Oakland standard metropolitan statistical area, Connecticut, Detroit [metropolitan], Hawaii, Iowa, New Mexico, Seattle [Puget Sound], Utah, Atlanta [metropolitan], San Jose–Monterey [SJM], Los Angeles, Alaska Natives, rural Georgia, California excluding SF/SJM/Los Angeles, Kentucky, Louisiana, New Jersey, greater Georgia) cover 28% of the United States population. Analytic cohorts included in this report used similar exclusion criteria for cancers of 1) the renal parenchyma (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3]5 topographic code: C64.9) and 2) renal pelvis (C65.9) and ureter (C66.9) (Table 1). Invasive renal parenchyma and both in situ and invasive renal pelvis cancer cases diagnosed in 2010 were included for the comparison of stage distribution between the AJCC 6th and 7th editions. The AJCC 7th edition schemas for these sites include identical ICD-O-3 histological types: 8000–8576, 8940–8950, 8980–8981. Autopsy- and death certificate–only cases were excluded, as were cases with histologies that were not included in the AJCC 6th and 7th editions. For the purpose of evaluating the completeness and quality of SSFs, only invasive cases diagnosed in 2010 were included.
TABLE 1.
Exclusion Criteria for Renal Parenchyma and Renal Pelvis and Ureter Cancer Analytic Cohorts, SEER, 2010
| Exclusion Criteria | AJCC 6th & 7th Stage Cohort | SSF Cohort |
|---|---|---|
| A. Renal parenchymaa | ||
| In situ cases | Yesa | Yes |
| Autopsy- or death certificate–only cases | Yes | Yes |
| Histologies for which AJCC 6th | Yes | Yes |
| & 7th stage not defined | ||
| Code 988, blank for each SSF | No | Yes |
| Arizona Indians and Cherokee | Yes | Yes |
| Final sample size | 12,218 | 12,203 |
| B. Renal pelvis and ureter | ||
| In situ cases | No | Yes |
| Autopsy or death certificate only cases | Yes | Yes |
| Histologies for which AJCC 6th and 7th stage not defined | Yes | Yes |
| Code 988, blank for each SSF | No | Yes |
| Arizona Indians and Cherokee | Yes | Yes |
| Final sample size | 1684 | 1193 |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results; SSF, site-specific factor.
Papillary noninvasive carcinoma (0a) and in situ (0is) not applicable to renal parenchyma
Key Changes in AJCC Staging Between the 6th and 7th Editions
AJCC staging is based on detailed information regarding tumor size (T), the extent of lymph node involvement (N), and metastasis (M). Changes in the AJCC 7th edition3 for renal parenchymal tumors divided T2 (tumor >7 cm in greatest dimension, limited to the kidney) into T2a (tumor size >7 but ≤10 cm, limited to the kidney) and T2b (tumor size >10 cm, limited to the kidney). Furthermore, ipsilateral (same side) adrenal gland involvement was reclassified as T4 if there was contiguous invasion and as M1 if invasion was not contiguous; renal vein involvement was reclassified as T3a from T3b; and nodal involvement was simplified to N0 versus N1, without distinguishing metastasis in a single regional lymph node from metastasis in more than 1 regional lymph node as in the AJCC 6th edition. Since the publication of the AJCC 6th edition, new evidence supported the division of T2 tumors and reclassification of the T3a and N categories. The rationale for dividing T2 into T2a and T2b is based on large, retrospective cohort studies6–10 with extended follow-up that demonstrate substantially different outcomes for these subgroups. Furthermore, multiple studies11–18 have documented a poor prognosis for patients with ipsilateral adrenal involvement similar to patients with T4 or M1 disease; these tumors are now reclassified to reflect current concepts about likely mechanisms of spread. In addition, tumors with isolated renal vein thrombus are known to have a relatively favorable prognosis19,20 and are now staged as T3a rather than T3b. Last, nodal involvement is now consolidated as N1 because most studies21–24 suggest a relatively poor prognosis among patients with nodal involvement. For renal pelvis and ureter cancers, the definition of TNM and the stage grouping have not changed from the AJCC 6th edition.
Summary of SSFs
Renal parenchyma
SSFs for renal parenchyma include invasion beyond capsule (SSF1), vein involvement (SSF2), ipsilateral adrenal gland involvement (SSF3), sarcomatoid features (SSF4), Fuhrman nuclear grade (SSF6), and extranodal extension of regional lymph nodes (SSF8). Collection of SSF5 (histologic tumor necrosis) and SSF7 (size of metastasis in lymph nodes) was never required by the SEER Program, but they were voluntarily reported to SEER in some instances, primarily by Commission on Cancer–accredited facilities.25
The kidney is encased by a fibrous capsule and surrounded by perirenal fat. Information about invasion beyond the capsule and involvement of the ipsilateral adrenal gland and/or vein has been collected in the CS extension field. The CS extension field identifies the primary tumor growth within the organ or its extension into neighboring organs. In CSv2, 3 SSFs were added to more specifically define tumor extension. SSF1, invasion beyond capsule, provides the specific depth of tumor extension outside the kidney capsule. SSF2 (vein involvement) records the presence and level of involvement of specific major named blood vessels. Although contiguous and noncontiguous ipsilateral adrenal gland involvement is collected in the CS extension and in the CS metastasis at diagnosis field, SSF3 (ipsilateral adrenal gland involvement) provides detailed information about adrenal gland involvement.26
The other 3 SSFs affect prognosis. SSF4, sarcomatoid features, documents any sarcomatoid or spindle cell features in any renal cell carcinoma. Patients with renal cell carcinoma with sarcomatoid differentiation tend to have worse outcomes than renal cell carcinoma without sarcomatoid differentiation.27,28 Fuhrman nuclear grade, SSF6, is a 4-grade system based on nuclear diameter and shape and the prominence of nucleoli in the tumor cells. Patients with higher grades had worse survival rates.29 The definitions of nuclear grade are documented in the kidney cancer protocol of the College of American Pathologists checklist30: G1, nuclei round, uniform, approximately 10 μm, nucleoli inconspicuous or absent; G2, nuclei slightly irregular, approximately 15 μm, nucleoli evident; G3, nuclei very irregular, approximately 20 μm, nucleoli large and prominent, G4, nuclei bizarre and multilobated, 20 μm or greater, nucleoli prominent, chromatin clumped.
