Abstract
Oxirane derivatives are the most used benchmarks for chiroptical spectroscopies in view of their small size and relative rigidity. The molecular structure, vibrational harmonic and anharmonic frequencies, and infrared intensities of the ground electronic states are analyzed in this paper. Equilibrium structure and harmonic force fields have been evaluated by means of high-level quantum-chemical calculations at the coupled-cluster level including single and double excitations together with a perturbative treatment of triples (CCSD(T)). Extrapolation to the complete basis-set limit as well as core-correlation effects have also been taken into account. Anharmonic contributions have been computed at the CCSD(T)/cc-pVTZ level for trans-2,3-dideuterooxirane. These data can serve as references to evaluate the accuracy of less expensive computational approaches rooted in the density functional theory (DFT). The latter have been used within hybrid CC/DFT approaches, which have been applied to simulate fully anharmonic infrared (IR) spectra. Finally, the best theoretical estimates of the equilibrium structures and vibrational wavenumbers are compared to the most accurate experimental data and show in all cases very good agreement, i.e., within 0.001 Å, 0.1 degrees and 10 cm−1 and 0.5 km mol−1, for bond lengths, angles, wavenumbers and IR intensities, respectively.
Keywords: oxiranes, accurate structure, IR spectra, anharmonicity
I. INTRODUCTION
Substituted oxiranes have become the de facto standards to benchmark theoretical and experimental methodologies intended for chiroptical spectroscopies1,2. In particular, trans-2,3-dideuterooxirane (D2Ox), methyloxirane (MeOx), and trans-2,3-dimethyloxirane (Me2Ox) have been extensively investigated with both density functional theory (DFT) and post-Hartree-Fock approaches by comparing the computed optical rotation, electronic and vibrational circular dichroism (ECD and VCD, respectively) spectra with their experimental counterparts, recorded in the gas phase and/or in solution3–27. Although satisfactory results have been obtained with different approaches, thorough studies proceeding from accurate equilibrium structures to anharmonic force fields and further to electric and magnetic moments, excited electronic states and environmental effects are still lacking. Accurate molecular structures and vibrational spectra are the mandatory starting points to an accurate treatment of chiroptical spectroscopies. In fact, the positions of the VCD bands are the same as those of their infrared (IR) counterparts and the VCD intensities depend, in addition to the magnetic moments, on the electric moments involved in the IR intensities. At the same time, optical activity is ruled by molecular structure and vibrational effects. Furthermore, we point out that the vibrational corrections to rotational constants, chiral and non-chiral molecular properties, and thermodynamical functions are in all cases obtained from the same anharmonic force field employed in the IR spectra determination. Finally, vibronic contributions to ECD spectra originate from ground- and excited-state vibrational frequencies (see Ref.28 for an extensive review on different effects underlying the overall spectral phenomena). On these grounds, we decided to undertake a comprehensive study of the substituted oxiranes mentioned above aiming at providing accurate structures and vibrational contributions as a first step toward the determination of reliable chiroptical properties.
Accurate structural determinations are still a challenge for both experiment and theory. The best choice to report structural information is the equilibrium structure since it is well defined (minimum of the Born-Oppenheimer (BO) potential energy surface), it excludes vibrational effects in a rigorous manner and, within the BO approximation, it is independent of the considered isotopic species. In view of the difficulties in dealing with vibrational effects, the so-called semi-experimental equilibrium structure 29 probably provides the most reliable route toward the accurate determination of equilibrium geometries30–33. This requires the determination of experimental ground-state rotational constants (B0) for different isotopic species, their correction for vibrational effects by means of anharmonic force-field computations, and then a least-square fit of the geometrical parameters to the resulting semi-experimental equilibrium rotational constants . Unfortunately, this approach is limited by the availability of rotational constants for different isotopic species, which are often not sufficient for determining all geometrical parameters, thus requiring debatable constraints for some of them30,32,34–36. In the present context, sufficient information is available to obtain the semi-experimental equilibrium structure of oxirane37–39 (which is the same of trans-2,3-dideuterooxirane within the BO approximation) and methyloxirane40, but this is not the case for dimethyloxirane. Under such circumstances, one has to resort to pure theoretical geometries. Several studies have shown that, in the absence of a strong multireference character, the coupled-cluster (CC) singles and doubles approximation augmented by a perturbative treatment of triple excitations, CCSD(T)41, in conjunction with extrapolation to the complete basis-set limit and a proper account of core correlation is able to provide accurate structures, rivaling their best experimental counterparts (see, for example, Refs.30,42–45). Despite the high computational cost of such an approach, the latest developments in hardware and software permit its use for medium-sized systems46–48. Recently, we have shown that the dimensions of systems amenable to such accurate analysis can be further extended by performing basis-set extrapolation and inclusion of core correlation by means of second-order Møller-Plesset perturbation theory (MP2)49 in place of the much more expensive (and poorly scaling) coupled-cluster ansatz36,42. This cheaper approach will be further validated in the present work by comparison with full coupled-cluster and semi-experimental results for the two smaller substituted oxiranes.
