Abstract
Objective
Postpartum infections are polymicrobial and typically include Ureaplasma, an intracellular microbe treated by macrolides such as azithromycin. The aim of this study was to evaluate the perinatal pharmacokinetics of azithromycin following a single pre-incision dose prior to cesarean delivery.
Study Design
Thirty women undergoing scheduled cesarean delivery were randomized to receive 500 mg of intravenous azithromycin initiated 15, 30, or 60 minutes prior to incision and infused over one hour. Serial maternal plasma samples were drawn from the end of infusion up to 8 hours after the infusion. Samples of amniotic fluid, umbilical cord blood, placenta, myometrium, and adipose tissue were collected intraoperatively. Breast milk samples were collected 12-48 hours after the infusion in 8 women who were breastfeeding. Azithromycin was quantified using high performance liquid chromatography separation coupled with tandem mass spectrometry detection. Plasma pharmacokinetic parameters were estimated using non-compartmental analysis and compartmental modeling and simulations.
Results
The maximum maternal plasma concentration was reached within 1 hour and exceeded the in vitro minimum inhibitory concentration (MIC50) of 250 ng/mL of Ureaplasma spp in all 30 patients. The concentrations were sustained with a half-life of 6.7 hours. The median concentration (Cmed) of azithromycin in adipose was 102 ng/g, which was below the MIC50. The Cmed in myometrium was 402 ng/g, which exceeded the MIC50. Azithromycin was detectable in both the umbilical cord plasma and amniotic fluid following the single pre-operative dose. Azithromycin concentrations in breast milk were high and sustained up to 48 hours following the single dose. Simulations demonstrated accumulation in breast milk following multiple doses.
Conclusion
A single dose of azithromycin achieves effective plasma and tissues concentrations and is rapidly transported across the placenta. The tissue concentrations achieved in the myometrium exceed the MIC50 for Ureaplasma spp
Keywords: cesarean section, endometritis, wound infection, azithromycin, pharmacokinetics, Ureaplasma, breast milk
Introduction
Post-cesarean infections, including endometritis and wound infections, represent a significant health and economic burden. Studies have demonstrated up to a 50% reduction in postpartum infections when cephalosporin antibiotics are administered before skin incisions with no apparent adverse effects in neonates1, 2. Current guidelines recommend administration of a first-generation cephalosporin within 60 minutes prior the start of cesarean delivery3. Despite recent advances, surgical site infections remain a significant problem. Postpartum infections are polymicrobial, and intracellular microbes such as Ureaplasma spp and Mycoplasma spp, which are not effectively treated by cephalosporins, are significant pathogens in endometritis4, 5. Extended-spectrum antibiotic prophylaxis with both a cephalosporin and azithromycin, which has antimicrobial activity against Ureaplasma spp, has been associated with a significant reduction in post-cesarean endometritis and shorter hospital stays when given after cord clamp.6-8 An ongoing large clinical trial is investigating if the addition of azithromycin to the standard regimen of a cephalosporin prior to skin incision further decreases post-cesarean infections.
Ureaplasma spp has also been implicated in significant neonatal infections, such as pneumonia, meningitis, and bacteremia.9 Multiple studies have shown that respiratory tract colonization with Ureaplasma spp is associated with an increased risk of bronchopulmonary dysplasia (BPD).10 Postnatal treatment with azithromycin may prevent BPD in preterm infants with Ureaplasma spp colonization or infection.11 These infections often result from perinatal transmission, as Ureaplasma spp are commensal organisms of the lower genital tract and are implicated in chorioamnionitis, pregnancy loss and spontaneous preterm birth.12 Therefore, perinatal treatment of select populations with azithromycin may potentially reduce the risk of both maternal and neonatal complications caused by these organisms if transplacental transfer occurs. These benefits must be carefully weighed against the potential for antimicrobial resistance, thereby selecting for more virulent maternal and neonatal pathogens.