SSF8, extranodal extension of regional lymph nodes, is defined as a metastatic tumor growing from within the lymph node outward through the lymph node capsule and into surrounding connective tissues. Extranodal extension can be detected clinically, on gross examination of dissected lymph nodes, or microscopically. Kidney cancer patients with extranodal extension have a worse prognosis than those without nodal involvement.
Renal pelvis and ureter
Kidney and renal pelvis cancers often are combined for the purposes of cancer surveillance, with ureter cancer presented separately. Renal pelvis and ureter cancers share CSv2 SSFs, however, and are grouped in the AJCC stage coding schema; cancers of the renal parenchyma have a different set of SSFs.3 Findings for both renal pelvis and ureter cancers are presented in this report.
The SSFs for these cancer sites are World Health Organization or International Society of Urological Pathology (WHO/ISUP) grade31 (SSF1) and depth of renal parenchymal invasion (SSF2).32 Both SSFs for these sites affect prognosis. SSF1 for renal pelvis and ureter is the WHO/ISUP grade, a 2-grade system (low and high grade). This grading system was proposed by ISUP in 1998 and adopted by WHO in 2004 to better classify the tumor grade for urothelial carcinomas of the renal pelvis, ureter, bladder, and urethra.33,34 The strengths of the WHO/ISUP grade’s clear-cut criteria and the elimination of subjective and arbitrary interpretation have greatly improved the ambiguous language that marked the 1973 WHO system.35 SSF2, depth of renal parenchymal invasion, records the depth of tumor invasion into the renal parenchyma in millimeters as documented in the pathology report.
Data Analysis
AJCC 6th edition stage distribution was examined by year of diagnosis from 2004 through 2010. The annual percent change (APC) in frequencies by stage and corresponding 95% confidence intervals (CIs) were calculated. Database queries were performed with SEER*Stat v 8.0.2 (IMS, Calverton, MD).
A Kappa statistic36 was used to measure staging agreement between the AJCC 6th and 7th editions for renal parenchyma and renal pelvis and ureter cancers (Proc Freq Agree option and Test Kappa command; SAS v 9.2 Cary, NC). Frequencies and percentage distribution of SSFs by known, unknown, and not applicable categories were computed, including analyses restricted to resected cases. Tables were populated using the Proc Freq procedure (SAS v 9.2 Cary, NC).
RESULTS
Incidence Cases
A total of 79,908 renal parenchymal cancer cases and 11,611 renal pelvis and ureter cases diagnosed during 2004–2010 were available for analysis in examining staging trends based on the AJCC 6th edition. For comparing AJCC 6th and 7th edition stage distributions, the analytic data sets included 12,218 invasive and in situ renal parenchymal cancer cases and 1684 invasive and in situ renal pelvis and ureter cancer cases diagnosed during 2010 (Table 1). Analyses of SSFs included only invasive cancer, yielding 12,203 renal parenchymal cancer cases and 1193 renal pelvis and ureter cancer cases diagnosed in 2010.
AJCC 6th Edition Stage Distribution Trends
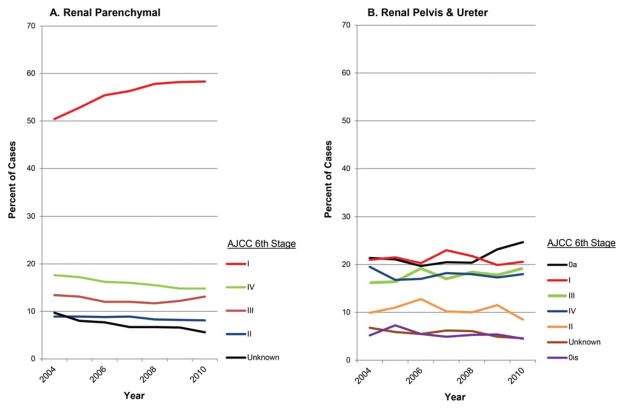
The percentage of stage I renal parenchymal cancer cases increased from 50% in 2004 to 58% in 2010 (APC, 6.9%; 95% CI, 3.8% to 10.0%; Fig. 1A). Statistically significant increases were also seen in the number of stage II cases (APC, 2.6%; 95% CI, 0.3% to 5.0%) and in stage III cases (APC, 3.6%; 95% CI, 2.4% to 4.9%). Although not statistically significant, from 2004 to 2010, the percentage of tumors classified as stage IV decreased from 18% to 15%, and the percentage designated as unknown stage decreased from 10% to 6%.