The situation is more involved for IR spectra when the sought accuracy implies going beyond the harmonic approximation for frequencies and, especially, for intensities50. From a methodological point of view, second-order vibrational perturbation theory (VPT2)51,52 based on Cartesian normal modes still represents, in our opinion, the best compromise between accuracy and efficiency, at least for semi-rigid molecules of medium-to-large size53–61. Several studies have shown that electron correlation and basis-set extension are of paramount importance for the harmonic contributions, whereas anharmonic corrections can be reliably obtained at lower computational levels62–70. This led to the introduction of hybrid schemes62,69,71,72, which are based on the close correspondence between the normal modes obtained at the two different levels of theory and on the hypothesis that the largest part of the differences in calculated observables lies in the harmonic part. While this strategy can be considered well tested for vibrational energies42,50,62,69–73, the corresponding approach for IR intensities has seen limited applications till now, also due to the fact that it has become only recently available in general-purpose computational codes59,74. However, our first results are promising also in this direction. As a result, hybrid CC/DFT schemes turn out to combine accuracy and computational efficiency42,50,75. In this context, hybrid density functionals (especially B3LYP) provide particularly good results when coupled with polarized double-ζ basis sets augmented by diffuse functions50,69,70. Accuracy can be further improved by using the so-called double hybrid functionals (especially B2PLYP), but at the price of increased basis-set requirements (at least of augmented doubly polarized triple-ζ quality) and computational cost50,76. In the present study, the small size of the dideuterated oxirane allows us to further investigate the accuracy reachable with hybrid CC/DFT schemes with respect to accurate anharmonic force fields derived from best-estimated harmonic force fields with cubic and semi-diagonal quartic force constants computed at the CCSD(T) level.
This manuscript is organized as follows: after an extensive description of the computational protocol used in this work, the equilibrium structures obtained with different computational models are compared to our best theoretical estimates and, where available, to the semi-experimental structures. Subsequently, the accuracy of the harmonic vibrational wavenumbers and IR intensities is discussed, and followed by the analysis of the corresponding anharmonic contributions. Finally, the fully anharmonic IR spectra are simulated and the accuracy of different methods and hybrid approaches is assessed by comparison with the available experimental data and our best theoretical estimates.
II. COMPUTATIONAL DETAILS
The MP249 and CCSD(T)41 methods were employed in molecular structure and force-field calculations, as described below. Correlation-consistent basis sets, (aug)-cc-p(C)VnZ (n=T,Q,5)77–79, were used in conjunction with the aforementioned methods. MP2 and CCSD(T) calculations were carried out with the quantum-chemical package CFour80.
Density Functional Theory was employed to compute equilibrium geometries, quadratic, cubic and semi-diagonal quartic force fields together with up to the third derivatives of the dipole moment, needed for the computation of fully anharmonic IR spectra. In view of its efficiency in computational spectroscopy studies of relatively large molecular systems,69,76,81,82 including IR and Raman intensities42,48,50,75, the standard functional B3LYP83 was chosen in conjunction with a polarized basis set of double-ζ quality supplemented by diffuse functions, SNSD50,84. Moreover, functional CAM-B3LYP85, which has shown a good performance for the computations of chiroptical properties82,86, has been considered. For both B3LYP and CAM-B3LYP, the basis set convergence has been assessed by comparison with the results obtained with basis set aug-cc-pVTZ. Finally, the double-hybrid functional B2PLYP87,88 was considered in conjunction with basis set aug-cc-pVTZ because of its well-proved accuracy50,76. Computations at the B2PLYP level are significantly more expensive than those performed with B3LYP or CAM-B3LYP because of both the inclusion of a second-order perturbation treatment of the electron correlation and larger basis set requirements. On the other hand, they remain very cost-effective alternatives with respect to CCSD(T)50,76,89,90. All DFT computations were performed employing a locally modified version of the suite of programs for quantum chemistry GAUSSIAN91.
A. Molecular Structure
Two different composite approaches were employed in view of accurately determining equilibrium structures. Within these composite schemes, the contributions that are considered to be the most important are evaluated separately at the highest possible level and then, by resorting to the additivity approximation, combined in order to obtain the best theoretical estimates.
The first approach considered is the so-called “gradient scheme”. It is a rigorous approach based on the additivity at an energy-gradient level,43,44 and the included contributions are: the Hartree-Fock self-consistent-field (HF-SCF) energy extrapolated to the complete basis-set (CBS) limit, the valence correlation energy at the CCSD(T) level extrapolated to the CBS limit as well, and the core-correlation correction. The energy gradient used in the geometry optimization is therefore given by
| (1) |
where dE∞(HF-SCF)/dx and dΔE∞(CCSD(T))/dx are the energy gradients corresponding to the exp(−Cn) extrapolation scheme for HF-SCF92 and to the n−3 extrapolation formula for the CCSD(T) correlation contribution,93 respectively. In the expression given above, n=T, Q and 5 were chosen for the HF-SCF extrapolation, while n=T and Q were used for CCSD(T) for all molecules, except oxirane for which n=Q and 5 were also considered. Core-correlation effects were included by adding the corresponding correction, dΔE(CV)/dx, with the core-correlation energy correction, ΔE(CV), being obtained as the difference between the all-electron and frozen-core CCSD(T) energies using core-valence basis sets (either cc-pCVTZ or cc-pCVQZ). The resulting structure, which is usually referred to as “CCSD(T)/CBS+CV”, will be simply denoted as “bestCC”.