Depending on the clinical isolate, the in vitro minimal inhibitory concentration (concentration of drug required to inhibit 50% of growth, MIC50) of azithromycin against Ureaplasma spp ranges from 250 ng/mL to 1000 ng/mL. For example, the MIC50 of azithromycin is 250 ng/mL for Ureaplasma parvum isolated from the placenta,13 while the MIC50 of AZI for Ureaplasma spp isolated from the adult genital tract is 500 ng/mL.14 Neonatal isolates require higher concentrations of the antibiotic as the MIC50 for Ureaplasma spp isolated from neonatal respiratory tracts is 1000 ng/mL.15
There are limited data regarding the perinatal pharmacokinetics of azithromycin.16 Given the multiple potential applications for the use of azithromycin during pregnancy, we sought to evaluate the perinatal pharmacokinetics of AZI following a single pre-incision intravenous dose. Intravenous azithromycin administration at different time points for pre-incision prophylaxis provides a model to study the maternal-fetal pharmacokinetics of intravenous azithromycin, which could enhance our understanding of appropriate dosing strategies during pregnancy.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Alabama at Birmingham (F101111007) and was registered at ClinicalTrials.gov (NCT01464840). An Investigational New Drug application was approved by the Food and Drug Administration (IND 111917). Women undergoing a planned cesarean delivery at term (≥ 37 weeks) with a singleton gestation were eligible for the study. Exclusion criteria included: multiple gestation, preterm (< 37 weeks) gestation, ruptured membranes or labor, known fetal anomalies, oligo- or polyhydramnios, azithromycin exposure within 2 weeks, allergy to macrolide antibiotics, significant medical or obstetric co-morbidities, hepatic or renal impairment, concurrent treatment with medications that prolong the QT interval (such as ondansetron), concurrent treatment with nelfinavir, efavirenz, or fluconazole, structural heart defects, or known arrhythmias. Signed informed consent was obtained at least 24 hours prior to delivery. The participants were contacted and charts were reviewed 1 week and 3 months after completion of the study for any study-related maternal and fetal adverse events.
Women were randomized to receive 500 mg of azithromycin intravenously initiated 15, 30, or 60 minutes prior to the planned incision time. The infusion was given over 1 hour. Due to clinical constraints, the actual timing of the incision may have deviated from the planned interval. Each participant had a second IV line designated for phlebotomy. Maternal blood samples for azithromycin concentration determination in plasma were scheduled to be drawn prior to the infusion, at the conclusion of the infusion, at the time of incision, and 30 minutes, 1 hour, 3 hours, 5 hours, and 7 hours after the conclusion of the infusion. Amniotic fluid, umbilical cord blood, placental tissue, myometrial tissue, and adipose tissue samples were collected intraoperatively. Breast milk specimens were collected from pumped samples 12-48 hours after the infusion from breastfeeding participants. All samples were stored at -80°C until analysis.
Azithromycin and its added internal standard clarithromycin were quantified using high performance liquid chromatography (HPLC) separation coupled with tandem mass spectrometry detection. Tissue samples were weighed and homogenized in 4 volumes of 50 mM ammonium acetate. A standard curve (range 2.5 – 5000 ng/mL) was prepared in plasma, and the plasma curve was used as a surrogate for all other matrices. Quality control samples were prepared by spiking plasma with azithromycin, for final concentrations of 7, 450, and 4500 ng/mL, and the internal standard clarithromycin (250 ng/mL). Azithromycin and the internal standard were extracted from all unknown samples, standards, and quality control samples by addition of 50 μL of sample to 500 μL of acetonitrile in microcentrifuge tubes. The tubes were centrifuged and the supernatant was diluted (1:2 dilution) in a mixture of 50 mM ammonium acetate and methanol (1:1). Reversed phase chromatographic separation of azithromycin and the internal standard was performed on a XTerra® MS C8 column (5 urn, 2.1 × 100mm, Waters Corp, Milford, MA) under isocratic conditions. A binary mobile phase consisting of 50 mM ammonium acetate, acetonitrile, and methanol (50:31:19) was used. The detection and quantitation was achieved for azithromycin and the internal standard by multiple reaction monitoring (MRM). The de-protonated molecular ions [M-H]-were monitored at m/z 749.6 > 573.2 for AZI, and m/z 748.5 >157.9 for clarithromycin. These provided adequate sensitivity with minimal interference from endogenous matrix components. Plasma pharmacokinetic parameters were estimated using noncompartmental methods (Phoenix WinNonlin, Certara USA, Inc., St. Louis, MO). Modeling and simulations of plasma and breast milk data were performed using ADAPT 5.17
Results
Thirty women undergoing scheduled cesarean deliveries completed the study. The baseline characteristics of the participants are shown in Table 1. The median time between initiation of the infusion and the skin incision was 51 minutes with a range of 10-219 minutes. The incision time was within 15 minutes or less of the planned interval in 20 of the 30 patients. There were no significant adverse events related to azithromycin exposure reported in the women or infants.