Figure 1.
Stage distribution trends for (A) renal parenchymal cancer and (B) renal pelvis and ureter cancer. Data from SEER registries during 2004–2010, AJCC 6th edition.
For renal pelvis and ureter cancer but not renal parenchymal cancer, stage designations included 0a and 0is (Fig 1B). The percentage of cases that were diagnosed at stage 0a increased to 25% in 2010, surpassing case counts for all other stages (APC, 3.8%; 95% CI, 0.6% to 7.0%). Stage 0a, or abnormal cells, differs from stage 0is, or carcinoma in situ. Among other stages, only the increase in counts of stage III tumors was significant (APC, 3.4%; 95% CI, 0.7% to 6.3%). Stage III and IV tumors accounted for between 15% and 20% of cases throughout the surveillance period, and stage II tumors accounted for fewer than 10% of cases in 2009 and 2010. Unknown and 0is stage tumors both accounted for fewer than 5% of cases in 2010.
Comparison of Stage Distributions
The impact of changes in definitions between the AJCC 6th and 7th editions for cancers of the renal parenchyma diagnosed during 2010 is shown in Table 2. For renal parenchymal cancers, slightly more cases were classified as stage I under the 7th edition guidelines than under the 6th (58.6% versus 58.3%) and as stage III (13.6% versus 13.1%). Fewer cases were classified as unknown stage (5.0% versus 5.6%). Differences in other staging categories were even more modest. With respect to renal pelvis and ureter tumors, only 3 tumors were reclassified—all from an unknown stage under the AJCC 6th edition to stage I under the 7th edition.
TABLE 2.
Distribution of AJCC 6th and 7th Edition Staging for Renal Parenchyma and Renal Pelvis and Ureter Cancer Cases, SEER, 2010
| A. Renal Parenchyma
| ||||
|---|---|---|---|---|
| Stage | AJCC 6th | AJCC 7th | ||
|
| ||||
| n | % | n | % | |
| I | 7127 | 58.3 | 7156 | 58.6 |
| II | 995 | 8.1 | 1004 | 8.2 |
| III | 1601 | 13.1 | 1658 | 13.6 |
| IV | 1805 | 14.8 | 1784 | 14.6 |
| Unknown | 690 | 5.6 | 616 | 5.0 |
| Total | 12,218 | 100.0 | 12,218 | 100.0 |
| B. Renal Pelvis and Ureter
| ||||
|---|---|---|---|---|
| Stage | AJCC 6th | AJCC 7th | ||
|
| ||||
| n | % | n | % | |
| 0a | 416 | 24.7 | 416 | 24.7 |
| 0is | 75 | 4.5 | 75 | 4.5 |
| I | 347 | 20.6 | 350 | 20.8 |
| II | 143 | 8.5 | 143 | 8.5 |
| III | 323 | 19.2 | 323 | 19.2 |
| IV | 303 | 18.0 | 303 | 18.0 |
| Unknown | 77 | 4.6 | 74 | 4.4 |
| Total | 1684 | 100.0 | 1684 | 100.0 |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results.
Table 3 provides a more detailed cross-tabulation of AJCC 6th and 7th edition staging for cancers of the renal parenchyma and renal pelvis and ureter diagnosed during 2010. Almost perfect agreement was seen for both cancer sites (Kappa, 0.98 and 0.99, respectively). Some discordance was observed, however. For renal parenchymal tumors, 75 of 690 cases (11%) classified as unknown stage under the AJCC 6th edition guidelines were reassigned to specified stages under the 7th edition. In addition, 41 of 1805 cases (2%) classified as stage IV under the AJCC 6th edition were reclassified to stage III under the 7th edition. Of 1684 cases with renal pelvis and ureter cancers, 1681 (99.99%) had identical stage classifications under both the AJCC 6th and 7th editions.
TABLE 3.
Comparison of AJCC 6th and 7th Edition Staging Distributions for Renal Parenchyma and Renal Pelvis and Ureter Cancer Patients Diagnosed in 2010, SEER
| A. Renal Parenchyma (Kappa, 0.98; P < .05)
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC 7th
| |||||||||||
| AJCC 6th | I | II | III | IV | Unknown | Total | |||||
|
| |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | |
| I | 7127 | 99.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7127 |
| II | 0 | 0 | 995 | 99.1 | 0 | 0 | 0 | 0 | 0 | 0 | 995 |
| III | 0 | 0 | 0 | 0 | 1581 | 95.4 | 20 | 1.1 | 0 | 0 | 1601 |
| IV | 0 | 0 | 0 | 0 | 41 | 2.5 | 1763 | 98.8 | 1 | 0.2 | 1805 |
| Unknown | 29 | 0.4 | 9 | 0.9 | 36 | 2.2 | 1 | 0.1 | 615 | 99.8 | 690 |
| Total | 7156 | 100.0 | 1004 | 100.0 | 1658 | 100.0 | 1784 | 100.0 | 616 | 100.0 | 12218 |
| B. Renal Pelvis and Ureter (Kappa, > 0.99; P <.05)
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC 7th
| |||||||||||||||
| AJCC 6th | 0a | 0is | I | II | III | IV | Unknown | Total | |||||||
|
| |||||||||||||||
| n | % | n | % | n | % | N | % | n | % | n | % | n | % | n | |
| 0a | 416 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 416 |
| 0is | 0 | 0 | 75 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75 |
| I | 0 | 0 | 0 | 0 | 347 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 347 |
| II | 0 | 0 | 0 | 0 | 0 | 0 | 143 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 143 |
| III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 323 | 0 | 0 | 0 | 0 | 0 | 323 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 303 | 100 | 0 | 0 | 303 |
| Unknown | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 74 | 95 | 77 |
| Total | 416 | 100 | 75 | 100 | 350 | 100 | 143 | 100 | 323 | 100 | 303 | 100 | 74 | 100 | 1684 |
Abbreviations: AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results.