The second composite approach employed is a cheaper computational scheme recently introduced to obtain accurate equilibrium structures of large molecules42,94, which relies on the additivity approximation applied directly to geometrical parameters. This scheme mainly involves geometry optimizations at the MP249 level. Within this approach, the CBS limit is evaluated by assuming that the convergence behavior of the structural parameters mimics that of the energy. On a purely empirical (but well tested, see Ref.95) basis, the consolidated n−3 extrapolation form93 was applied to the case n=T and Q:
| (2) |
where n=4, and thus r(n) and r(n − 1) denote the MP2/cc-pVQZ and MP2/cc-pVTZ optimized parameters, respectively. The effects due to the CV correlation were included by means of the corresponding correction, Δr(CV), derived by analogy with the energy-gradient corrections, but at the MP2 level (with the cc-pCVTZ basis set). The effect of diffuse functions (Δr(aug)) was considered through geometry optimizations at the MP2/aug-cc-pVTZ level within the frozen-core approximation, in order to complement the extrapolation described above (see Refs.42,94). Higher-order correlation energy contributions to molecular structure (Δr(T)) were derived from the comparison of the geometries optimized at the MP2 and CCSD(T) levels, both with the cc-pVTZ basis set. On the whole, our best-estimated equilibrium structure was thus determined as
| (3) |
This structure will be denoted as “best estimate” or, in equations and tables, more simply as “best”.
B. Harmonic force fields
Similarly to molecular structure determination, two different composite schemes were employed to derive best-estimated harmonic force fields. The first approach relies only on CCSD(T) calculations and follows the first composite scheme introduced in the previous section, while the second (“cheap”) one is based on the additivity scheme summarized in Eq. (3). While the former could be applied only to the study of oxirane, the second approach was used for all three investigated molecules. The procedure introduced in Ref.96 was employed to perform the extrapolation to the CBS limit of the harmonic wavenumbers, ω, at both the MP2 and CCSD(T) levels (ω(CBS(T, Q))). The extrapolated harmonic wavenumbers were then corrected for the effects of core correlation (Δω(CV)) and diffuse functions (Δω(aug)), obtained at either the CCSD(T) or the MP2 level. For the second approach, the so-called “cheap scheme”, higher-order electron-correlation energy contributions (Δω((T))) were also considered. The best-estimated harmonic wavenumbers obtained by means of the two composite schemes, ω(bestCC) and ω(best), respectively, were thus obtained by applying the additivity approximation and putting together the contributions considered as follows:
| (4) |
and
| (5) |
All harmonic force fields were obtained using the analytic second derivatives80,97.
A composite scheme was also used to determine the best estimates for IR intensities, I(best), within the harmonic approximation. Even if extrapolation schemes have not been formulated yet for such a property, on an empirical basis, the approaches introduced above for wavenumbers were applied:
| (6) |
for the scheme exclusively based on CCSD(T) calculations and
| (7) |
for the “cheap scheme”.
C. Anharmonic force fields
For trans-2,3-dideuterooxirane, thanks to its limited molecular size, the anharmonic (full cubic and semi-diagonal quartic) force field was calculated at the CCSD(T)/cc-pVTZ level, within the frozen-core approximation. As implemented in CFour, the harmonic force fields were obtained using analytic second derivatives as described above, whereas the corresponding cubic and semi-diagonal quartic force fields were determined in a normal-coordinate representation via numerical differentiation of the analytically evaluated force constants98–101.
The DFT cubic and semi-diagonal quartic force fields and up to the third derivatives of the electric dipole moment, were determined by numerical differentiations of analytic force constants matrix and first derivatives of the electric dipole at displaced geometries along the normal modes (with a 0.01 Å step), with the equilibrium structure optimized using tight convergence criteria (maximum forces and displacements smaller than 1.5 × 10−5 Hartree/Bohr and 6 × 10−5 Å, respectively). To get accurate results, all computations were carried out with an ultrafine integration grid (99 radial shells and 590 angular points per shell) for the numerical integrations to obtain the two-electron integrals and their derivatives.
The hybrid force fields were obtained in a normal-coordinate representation by replacing in the full anharmonic force fields (computed mainly at the DFT level) the harmonic wavenumbers calculated at a higher lever of theory (mainly the best theoretical estimates). When the normal modes are very similar, which is the present case, DFT cubic and quartic force constants can be used without any transformation. These hybrid force fields were then employed in anharmonic computations within the VPT2 approach51,52. This procedure also allows us to identify resonances based on the most accurate results.
Vibrational wavenumbers were obtained within the generalized VPT2 model (GVPT2), where nearly-resonant contributions are removed from the perturbative treatment (leading to the deperturbed model, DVPT2) and treated variationally in a second step55,58. Such an approach relies on semi-empirical thresholds for Fermi and Darling-Denninson resonances. In the present work, the criteria proposed by Martin et al.102 for Fermi resonances have been chosen as they provide accurate results for fundamental50 and non-fundamental75 transitions. IR intensities with full account of both mechanical and electrical anharmonicities were computed at the DVPT2 level74,90, along with the criteria proposed by some of us for 1-1 resonances74. In addition to the simulation of fully anharmonic IR spectra, VPT2 computations also provide vibrational corrections to rotational constants.
VPT2 computations were mostly performed employing the GAUSSIAN suite of programs for quantum chemistry (G09 Rev: D.01)91.
III. RESULTS AND DISCUSSION
A. Equilibrium structures
As mentioned in the computational details section, the equilibrium structures of dideuterooxirane, methyloxirane and dimethyloxirane have been investigated by means of different composite schemes. This allows us to further check the reliability of the “cheap” scheme already introduced and tested, for instance, for uracil36, glycine42 and thiouracil94. An additional test opportunity is offered by the availability of semi-experimental equilibrium geometries, which are known to have an accuracy of 0.001 Å for bond distances30–33. For oxirane, the equilibrium structure was investigated in detail by Demaison et al.39, who derived the semi-experimental equilibrium geometry. For methyloxirane, such determination has been carried out in the present work based on the experimental data from Ref.40.