Table 1.
Baseline demographics of the study population.
| Characteristic | Result |
|---|---|
|
| |
| Maternal age, years – mean ± SD (range) | 28.1 ± 5.4 (20-41) |
| Parity – median (interquartile range; range) | 2 (1-2; 0-6) |
| Race – no. (%) | |
| African-American | 17 (56.7%) |
| Hispanic | 8 (26.7%) |
| Caucasian | 4 (13.3%) |
| Asian | 1 (3.3%) |
| Pre Preg Body mass index, kg/m – mean ± SD (range) | 30.1 ± 6.0 (19.8-43.1) |
| Body mass index, kg/m2 – mean ± SD (range) | 36.6 ± 7.0 (26.5-59.3) |
| Indication for cesarean delivery – no. (%) | |
| Elective repeat cesarean | 27 (90%) |
| Malpresentation | 1 (3.3%) |
| Prior classical, vertical, T or J | 1 (3.3%) |
| Other | 1 (3.3%) |
| Gestational age at delivery, weeks – mean ± SD (range) | 39.1 ± 0.3 (39-40) |
| Birthweight, grams – mean ± SD (range) | 3318 ± 359 (2800-4290) |
| Dose timing*, min – mean, range | 51 (10-219) |
time between initiation of infusion and skin incision
SD, standard deviation
Maternal serum concentrations peaked within 1 hour and were sustained over the study period (Fig.1) with a half-life of 6.7 hours (Table 2). Pharmacokinetic parameters (Table 2) were estimated using a 2-compartment plasma model linked to a 1-compartment breast milk model with an intermediate delay compartment between the plasma and breast milk. The last plasma sample collection in our dataset was at approximately 8 hours, thus a 2 compartment model adequately fit the data in this case as no points in the terminal elimination phase were collected. Figure 1 depicts the raw AZI plasma and breast milk concentrations and the best model fit for each. The mean (standard deviation) plasma area under the concentration-time curve from time 0 to infinity (AUC0-∞), minimum concentration (Cmin), and maximum concentration (Cmax) were 6030 (2170) ng × hr/mL, 147 (43) ng/mL, and 4500 (2430) ng/mL, respectively. Azithromycin was rapidly distributed into tissues (Fig. 2). The median concentrations (Cmed) of AZI in adipose, placental, and myometrial tissue were 102, 221, and 402 ng/g, respectively (Fig. 2). The highest concentration of AZI was achieved in myometrial tissue, with a Cmax of 7774 ng/g. The Cmax in adipose and placental tissue were 717 and 961 ng/g, respectively.
Figure 1.
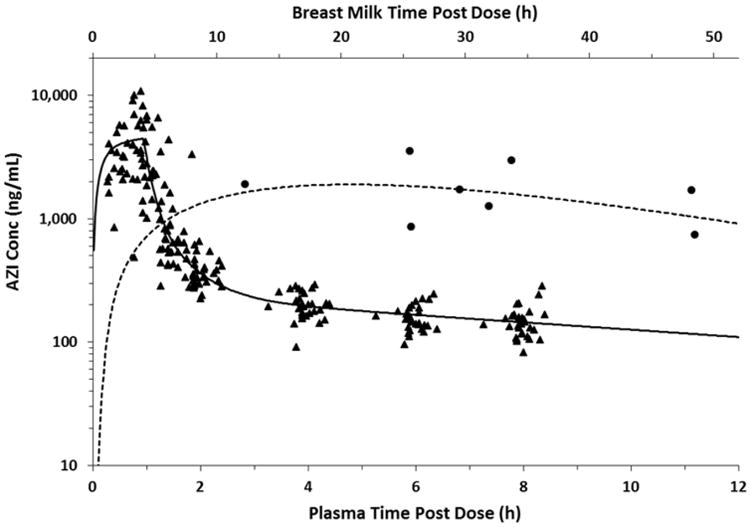
Concentration-time profile of maternal plasma AZI (triangles) and breast milk (circles) following a single intravenous dose. Solid line represents the best model fit of plasma data and dashed line is the best model fit of breast milk data.
Table 2.