Completeness of SSFs
A summary of codes corresponding with not applicable, known, and unknown values for SSFs is presented in Table 4. Fewer than 1% of renal parenchymal cancers were reported as not applicable. Known responses ranged from a low of 70.5% for SSF6 (Fuhrman nuclear grade) to a high of 90.3% for SSF8 (extranodal extension). Data present beyond the known, unknown, and not applicable levels are not shown in Table 4.
TABLE 4.
SSFs for Cancers of the Renal Parenchyma and Renal Pelvis and Ureter, SEER, 2010
| A. Renal Parenchyma
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSF | Not Applicablea | Known | Unknown | Total | ||||||
|
| ||||||||||
| Codes | n | % | Codes | n | % | Codes | n | % | n | |
| 1. Invasion beyond capsule | 888 | 0 | 0.0 | 000, 010, 020, 030, 991 | 9426 | 77.2 | 998, 999 | 2777 | 22.8 | 12,203 |
| 2. Vein involvement | 888 | 0 | 0.0 | 000, 010, 020, 030, 040, 050, 060,070, 080, 090 | 9586 | 78.6 | 998, 999 | 2617 | 21.4 | 12,203 |
| 3. Ipsilateral adrenal gland involvement | 888 | 0 | 0.0 | 000, 010, 020, 030, 040 | 9932 | 81.4 | 998, 999 | 2271 | 18.6 | 12,203 |
| 4. Sarcomatoid features | 888, 987 | 13 | 0.1 | 000, 010 | 9611 | 78.8 | 998, 999 | 2579 | 21.2 | 12,203 |
| 6. Fuhrman nuclear grade | 888, 987 | 69 | 0.6 | 010, 020, 030, 040 | 8607 | 70.5 | 998, 999 | 3527 | 28.9 | 12,203 |
| 8. Extranodal extension of regional lymph nodes | 0 | 0.0 | 000, 010, 020, 030 | 11,015 | 90.3 | 999 | 1188 | 9.7 | 203 | |
| B. Renal Pelvis and Ureter
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSF | Not Applicablea | Known | Unknown | Total | ||||||
|
| ||||||||||
| Codes | n | % | Codes | n | % | Codes | n | % | n | |
| 1. WHO/ISUP grade | 888 987 | 16 | 1.3 | 010, 20 | 874 | 73.3 | 998, 999 | 303 | 25.4 | 1193 |
| 2. Depth of renal parenchymal invasion | 0 | 0.0 | 000, 001–979, 980 | 560 | 46.9 | 991,998, 999 | 633 | 53.1 | 1193 | |
Abbreviations: ISUP, International Society of Urological Pathology; SEER, Surveillance, Epidemiology, and End Results; SSF, site-specific factor; WHO, World Health Organization.
Excluded from SSF-related analyses were 988 and blank responses (see Table 1).
Renal parenchyma
Among the 12,203 renal parenchymal cancer cases, 9426 cases (77.2%) had known values for invasion beyond capsule (SSF1; Table 4). This includes 8068 cases (66.1%) with no reported invasion beyond the capsule and 1358 (13.8%) with reported capsular invasion. Of these 1358 cases, 1307 (96.2%) had detailed information on the extent of invasion, and 51 (3.7%) had invasion beyond the capsule that was not otherwise specified (NOS). Of the 1307 cases with detailed information on invasion, 807 cases had lateral invasion, 311 had medial invasion, and 189 had both lateral and medial invasion. Among 2777 cases (22.8%) without data on invasion beyond the capsule, 2212 (79.7%) did not have a surgical resection. Among cases with reported resections, large proportions had known SSF values, ranging from 87.2% for SSF 6 to 96.7% for SSF 2 (Table 5). Information on vein involvement (SSF2) was available for 9586 cases (78.6%) and missing for 2617 (21.4%) of the 12,203 cases. Of the 9586 cases with information, 8573 (89.4%) did not present with vein involvement, and 1013 (10.6%) had reported vein involvement. The majority of these cases (81%) did not receive a surgical resection.
TABLE 5.