The results for D2Ox, MeOx, and Me2Ox, collected in Tables I, II, and III, respectively, show that there is a very good agreement between the structural parameters obtained by means of the so-called “cheap” scheme and those derived with the gradient scheme. The only relevant deviation is observed for the C-O distances for all molecules: they turn out to be overestimated with the “cheap” scheme by about 0.004 Å. This is a consequence of rather large corrections due to the inclusion of diffuse functions in the basis set; in fact, the latter result to be one order of magnitude larger for the C-O distances. For oxirane, we furthermore note that the two equilibrium structures obtained by means of the gradient scheme using different sets of basis (see the “Computational details” section) are in very good agreement, with differences smaller than 0.001 Å for the bond lengths and 0.1 degrees for the angles. This confirms that the triple- and quadruple-zeta basis sets are suitable for the extrapolation to the CBS limit and that a triple-ζ quality set is able to provide reliable core-correlation corrections. Moving to the comparison of our computed geometries with the semi-experimental ones, we point out a very good agreement as well, i.e., bond distances agree within 0.001 Å and angles within 0.1 degrees. The overall conclusion is that the present study further confirms the reliability of the so-called “cheap” scheme. For all molecules, the various contributions are collected in the Supplementary Material103.
TABLE I.
Equilibrium structure of trans-2,3-dideuterooxirane. Distances are in Å, angles in degrees.
| Parameters | B3LYP/ SNSD |
B3LYP/ AVTZ |
CAM-B3LYP/ SNSD |
CAM-B3LYP/ AVTZ |
B2PLYP/ AVTZ |
MP2/ AVTZ |
best “cheap”a |
bestCC (TZ,QZ)/(CT)b |
bestCC (QZ,5Z)/(CQ)c |
semi-exp red |
|---|---|---|---|---|---|---|---|---|---|---|
| Bonds | ||||||||||
| C-O | 1.4320 | 1.4296 | 1.4225 | 1.4198 | 1.4330 | 1.4359 | 1.4303 | 1.4259 | 1.4263 | 1.42726(2) |
| C-C | 1.4670 | 1.4632 | 1.4623 | 1.4581 | 1.4619 | 1.4625 | 1.4602 | 1.4601 | 1.4606 | 1.46082(2) |
| C-H/D | 1.0890 | 1.0844 | 1.0878 | 1.0834 | 1.0824 | 1.0819 | 1.0816 | 1.0819 | 1.0817 | 1.08209(2) |
| MAE-re(SE)e | 0.0059 | 0.0024 | 0.0040 | 0.0038 | 0.0024 | 0.0035 | 0.0014 | 0.0008 | 0.0005 | |
| ∣MAX∣-re(SE)e | 0.0069 | 0.0024 | 0.0057 | 0.0075 | 0.0057 | 0.0087 | 0.0031 | 0.0013 | 0.0009 | |
| MAE-bestCC f | 0.0065 | 0.0029 | 0.0039 | 0.0036 | 0.0029 | 0.0039 | 0.0015 | 0.0004 | ||
| ∣MAX∣bestCC f | 0.0073 | 0.0033 | 0.0061 | 0.0066 | 0.0067 | 0.0096 | 0.0040 | 0.0005 | ||
|
| ||||||||||
| Angles | ||||||||||
| DCH | 115.75 | 115.65 | 115.81 | 115.73 | 115.94 | 116.42 | 116.33 | 116.16 | 116.21 | 116.189(3) |
| θ | 158.06 | 157.98 | 157.97 | 157.88 | 158.13 | 158.12 | 158.45 | 157.98 | 158.05 | 157.951(8) |
Best-estimated equilibrium structure obtained by means of the “cheap” scheme (Eq. (3)).
Best-estimated “bestCC” equilibrium structure (Eq. (1)) using the cc-pVTZ and cc-pVQZ basis sets for the CBS extrapolation and the cc-pCVTZ set for the core-correlation correction.
Best-estimated “bestCC” equilibrium structure (Eq. (1)) using the cc-pVQZ and cc-pV5Z basis sets for the CBS extrapolation and the cc-pCVQZ set for the core-correlation correction.
Semi-experimental equilibrium structure: Ref.39.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the semi-experimental equilibrium structure.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (bestCC (QZ,5Z)/(CQ)) parameters.
TABLE II.