Plasma and breast milk (BM) pharmacokinetic parameters of AZI for cesarean prophylaxis.
| Parameter | Median (range) |
|---|---|
|
| |
| T1/2 – plasma half-life (hr) | 6.7 (6.4-7.6) |
| CL - plasma clearance (L/hr) | 73.2 (59.0-87.1) |
| Vc – central volume of distribution in plasma (L) | 27.7 (24.0-35.0) |
| Vp – peripheral volume of distribution in plasma (L) | 290 (286-292) |
| CLd – intercompartmental clearance (L/hr) | 51.0 (43.9-56.0) |
| Tau – rate constant for delay compartment (hr-1) | 0.096 (0.06-0.22) |
| T1/2bm – BM half-life (hr) | 15.6 (15.5-15.8) |
| CLbm – BM clearance (L/hr) | 0.17 (0.17-0.17) |
| Vbm – BM distribution volume (L) | 3.87 (3.85-3.88) |
Figure 2.
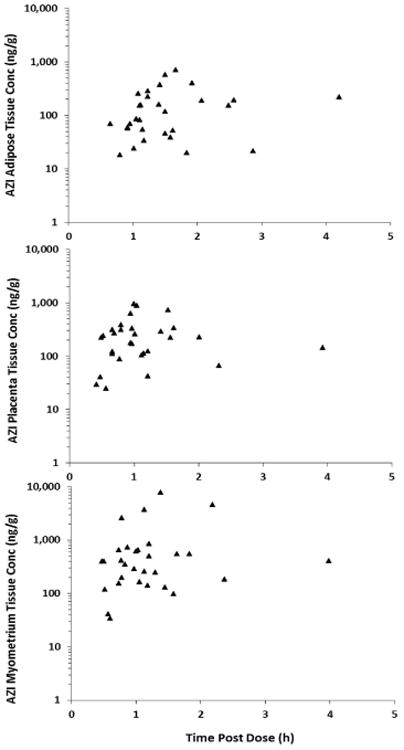
AZI concentrations (triangles) in a) adipose, b) placental, and c) myometrial tissues.
AZI was transported across the placenta with concentrations detectable in fetal compartments within 30 minutes (Fig. 3). The Cmed in amniotic fluid was 33 ng/mL at a median time of 0.92 hours post-dose. The Cmed in venous umbilical cord plasma was 150 ng/mL at a median time of 0.95 hours post-dose.
Figure 3.
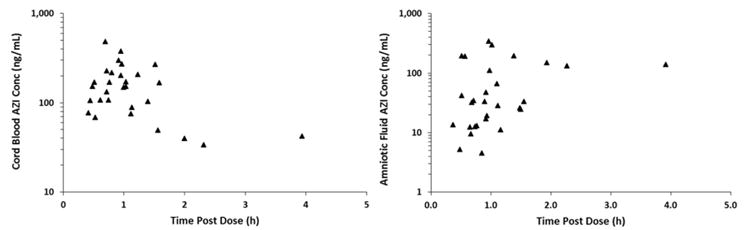
AZI concentrations (triangles) in a) venous umbilical cord plasma and b) and amniotic fluid.
AZI achieved sustained concentrations in breast milk up to 48 hours after the single dose (Fig. 4). At a median of 30.7 hours post-dose, the median breast milk concentration was 1713 ng/mL. The model used to fit the concentration-time data was also used to simulate plasma and breast milk concentrations following multiple IV doses of 500 mg every 12 hours (Fig 4). Simulations predicted accumulation in breast milk following 3 doses with steady-state achieved at approximately 3 days. Assuming an intake of 150 mL/kg/day and bioavailability of 38%, the daily AZI dose of a 3.5 kg exclusively-breast fed infant is estimated to be 340 μg (∼0.1 mg/kg).
Figure 4.
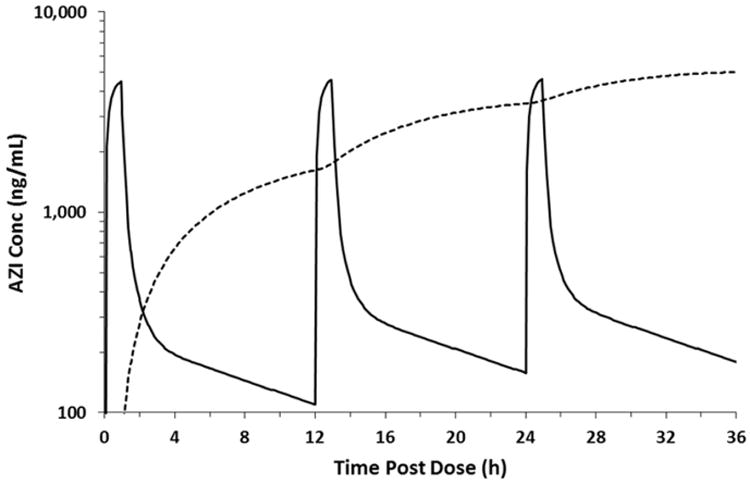
Simulated plasma and breast milk concentration-time curves following 3 IV doses.