SSFs for Cancers of the Renal Parenchyma, and Renal Pelvis and Ureter, SEER-18, 2010 (Limited to Cases With a Resection Based on SEER 2003+ Site-Specific Surgery of Primary Site Codes 20–80)
| A. Renal Parenchyma
| |||||||
|---|---|---|---|---|---|---|---|
| SSF | Not Applicable | Known | Unknown | Total | |||
|
| |||||||
| n | % | n | % | n | % | n | |
| 1. Invasion beyond capsule | 0 | 0.0 | 9033 | 96.4 | 340 | 3.6 | 9373 |
| 2. Vein involvement | 0 | 0.0 | 9070 | 96.7 | 303 | 3.2 | 9373 |
| 3. Ipsilateral adrenal gland involvement | 0 | 0.0 | 8660 | 92.4 | 713 | 7.6 | 9373 |
| 4. Sarcomatoid features | 7 | 0.1 | 8917 | 95.1 | 449 | 4.8 | 9373 |
| 6. Fuhrman nuclear grade | 54 | 0.6 | 8176 | 87.2 | 1143 | 12.2 | 9373 |
| 8. Extranodal extension of regional lymph nodes | 0 | 0.0 | 9010 | 96.1 | 363 | 3.9 | 9373 |
| B. Renal Pelvis and Ureter
| |||||||
|---|---|---|---|---|---|---|---|
| SSF | Not Applicable | Known | Unknown | Total | |||
|
| |||||||
| n | % | n | % | n | % | n | |
| 1. WHO/ISUP grade | 16 | 1.4 | 805 | 84.7 | 132 | 13.9 | 950 |
| 2. Depth of renal parenchymal invasion | 0 | 0.0 | 546 | 57.5 | 404 | 42.5 | 950 |
Abbreviations: ISUP, International Society of Urological Pathology; SEER, Surveillance, Epidemiology, and End Results; SSF, site-specific factor; WHO, World Health Organization.
Ipsilateral adrenal gland involvement (SSF3) data were reported for 9932 cases (81.4%); 18.6% of cases had no relevant information. Among cases with data, 9679 (97%) did not present with ipsilateral adrenal gland involvement, 184 (1.9%) had contiguous or noncontiguous involvement, and 69 (0.69%) had unknown contiguous/noncontiguous involvement.
Known sarcomatoid features (SSF4) data were available for 9624 cases (78.8%), with data unknown results for 2579 cases (21.2%). Among cases with information, 9252 (96.2%) did not present with sarcomatoid features; 359 (3.7%) did present with these features. The majority of the cases with unknown values (65%) had no pathologic examination of the primary site. This variable was not applicable to 13 cases (0.1%) without renal cell carcinoma morphology.
Fuhrman nuclear grade (SSF6) was known for 8607 cases (70.5%). Of these, 1030 (12%) were grade 1 cases, 4525 (52.6%) were grade 2 cases, and 2436 (28.3%) were grade 3 cases, with the remaining 616 (7.2%) being grade 4 cases. Another 3527 cases (29%) were missing this information, including 1675 (13.7%) that did not have a histologic examination. The remaining 69 cases (0.57%) did not have renal cell carcinoma morphologies.
Extranodal extension of regional lymph nodes (SSF8) was known for 11,015 cases (90.3%). The SSF8 value was unknown for 1188 cases (9.7%). There were no cases with missing or blank values for this data item.
Renal pelvis and ureter
Among renal pelvis and ureter cancers, 73.3% had known values, including 61.3% high-grade urothelial carcinomas and 12% low-grade urothelial carcinomas. Another 1.3% of WHO/ISUP grade (SSF1) responses were reported as not applicable, and the remaining 25.4% were unknown. The majority of responses for depth of renal parenchymal invasion (SSF2; 53.1%) were unknown. All remaining responses (46.9%) were listed as known. Among the known responses, 549 of 560 (98%) had no parenchymal invasion present/identified. When analysis was restricted to cases with reported resections, the majority had known SSF values, ranging from 57.5% for SSF 2 to 84.7% for SSF 1 (Table 5).
Quality assessment, renal parenchyma
For quality control purposes, cross-tabulations of SSFs and existing SEER variables were performed when possible (data not shown). For renal parenchyma SSF1 (invasion beyond the capsule), results were consistent with those for CS-extension-confirmed invasion. Cases with T3a and more advanced stage tumors (invasion beyond the capsule) generally had invasive cancer. Similarly, comparisons of Fuhrman nuclear grade (SSF6) and ICD-O grade/differentiation code revealed a high level of agreement among cases with known grade. When compared with existing staging variables, inconsistencies were seen for SSFs 2, 3, and 8, which remain the subjects of investigation.
Quality assessment, renal pelvis and ureter
Cross-tabulations for renal pelvis and ureter WHO/ISUP grade (SSF1) and ICD-O grade/differentiation code also showed agreement among cases with known grade.
DISCUSSION
Stage distributions under the AJCC 6th and 7th editions were similar. As previously reported,37 the annual increase in stage I cases explains the overall increase in renal parenchyma cancer incidence rates. Of the 6 renal parenchyma and 2 renal pelvis and ureter SSFs that currently are collected by the SEER Program, 4 were considered ready for release based on completeness, consistency of results compared with existing and closely related variables, and the insight they provide into staging and prognosis. This includes 3 SSFs for renal parenchymal cancer: SSF1 (invasion beyond capsule), SSF4 (sarcomatoid features), and SSF6 (Fuhrman nuclear grade). Of these, sarcomatoid differentiation and Fuhrman nuclear grade have particular prognostic value; invasion beyond the capsule is an anatomic field that largely duplicates existing staging data items. Although both renal pelvis and ureter cancer SSFs have prognostic value, only SSF1 (WHO/ISUP grade) was recommended for release, given the partial completeness of SSF2 (renal parenchymal invasion) data. For an SSF to be retained in the SEER data collection, not only does the SSF need to have a high degree of completeness, but the known value should provide added insight, such as enabling classification of patients into refined and specific categories for projecting prognosis and recurrence and guiding treatment options.