Equilibrium structure of methyloxirane. Distances are in Å, angles in degrees.
| Parameters | B3LYP/ SNSD |
B3LYP/ AVTZ |
CAM-B3LYP/ SNSD |
CAM-B3LYP/ AVTZ |
B2PLYP/ AVTZ |
MP2/ AVTZ |
best “cheap”a |
bestCC (TZ,QZ)/(CT)b |
semi-exp rec |
|---|---|---|---|---|---|---|---|---|---|
| Bonds | |||||||||
| O1-C2 | 1.4371 | 1.4344 | 1.4262 | 1.4235 | 1.4372 | 1.4392 | 1.4331 | 1.4289 | 1.4305 |
| O1-C3 | 1.4354 | 1.4326 | 1.4257 | 1.4299 | 1.4366 | 1.4404 | 1.4341 | 1.4299 | 1.4323 |
| C2-C3 | 1.4684 | 1.4644 | 1.4628 | 1.4587 | 1.4627 | 1.4630 | 1.4605 | 1.4600 | 1.4601 |
| C2-C7 | 1.5057 | 1.5017 | 1.5005 | 1.4966 | 1.4997 | 1.4972 | 1.4968 | 1.4969 | 1.4985 |
| C2-H6 | 1.0919 | 1.0872 | 1.0906 | 1.0861 | 1.0854 | 1.0853 | 1.0851 | 1.0852 | 1.0847 |
| C3-H4 | 1.0898 | 1.0853 | 1.0887 | 1.0843 | 1.0834 | 1.0833 | 1.0831 | 1.0837 | 1.0827 |
| C3-H5 | 1.0893 | 1.0849 | 1.0881 | 1.0837 | 1.0828 | 1.0824 | 1.0822 | 1.0826 | 1.0824 |
| C7-H8 | 1.0942 | 1.0849 | 1.0931 | 1.0886 | 1.0884 | 1.0886 | 1.0886 | 1.0881 | 1.0871 |
| C7-H9 | 1.0964 | 1.0918 | 1.0949 | 1.0903 | 1.0904 | 1.0903 | 1.0902 | 1.0900 | 1.0897 |
| C7-H10 | 1.0956 | 1.0912 | 1.0942 | 1.0898 | 1.0896 | 1.0895 | 1.0893 | 1.0890 | 1.0882 |
| MAE-re(SE)d | 0.0068 | 0.0027 | 0.0050 | 0.0028 | 0.0020 | 0.0026 | 0.0011 | 0.0009 | |
| ∣MAX∣-re(SE)d | 0.0084 | 0.0043 | 0.0066 | 0.0093 | 0.0067 | 0.0087 | 0.0026 | 0.0024 | |
| MAE-bestCCe | 0.0070 | 0.0031 | 0.0044 | 0.0018 | 0.0022 | 0.0026 | 0.0011 | ||
| ∣MAX∣bestCCe | 0.0088 | 0.0055 | 0.0055 | 0.0070 | 0.0083 | 0.0105 | 0.0042 |
| Angles | |||||||||
| C2-O1-C3 | 61.49 | 61.43 | 61.72 | 61.66 | 61.19 | 61.07 | 61.12 | 61.44 | 61.33 |
| C3-C2-C7 | 122.54 | 122.63 | 122.28 | 122.33 | 122.27 | 121.66 | 121.88 | 121.73 | 121.68 |
| C3-C2-H6 | 116.99 | 116.98 | 117.09 | 117.09 | 117.06 | 117.10 | 117.15 | 117.22 | 117.31 |
| C2-C3-H4 | 119.26 | 119.28 | 119.14 | 119.14 | 119.09 | 118.71 | 118.88 | 118.84 | 118.96 |
| C2-C3-H5 | 120.10 | 120.14 | 120.10 | 120.15 | 120.12 | 120.02 | 120.14 | 120.07 | 119.89 |
| C2-C7-H8 | 110.75 | 110.80 | 110.53 | 110.56 | 110.52 | 110.07 | 110.24 | 110.17 | 110.29 |
| C2-C7-H9 | 110.75 | 110.43 | 110.53 | 110.51 | 110.43 | 110.48 | 110.39 | 110.48 | 110.43 |
| C2-C7-H10 | 110.61 | 110.59 | 110.59 | 110.56 | 110.53 | 110.48 | 110.39 | 110.54 | 110.31 |
| H4-C3-C2-O1 | 103.24 | 103.33 | 103.29 | 103.39 | 103.18 | 103.09 | 102.88 | 103.21 | 103.04 |
| H5-C3-C2-O1 | −103.03 | −103.09 | −103.02 | −103.08 | −102.88 | −102.66 | −102.53 | −102.84 | −102.66 |
| H6-C2-C3-O1 | 101.92 | 101.97 | 102.01 | 102.09 | 101.90 | 101.93 | 101.79 | 102.04 | 101.91 |
| H8-C7-C2-C3 | 25.02 | 25.15 | 25.19 | 25.25 | 25.02 | 25.12 | 23.89 | 24.84 | 25.35 |
| H9-C7-C2-C3 | −95.44 | −95.31 | −95.25 | −95.18 | −95.39 | −95.23 | −96.46 | −95.47 | −95.20 |
| H10-C7-C2-C3 | 145.12 | 145.29 | 145.14 | 145.24 | 145.05 | 144.94 | 143.84 | 144.73 | 145.04 |
| MAE-re(SE)d | 0.30 | 0.29 | 0.23 | 0.25 | 0.19 | 0.12 | 0.38 | 0.18 | |
| ∣MAX∣-re(SE)d | 0.87 | 0.95 | 0.60 | 0.65 | 0.59 | 0.26 | 1.45 | 0.51 | |
| MAE-bestCCe | 0.24 | 0.28 | 0.21 | 0.24 | 0.17 | 0.15 | 0.34 | ||
| ∣MAX∣bestCCe | 0.82 | 0.90 | 0.55 | 0.60 | 0.54 | 0.37 | 0.99 |
Best-estimated equilibrium structure obtained by means of the “cheap” scheme (Eq. (3)).
Best-estimated “bestCC” equilibrium structure (Eq. (1)) using the cc-pVTZ and cc-pVQZ basis sets for the CBS extrapolation and cc-pCVTZ for the core-correlation correction.