Comment
A single intravenous dose of 500 mg of azithromycin administered just prior to skin incision achieved effective maternal plasma concentrations that exceeded the MIC50 for most Ureaplasma spp isolates. Assuming an average tissue density of 1 g/mL, the Cmed of azithromycin in myometrium of approximately 402 ng/mL exceeded the MIC50 (250 mg/mL) of Ureaplasma parvum isolated from the reproductive tract.13 However, the Cmed of all tissues was below the MIC50 (1000 mg/mL) for Ureaplasma spp derived from neonatal tracheal aspirates.15 Azithromycin was rapidly transferred across the placenta, with concentrations detectable in the umbilical cord plasma and amniotic fluid within approximately 20-30 minutes. However, these median concentrations following this single dose were well below the MIC50 for Ureaplasma spp. In breast milk, azithromycin exhibited high and sustained concentrations over the sampling period.
There are limited data regarding the pharmacokinetics of azithromycin during pregnancy and lactation. In one study of oral azithromycin administered 6 to 72 hours prior to cesarean delivery in humans, drug concentrations in myometrium, adipose tissue, and placenta were 900-2100 ng/mL,16 thus exceeding the MIC50 for most Ureaplasma spp. Maternal serum, amniotic fluid, and umbilical cord blood levels were 19-311 ng/mL. In a primate model of intra-amniotic Ureaplasma infection, multiple doses of AZI, administered every 12 hours intravenously for 10 days, resulted in a prolonged plasma half-life of 66 hours and accumulation of the drug in the amniotic fluid with a half-life of 129 hours.18 Importantly, this multi-dose regimen resulted in eradication of Ureaplasma with a 95% effective concentration of 39 ng/mL. Also, there is currently only one case report of a single patient which analyzed the transfer of AZI into breast milk.19
This pharmacokinetic study has some strengths and limitations. The dose timing, administered just prior to delivery, is clinically relevant, mirroring real-world dosing of prophylactic antibiotics for cesarean. Multiple maternal and fetal compartments were sampled in this study. Although many of the measured concentrations were below the MIC50 for Ureaplasma spp, the in vitro MIC may not correlate with the in vivo microbial activity of azithromycin in this setting. Additionally, the single early time points of tissue concentrations are not reflective of the continued tissue uptake of azithromycin, which was demonstrated in a previous study of oral azithromycin.16 In addition to the higher tissue concentrations of azithromycin attained in the previous study, the cost of oral azithromycin is significantly less than the intravenous formulation. However, administration of prophylactic antibiotics several hours to several days prior to cesarean delivery is not practical, especially for non-scheduled deliveries with the highest risk of infection. As this was designed as a pharmacokinetic study, we did not assess the clinical or microbial efficacy of azithromycin in post-surgical infections. Primate models of intra-amniotic Ureaplasma infection have indicated that multiple doses of azithromycin leads to eradication of the infection with drug concentrations below 100 ng/mL.18, 20 These amniotic fluid concentrations are similar to those in the current study (Cmed of 33 ng/mL).
This study also represents the first report of breast milk concentrations of azithromycin measured in multiple patients. Following a single dose, azithromycin exhibited high and sustained concentrations in breast milk. Pharmacokinetic simulations showed that multiple doses of azithromycin would result in further accumulation with higher concentrations during the treatment period. The estimated daily neonatal exposure following a single dose was calculated at approximately 340 μg. Although this is below the treatment dose recommended for neonates at 10 mg/kg/day, this dose would be additive to the transplacental exposure of azithromycin.