For kidney parenchyma, there are 6 SSFs. Invasion beyond capsule (SSF1) is included in both the AJCC 6th and 7th editions and has been shown to be prognostic. SSF1 captures the anatomic location of invasion beyond the capsule such as lateral invasion to perinephric fat or medial invasion to the renal sinus. Invasion beyond the capsule corresponds to a pathologic T stage designation of at least T3 and T4 if invading adjacent organs. Collection of SSF1 in addition to staging under the AJCC 6th or 7th edition added new and prognostic information on the anatomic location of invasion for 1307 of 1358 cases (96%) with reported invasion beyond the capsule. Vein involvement (SSF2) is somewhat redundant because it is a component of the AJCC 7th edition staging system, which updates the AJCC 6th edition to reflect the prognostic implications of various levels of vein involvement.38 Cases are assigned into 3 categories: T3a (renal vein only), T3b (inferior vena cava [IVC] or IVC below the diaphragm), and T3c (IVC above the diaphragm). Interest in ipsilateral adrenal involvement (SSF3) reflects awareness that outcomes for patients with adrenal gland invasion are significantly worse than for patients with extension of carcinoma only into the perinephric adipose tissue,11 and prompted the change in the AJCC 7th edition classification for adrenal involvement in renal cell carcinoma. Tumors with direct extension through the kidney into the adrenal gland are T4 lesions, and tumors with ipsilateral adrenal metastasis not secondary to direct extension are categorized as M1 lesions. With the updates in the AJCC 7th edition, SSF3 is redundant and adds no additional prognostic information.
Unlike SSFs 1, 2, and 3, the presence of sarcomatoid features (SSF4) and Fuhrman nuclear grade (SSF6) are anatomic-independent prognostic factors. Both these SSFs are of considerable clinical value.39,40 Although initially validated as a prognostic factor in clear cell renal cell carcinoma, contemporary studies have shown the prognostic value of nuclear grading in both clear cell and papillary renal cell carcinoma.41 Although a higher nuclear grade and the presence of sarcomatoid features are associated with a more advanced T stage, these factors are independently associated with outcomes among cases matched by stage. For these reasons, SSFs 4 and 6 are the most clinically useful and validated SSFs for kidney parenchymal tumors.
With respect to renal pelvis and ureter cancer, a high WHO/ISUP grade (SSF1) is independently associated with worse outcomes in surgical cases.42 WHO/ISUP grade was known for 85% of cases with resection. SSF2 (depth of renal parenchymal invasion as a marker of recurrence) also has been validated43; however, most values for this variable were unknown. Additional SSFs for renal pelvis and ureter cancer might be considered based on prognostic value. Promising markers for these understudied cancers include tumor architecture,44,45 multifocality,46 and the presence of concomitant carcinoma in situ.47 In some studies,48 tumor location (ie, ureter, renal pelvis, or both) has also been suggested to have prognostic value.
Alternative approaches to efficiently collecting new SSFs may have merit, including the use of pilot studies to assure the feasibility of recording promising biomarkers.49 The AJCC 7th edition CS manual incorporated enhanced registry data standards including nonanatomic prognostic factors to provide evidence-based staging. The 4 SSFs that were recommended to be added to the SEER public use research database by its internal data release committee based on considerations such as completeness and inherent value include all 3 nonanatomic SSFs. These are renal parenchymal cancer SSF4 (sarcomatoid features), SSF6 (Fuhrman nuclear grade), and renal pelvis and ureter SSF1 (WHO/ISUP grade). The AJCC 7th edition schema was intended to leverage rapidly increasing specific knowledge of cancer biology and electronic data capture technology, with the goal of supporting personalized cancer care through outcome prediction models.3 Long-term follow-up of survival may demonstrate the prognostic value of the AJCC 7th edition SSFs. The assessment may also reveal synergies between SSFs that affect prognosis.
This study has both strengths and limitations. At the time of its publication, the AJCC 7th edition staging manual reflected a consensus understanding of cancer staging. Adopting the major revisions included in the AJCC 7th edition staging manual as a central part of registry-based cancer surveillance was an expansion of concepts included in the AJCC 6th edition. The introduction of multiple SSFs was intended to refine stage classification and provide insight into prognosis and treatment decisions. Limitations include the cost of this endeavor and inconsistencies between SSF results compared with existing and related variables. A potential bias was introduced by the incompleteness of some SSFs, as demonstrated by results of modeling to impute missing values of progesterone and estrogen receptor status in breast cancer cases.50
In summary, results from this study suggest that changes in definitions between the AJCC 6th and 7th editions do not significantly affect stage distribution. Furthermore, it appears feasible to collect select SSFs for renal and ureter cancers within the SEER registries. Long-term follow-up of survival may confirm the prognostic value of these variables. Alternative strategies for the inclusion of proposed SSFs in programmatic SEER registry operations may include pilot studies of promising biomarkers so that informed decisions about the efficiency and value of future data collections may be made.