Semi-experimental equilibrium structure: this work.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the semi-experimental equilibrium structure.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (bestCC) parameters.
TABLE III.
Equilibrium structure of dimethyloxirane.a Distances are in Å, angles in degrees.
| Parameters | B3LYP/ SNSD |
B3LYP/ AVTZ |
CAM-B3LYP/ SNSD |
CAM-B3LYP/ AVTZ |
B2PLYP/ AVTZ |
MP2/ AVTZ |
best “cheap”b |
bestCC (TZ,QZ)/(CT)c |
|---|---|---|---|---|---|---|---|---|
| Bonds | ||||||||
| O1-C2 | 1.4402 | 1.4374 | 1.4295 | 1.4268 | 1.4410 | 1.4438 | 1.4369 | 1.4329 |
| C2-C3 | 1.4696 | 1.4658 | 1.4635 | 1.4596 | 1.4638 | 1.4637 | 1.4616 | 1.4611 |
| C2-C7 | 1.5060 | 1.5021 | 1.5007 | 1.4968 | 1.4998 | 1.4972 | 1.4970 | 1.4970 |
| C2-H6 | 1.0927 | 1.0881 | 1.0915 | 1.0870 | 1.0864 | 1.0866 | 1.0866 | 1.0864 |
| C5-H11 | 1.0941 | 1.0896 | 1.0930 | 1.0885 | 1.0883 | 1.0886 | 1.0886 | 1.0884 |
| C5-H12 | 1.0965 | 1.0920 | 1.0950 | 1.0905 | 1.0906 | 1.0905 | 1.0904 | 1.0902 |
| C5-H13 | 1.0958 | 1.0913 | 1.0943 | 1.0899 | 1.0898 | 1.0897 | 1.0895 | 1.0893 |
| MAEd | 0.0071 | 0.0030 | 0.0041 | 0.0013 | 0.0021 | 0.0021 | 0.0007 | |
| ∣MAX∣d | 0.0090 | 0.0051 | 0.0051 | 0.0061 | 0.0080 | 0.0109 | 0.0039 |
| Angles | ||||||||
| C2-O1-C3 | 61.36 | 61.31 | 61.58 | 61.53 | 61.05 | 60.91 | 61.14 | 61.31 |
| C3-C2-C7 | 123.13 | 123.22 | 122.92 | 122.97 | 122.91 | 122.40 | 122.55 | 122.44 |
| C3-C2-H6 | 116.69 | 116.67 | 116.71 | 116.7 | 116.67 | 116.55 | 116.68 | 116.71 |
| C2-C7-H8 | 110.82 | 110.87 | 110.60 | 110.64 | 110.58 | 110.13 | 110.27 | 110.23 |
| C2-C7-H9 | 110.52 | 110.50 | 110.60 | 110.57 | 110.48 | 110.52 | 110.43 | 110.51 |
| C2-C7-H10 | 110.58 | 110.55 | 110.55 | 110.53 | 110.50 | 110.45 | 110.38 | 110.52 |
| H4-C3-C2-O1 | 102.03 | 102.1 | 102.14 | 102.23 | 102.03 | 102.06 | 101.91 | 102.18 |
| H8-C7-C2-C3 | 24.33 | 24.51 | 24.45 | 24.56 | 24.38 | 24.34 | 23.50 | 24.17 |
| H9-C7-C2-C3 | −96.24 | −96.06 | −96.1 | −95.97 | −96.13 | −96.10 | −96.94 | −96.23 |
| H10-C7-C2-C3 | 144.34 | 144.57 | 144.31 | 144.48 | 144.34 | 144.11 | 143.37 | 144.01 |
| MAEd | 0.21 | 0.27 | 0.20 | 0.24 | 0.19 | 0.13 | 0.28 | |
| ∣MAX∣d | 0.69 | 0.78 | 0.47 | 0.53 | 0.47 | 0.39 | 0.71 |
By symmetry, we have the following relationships: O1-C2 = O1-C3; C2-C7 = C3-C5; C2-H6 = C3-H4; C5-H11 = C7-H8; C5-H12 = C7-H9; C5-H13 = C7-H10; C3-C2-C7 = C2-C3-C5; C3-C2-H6 = C2-C3-H4; H4-C3-C2-O1 = H6-C2-C3-O1.
Best-estimated equilibrium structure obtained by means of the “cheap” scheme (Eq. (3)).
Best-estimated “bestCC” equilibrium structure (Eq. (1)) using the cc-pVTZ and cc-pVQZ basis sets for the CBS extrapolation and cc-pCVTZ for the core-correlation correction.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (bestCC) parameters.
Regarding the comparison of DFT structures with our best-estimated ones, as already noted for instance in Ref.42, the B3LYP/SNSD level provides distances overestimated by about 0.007 Å, with the accuracy that improves once employing the aug-cc-pVTZ basis set (the averaged error thus reducing to about 0.003 Å). The CAM-B3LYP model shows a better agreement with the best theoretical estimates, bond lengths being overestimated by about 0.004 Å with the SNSD basis set and by only 0.002-0.0035 Å at the CAM-B3LYP/aug-cc-pVTZ level. An overall improvement is observed once we move to the B2PLYP/aug-cc-pVTZ level, with the overestimation reduced to 0.001-0.003 Å. Once again, the largest deviations are noted for the C-O distances. For bond angles, the largest discrepancies and mean absolute deviations do not exceed 1 and 0.3 degrees, respectively. Finally, we note that MP2/aug-cc-pVTZ structures are less accurate than their B2PLYP/aug-cc-pVTZ counterparts and show the largest maximum errors (for the C-O bond exceeding 0.01 Å) among the considered computational models.