This study shows that a single intravenous dose of 500 mg of azithromycin prior to cesarean delivery results in adequate plasma and myometrium concentrations to for prophylaxis against Ureaplasma spp. Administration of azithromycin 1 hour or greater prior to the surgery appears to be optimal given the continued tissue uptake of the drug. Although azithromycin concentrations are lower in the adipose tissue, they still may be adequate for wound infection prophylaxis, especially considering the accumulation of azithromycin in tissue over time. In fact, an ongoing multicenter trial is assessing the clinical efficacy of extended-spectrum prophylaxis including azithromycin for post-cesarean infections. Further understanding of the transplacental pharmacokinetics of multiple doses of azithromycin over a longer sampling period will provide guidance in determining the optimal dose and timing of the drug for other indications, such as preterm premature rupture of membranes or perinatal treatment of syphilis. Additionally, such studies may set the stage for perinatal treatment of fetal and neonatal infections with Ureaplasma associated with bronchopulmonary dysplasia.
Acknowledgments
We thank Rachel Copper LeDuke, Mickey Parks, Mona Wallace, Lee Ann Merin, Stacy Harris, Suzette Byars, and Gloria Adams (Department of Obstetrics and Gynecology, University of Alabama at Birmingham) for research nursing support, Victoria Jauk, Robin Steele, Cherry Neely, and Suzanne Cliver (Department of Obstetrics and Gynecology, University of Alabama at Birmingham) for database management, and the Pfizer Pharmaceutical Corporation for providing azithromycin for this study.
This work was supported by an award from the University of Alabama at Birmingham Health Services Foundation General Endowment Fund and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR00165.
Footnotes
Disclosure: The authors report no conflict of interest.
Presented in part at the 34th Annual Meeting of the Society for Maternal-Fetal Medicine in New Orleans, LA February 3-8, 2014.
The trial was registered with ClinicalTrials.gov (NCT01464840) http://clinicaltrials.gov/show/NCT01464840.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199:301 e1–6. doi: 10.1016/j.ajog.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 2.Baaqueel H, Baaqueel R. Timing of administration of prophylactic antibiotics for caesarean section: a systematic review and meta-analysis. BJOG. 2013;120:661–9. doi: 10.1111/1471-0528.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472–83. doi: 10.1097/AOG.0b013e3182238c31. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Shah SR, Goldenberg RL, Cliver SP, Haugh JC, Cassell GH. Association of post-cesarean delivery endometritis with colonization of the chorioamnion by Ureaplasma urealyticum. Obstet Gynecol. 1995;85:509–14. doi: 10.1016/0029-7844(94)00436-H. [DOI] [PubMed] [Google Scholar]
- 5.Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and Chlamydia trachomatis. Obstet Gynecol. 1989;73:52–60. [PubMed] [Google Scholar]
- 6.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183–9. doi: 10.1016/s0029-7844(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 7.Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51–6. doi: 10.1097/01.AOG.0000295868.43851.39. [DOI] [PubMed] [Google Scholar]
- 8.Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199:303 e1–3. doi: 10.1016/j.ajog.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 9.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009;14:190–9. doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis. 2005;24:1033–9. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 11.Ballard HO, Shook LA, Bernard P, et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr Pulmonol. 2011;46:111–8. doi: 10.1002/ppul.21352. [DOI] [PubMed] [Google Scholar]
- 12.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–89. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walls SA, Kong L, Leeming HA, Placencia FX, Popek EJ, Weisman LE. Antibiotic prophylaxis improves Ureaplasma-associated lung disease in suckling mice. Pediatr Res. 2009;66:197–202. doi: 10.1203/PDR.0b013e3181aabd34. [DOI] [PubMed] [Google Scholar]
- 14.Krausse R, Schubert S. In-vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma ssp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16:1649–55. doi: 10.1111/j.1469-0691.2009.03155.x. [DOI] [PubMed] [Google Scholar]
- 15.Hassan HE, Othman AA, Eddington ND. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J Clin Pharmacol. 2011;51:1264–75. doi: 10.1177/0091270010382021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol. 2003;188:714–8. doi: 10.1067/mob.2003.141. [DOI] [PubMed] [Google Scholar]
- 17.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: Biomedical Simulations Resource; 2009. [Google Scholar]
- 18.Acosta EP, Grigsby PL, Buckoreelall K, et al. Transplacental Transfer of Azithromycin and its Application For Eradicating Intraamniotic Ureaplasma Infection in a Primate Model. J Infect Dis. 2013;209:898–904. doi: 10.1093/infdis/jit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelsey JJ, Moser LR, Jennings JC, Munger MA. Presence of azithromycin breast milk concentrations: a case report. Am J Obstet Gynecol. 1994;170:1375–6. doi: 10.1016/s0002-9378(94)70161-x. [DOI] [PubMed] [Google Scholar]
- 20.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475 e1–75 e14. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]