Acknowledgments
This supplement was sponsored by the National Cancer Institute’s Surveillance Research Program.
FUNDING SUPPORT
This supplement edition of Cancer has been sponsored by the National Cancer Institute. Data used in the production of this supplement was supported under Contract HHSN261201300004I (University of Southern California), HHSN261201300005I (Cancer Prevention Institute of California, HHSN261201300009I (University of Hawaii), HHSN261201300010I (University of New Mexico), HHSN261201300021I (Rutgers University), HHSN261201300019I (Connecticut Department of Health), HHSN 261201300020I (University of Iowa), HHSN261201300015I (Emory University), HHSN261201300016I (Louisiana State University), HHSN 261201300017I (University of Utah), HHSN261201300011I (Wayne State University), HHSN261201300012I (Fred Hutchinson Cancer Center), HHSN261201300013O (University of Kentucky), and HHSN26120 1300014I (Public Health Institute). Technical support was provided under contract HHSN261201100007I (Information Management Services, Inc.).
Footnotes
The opinions or views expressed in this supplement are those of the authors and do not necessarily reflect the opinions or recommendations of the journal editors, the American Cancer Society, the publisher, or the National Cancer Institute.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Krapcho NA, Neyman N, et al., editors. [Accessed August 15, 2013];SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012 Available at: http://seer.cancer.gov/csr/1975_2009_pops09/
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. Chicago: Springer-Verlag; 2010. [Google Scholar]
- 4.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, editors. AJCC Cancer Staging Manual. 6. Chicago: Springer-Verlag; 2002. [Google Scholar]
- 5.Fritz A, Jack A, Parkin DM, et al., editors. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 6.Klatte T, Patard JJ, Goel RH, et al. Prognostic impact of tumor size on pT2 renal cell carcinoma: an international multicenter experience. J Urol. 2007;178:35–40. doi: 10.1016/j.juro.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Frank I, Blute ML, Leibovich BC, et al. pT2 classification for renal cell carcinoma. Can its accuracy be improved? J Urol. 2005;173:380–384. doi: 10.1097/01.ju.0000149937.75566.ac. [DOI] [PubMed] [Google Scholar]
- 8.Kontak JA, Campbell SC. Prognostic factors in renal cell carcinoma. Urol Clin North Am. 2003;30:467–480. doi: 10.1016/s0094-0143(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani L, Giberti C, Martorana G, Rovida S. Radical extensive surgery for renal-cell carcinoma—long-term results and prognostic factors. J Urol. 1990;143:468–474. doi: 10.1016/s0022-5347(17)39992-5. [DOI] [PubMed] [Google Scholar]
- 10.Guinan PD, Vogelzang NJ, Fremgen AM, et al. Renal-cell carcinoma—tumor size, stage and survival. J Urol. 1995;153:901–903. [PubMed] [Google Scholar]
- 11.Han KR, Bui MH, Pantuck AJ, et al. TNM T3a renal cell carcinoma: adrenal gland involvement is not the same as renal fat invasion. J Urol. 2003;169:899–903. doi: 10.1097/01.ju.0000051480.62175.35. [DOI] [PubMed] [Google Scholar]
- 12.Lam JS, Patard JJ, Leppert JT, et al. Prognostic significance of T3A renal cell carcinoma with adrenal gland involvement: an international multicenter experience. J Urol. 2005;173:269–270. [Google Scholar]
- 13.Paul R, Mordhorst J, Leyh H, Hartung R. Incidence and outcome of patients with adrenal metastases of renal cell cancer. Urology. 2001;57:878–882. doi: 10.1016/s0090-4295(01)00937-2. [DOI] [PubMed] [Google Scholar]
- 14.Sagalowsky AI, Kadesky KT, Ewalt DM, Kennedy TJ. Factors influencing adrenal metastasis in renal cell carcinoma. J Urol. 1994;151:1181–1184. doi: 10.1016/s0022-5347(17)35208-4. [DOI] [PubMed] [Google Scholar]
- 15.Siemer S, Lehmann J, Loch A, et al. Current TNM classification of renal cell carcinoma evaluated: revising stage T3a. J Urol. 2005;173:33–37. doi: 10.1097/01.ju.0000146719.43269.e8. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Cheville JC, Lohse CM, et al. Reclassification of patients with pT3 and pT4 renal cell carcinoma improves prognostic accuracy. Cancer. 2005;104:53–60. doi: 10.1002/cncr.21125. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Leibovich BC, Cheville JC, et al. Should direct ipsilateral adrenal invasion from renal cell carcinoma be classified as pT3a? J Urol. 2005;173:918–921. doi: 10.1097/01.ju.0000153419.98715.24. [DOI] [PubMed] [Google Scholar]
- 18.Von Knobloch R, Varga Z, Schrader AJ, Hofmann R. All patients with adrenal metastasis from RCC will eventually die in tumor progression: there is no cure or benefit from simultaneous adrenalectomy. J Urol. 2004;171:4–4. [Google Scholar]
- 19.Leibovich BC, Cheville JC, Lohse CM, et al. Cancer specific survival for patients with pT3 renal cell carcinoma—can the 2002 primary tumor classification be improved? J Urol. 2005;173:716–719. doi: 10.1097/01.ju.0000151830.27750.d2. [DOI] [PubMed] [Google Scholar]
- 20.Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol. 2004;171:598–601. doi: 10.1097/01.ju.0000108842.27907.47. [DOI] [PubMed] [Google Scholar]
- 21.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes. Impact on survival and benefits of immunotherapy. Cancer. 2003;97:2995–3002. doi: 10.1002/cncr.11422. [DOI] [PubMed] [Google Scholar]
- 22.Dimashkieh HH, Lohse CM, Blute ML, Kwon ED, Leibovich BC, Cheville JC. Extranodal extension in regional lymph nodes is associated with outcome in patients with renal cell carcinoma. J Urol. 2006;176:1978–1982. doi: 10.1016/j.juro.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 24.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 25.The National Cancer Institute. [Accessed August 16, 2013];Required SEER Site-Specific Factors for Collaborative Stage. Available at: http://seer.cancer.gov/tools/ssf/
- 26.Collaborative Stage Work Group of the American Joint Committee on Cancer. Collaborative Stage Data Collection System User Documentation and Coding Instructions, version 02.04.40, Part I, Section 2: Lab Tests, Tumor Markers, and Site-Specific Factor Notes. [Accessed January 22, 2014];Effective. 2012 Jan 1; Available at: https://cancerstaging.org/cstage/Pages/default.aspx.