B. Harmonic force fields
The harmonic wavenumbers of the dideuterated oxirane are reported in Table IV, together with (for all the three molecules considered) the analysis of the accuracy of the different levels of theory, as derived from the comparison with the best theoretical estimates. The results of this analysis are drawn in terms of the mean absolute error (MAE) and maximum absolute deviation (∣MAX∣). Harmonic wavenumbers of MeOx and Me2Ox, and the various contributions to the best-estimated harmonic wavenumbers of D2Ox are gathered in the Supplementary Material103. For all molecules, we note that the differences between the MP2/cc-pVQZ and CBS values are small, either positive or negative, ranging from −3.7 cm−1 to +3.5 cm−1, with a mean value of −0.06 cm−1. Core-correlation effects are slightly larger, with positive corrections ranging from ~1 to ~6 cm−1 and a mean value of 3.5 cm−1. On the contrary, the contributions due to the inclusion of diffuse functions in the basis set are mostly negative with a mean value of −3.3 cm−1 (from −12.6 to 4.8 cm−1). The largest terms are the higher-order correlation energy contributions, Δω((T)), with corrections mostly negative that can be as large as −50 cm−1.
TABLE IV.
Harmonic vibrational wavenumbers (in cm−1) of trans-2,3-dideuterooxirane. Mean and maximum absolute deviations for methyloxirane and dimethyloxirane are also reported.
| Mode | Symmetry | B3LYP/ SNSD |
B3LYP/ aug-cc-pVTZ |
CAM/ SNSD |
B2PLYP/ aug-cc-pVTZ |
MP2/ aug-cc-pVTZ |
CCSD(T)/ cc-pVTZ |
CCSD(T)/ cc-pVQZ |
besta cheap |
bestCC b | Assignment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| trans-2,3-dideuterooxirane | |||||||||||
| 1 | A | 3134.0 | 3130.7 | 3163.1 | 3167.2 | 3204.0 | 3159.3 | 3163.0 | 3170.2 | 3170.0 | CH sym stretch |
| 2 | A | 2309.1 | 2306.5 | 2331.8 | 2333.6 | 2360.7 | 2328.4 | 2330.8 | 2335.7 | 2335.6 | CD sym stretch |
| 3 | A | 1426.9 | 1427.1 | 1449.9 | 1434.7 | 1436.0 | 1437.5 | 1434.3 | 1426.6 | 1427.2 | CHD sym scissor |
| 4 | A | 1258.2 | 1258.1 | 1277.6 | 1261.9 | 1264.2 | 1259.1 | 1259.3 | 1254.2 | 1256.0 | ring breathing |
| 5 | A | 1130.8 | 1136.0 | 1147.7 | 1142.0 | 1139.2 | 1142.5 | 1141.8 | 1137.3 | 1138.1 | CH sym twisting |
| 6 | A | 974.2 | 976.1 | 994.9 | 976.7 | 974.9 | 982.0 | 981.8 | 972.4 | 976.5 | CHD wagging |
| 7 | A | 895.0 | 896.2 | 910.8 | 899.0 | 904.0 | 903.5 | 903.4 | 896.6 | 899.1 | ring CC stretching |
| 8 | A | 759.4 | 761.8 | 768.8 | 767.7 | 770.9 | 765.6 | 767.1 | 768.0 | 768.9 | CD sym twisting |
| 9 | B | 3139.8 | 3136.3 | 3167.7 | 3172.5 | 3208.8 | 3164.0 | 3167.7 | 3175.0 | 3174.8 | CH asym stretch |
| 10 | B | 2297.4 | 2295.4 | 2318.2 | 2321.5 | 2347.3 | 2315.4 | 2318.0 | 2323.1 | 2322.9 | CD asym stretch |
| 11 | B | 1359.9 | 1364.2 | 1367.9 | 1372.7 | 1371.8 | 1371.0 | 1367.0 | 1360.0 | 1359.3 | CHD asym scissor |
| 12 | B | 1121.8 | 1129.6 | 1136.4 | 1134.8 | 1129.9 | 1125.7 | 1128.1 | 1132.8 | 1133.0 | CH asym twisting |
| 13 | B | 925.9 | 930.7 | 940.0 | 932.3 | 927.8 | 930.0 | 930.2 | 927.6 | 928.5 | CD asym twisting |
| 14 | B | 838.7 | 837.1 | 880.9 | 832.0 | 840.5 | 844.4 | 845.1 | 832.7 | 839.7 | CO asym stretching |
| 15 | B | 662.5 | 665.3 | 669.0 | 668.5 | 668.6 | 662.8 | 663.6 | 665.0 | 665.0 | CHD rocking |
| MAEc | 11.1 | 10.8 | 11.7 | 3.8 | 11.0 | 6.3 | 4.7 | 1.3 | |||
| ∣MAX∣c | 36.0 | 39.3 | 41.1 | 13.3 | 34.0 | 11.7 | 7.7 | 7.0 | |||
| MAEd | 11.2 | 11.1 | 12.9 | 4.0 | 11.8 | 7.4 | 5.8 | ||||
| ∣MAX∣d | 36.2 | 39.5 | 48.2 | 12.6 | 33.8 | 11.8 | 12.4 | ||||
| methyloxirane | B3LYP/SNSD | CAM/SNSD | B2PLYP/aug-cc-pVTZ | MP2/aug-cc-pVTZ | CCSD(T)/cc-pVTZ | CCSD(T)/aug-cc-pVTZ | |||||
| MAEd | 12.2 | -.- | 9.5 | 3.9 | 12.4 | 4.1 | 3.4 | ||||
| ∣MAX∣d | 34.7 | -.- | 28.0 | 11.6 | 40.1 | 12.2 | 13.0 | ||||
| dimethyloxirane | |||||||||||
| MAEd | 12.2 | -.- | 8.4 | 4.0 | 11.9 | 3.4 | . | ||||
| ∣MAX∣d | 27.4 | -.- | 34.4 | 11.7 | 41.0 | 7.6 | . | ||||
Best-estimated (best) harmonic wavenumbers obtained by means of the “cheap” scheme according to Eq. (5).