- 27.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435–441. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bretheau D, Lechevallier E, de Fromont M, Sault MC, Rampal M, Coulange C. Prognostic value of nuclear grade of renal cell carcinoma. Cancer. 1995;76:2543–2549. doi: 10.1002/1097-0142(19951215)76:12<2543::aid-cncr2820761221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.College of American Pathologists. [Accessed August 16, 2013];Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of Renal Tubular Origin. 2012 Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Kidney_12protocol_3101.pdf.
- 31.Collaborative Stage Data Collection System. [Accessed August 16, 2013];KidneyRenalPelvis CS Site-Specific Factor 1 WHO/ISUP Grade. 2010 Available at: http://web2.facs.org/cstage0204/kidneyrenalpelvis/KidneyRenalPelvis_jpd.html.
- 32.Collaborative Stage Data Collection System. [Accessed August 16, 2013];KidneyRenalPelvis CS Site-Specific Factor 2 Depth of Renal Parenchymal Invasion. 2013 Available at: http://cstage2.com/drafts/html/kidneyrenalpelvis/Kid-neyRenalPelvis_kaj.html.
- 33.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Eble JN World Health Organization, International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 35.Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- 36.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 37.Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:135–141. doi: 10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Salamanca JI, Huang WC, Millan I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol. 2011;59:120–127. doi: 10.1016/j.eururo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Han KR, Bleumer I, Pantuck AJ, et al. Validation of an integrated staging system toward improved prognostication of patients with localized renal cell carcinoma in an international population. J Urol. 2003;170:2221–2224. doi: 10.1097/01.ju.0000096049.64863.a1. [DOI] [PubMed] [Google Scholar]
- 40.Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174:1759–1763. doi: 10.1097/01.ju.0000177487.64651.3a. discussion 1763. [DOI] [PubMed] [Google Scholar]
- 41.Delahunt B, McKenney JK, Lohse CM, et al. A novel grading system for clear cell renal cell carcinoma incorporating tumor necrosis. Am J Surg Pathol. 2013;37:311–322. doi: 10.1097/PAS.0b013e318270f71c. [DOI] [PubMed] [Google Scholar]
- 42.Holmang S, Johansson SL. Urothelial carcinoma of the upper urinary tract: comparison between the WHO/ISUP 1998 consensus classification and WHO 1999 classification system. Urology. 2005;66:274–278. doi: 10.1016/j.urology.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Shariat SF, Zigeuner R, Rink M, et al. Subclassification of pT3 urothelial carcinoma of the renal pelvicalyceal system is associated with recurrence-free and cancer-specific survival: proposal for a revision of the current TNM classification. Eur Urol. 2012;62:224–231. doi: 10.1016/j.eururo.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 45.Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol. 2010;184:453–458. doi: 10.1016/j.juro.2010.03.142. [DOI] [PubMed] [Google Scholar]
- 46.Chromecki TF, Cha EK, Fajkovic H, et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol. 2012;61:245–253. doi: 10.1016/j.eururo.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Wheat JC, Weizer AZ, Wolf JS, et al. Concomitant carcinoma in situ is a feature of aggressive disease in patients with organ confined urothelial carcinoma following radical nephroureterectomy. Urol Oncol. 2012;30:252–258. doi: 10.1016/j.urolonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Raman JD, Ng CK, Scherr DS, et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol. 2010;57:1072–1079. doi: 10.1016/j.eururo.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Reichman ME, Altekruse S, Li CI, et al. Feasibility study for collection of HER2 data by National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program central cancer registries. Cancer Epidemiol Biomarkers Prev. 2010;19:144–147. doi: 10.1158/1055-9965.EPI-09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howlader N, Noone AM, Yu MD, Cronin KA. Use of imputed population-based cancer registry data as a method of accounting for missing information: application to estrogen receptor status for breast cancer. Am J Epidemiol. 2012;176:347–356. doi: 10.1093/aje/kwr512. [DOI] [PMC free article] [PubMed] [Google Scholar]