Best-estimated (bestCC) harmonic wavenumbers obtained at the CCSD(T) level according to Eq. (4).
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (bestCC) harmonic wavenumbers.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (“cheap”) harmonic wavenumbers.
The MAE and MAX values given in Table IV allow us to draw general conclusions about the accuracy of the levels of theory used in the present work. We note that functionals B3LYP and CAM-B3LYP provide accurate harmonic wavenumbers with MAEs for all molecules of about 12 cm−1, already converged when employing basis set SNSD. The B2PLYP/aug-cc-pVTZ results are of the same quality as, or even better than (see D2Ox) CCSD(T) in conjunction with the cc-pVTZ basis set, but at a strongly reduced computational cost. Similar accuracy is not obtained at the MP2/aug-cc-pVTZ level which yields results comparable to B3LYP or CAM-B3LYP, but at a significantly higher computational cost.
For trans-2,3-dideuterooxirane, as described in the “Computational details” section, the best-estimated harmonic wavenumbers were also obtained by means of a composite scheme entirely relying on CCSD(T) calculations (Eq. (4)). In Table IV, the corresponding results, denoted as bestCC, are compared with those determined with the so-called “cheap” scheme (Eq. (5)). A very good agreement is observed, with the differences being 1-2 wavenumbers (for five modes they are even smaller than 1 cm−1), thus pointing out the accuracy and reliability of the “cheap” scheme. The largest difference is about 7 cm−1, which means in relative terms a discrepancy of about 8%, and it is observed for the ring deformation mode ν14, involving the asymmetric stretch of the C-O bonds. Therefore, it can be related to the difference already observed between the two composite schemes at the structural level. The different contributions to the best estimates obtained at the MP2 level are compared to the corresponding ones evaluated at the CCSD(T) level in the Supplementary Material103. It is evident that, with only very few exceptions, the two levels of theory provide very similar contributions, with differences usually being on the order of a few tenths of wavenumber. Slightly larger errors are observed for modes ν4, ν5, ν6 and ν7 regarding contributions due to the basis set enlargement, but both approaches still agree within 1 cm−1, as discussed above. Larger deviations are only observed for ν14 for both basis-set and higher order correlation contributions, thus leading to an overall difference of about 7 cm−1. In summary, the results for D2Ox allow us to point out that the so-called “cheap” scheme is able to provide results of the same quality as those obtained with composite approaches involving only CCSD(T) calculations.
As mentioned in the methodology section, the harmonic IR intensities at different levels were combined in order to derive best-estimated values, according to the composite scheme described in Eq. (7). As for the wavenumbers, a composite approach entirely based on CCSD(T) computations was also employed for trans-2,3-dideuterooxirane (Eq. (6)). The different contributions to the best estimates obtained at the MP2 level are compared to their CCSD(T) counterparts in the Supplementary Material103, showing that alike for wavenumbers the “cheap” scheme leads to results of the same quality as those obtained with the composite approach involving only CCSD(T) calculations (also for ν14). For all molecules, the IR intensities computed with DFT and CC approaches are compared to the best estimates in Table V, along with the results for D2Ox, while the results for MeOx and Me2Ox are found in the Supplementary Material103. Conclusions analogous to those deduced for the harmonic wavenumbers can be drawn based on the MAE and MAX values. The B3LYP/SNSD results turn out to be very accurate, similar to those obtainable at the CCSD(T) level and already converged with respect to the basis-set extension. The accuracy slightly improves when moving to CAM-B3LYP/SNSD, in particular as far as the maximum discrepancies (i.e., for the CH asymmetric stretches) are concerned, which become smaller than 10 km mol−1. Moving to the computationally more expensive hybrid functional B2PLYP, the noted improvement is limited; i.e., the deviations with respect to the best-estimated values decrease from 2-3 km mol−1 to 1-2 km mol−1. Concerning MP2, no particular improvement with respect to B3LYP/SNS is noted, despite the increased computational cost. As already noted for the wavenumbers, the “cheap” scheme provides results very similar to those from the full CCSD(T) approach, with a MAE of only 0.4 km mol−1 and a maximum absolute deviation of 1.6 km mol−1. In summary, all DFT methods yield accurate IR intensities, while no relevant improvements are offered by performing computations at the MP2/aug-cc-pVTZ level. As already noted for the harmonic wavenumbers, the comparison of the different contributions to the “cheap” and CCSD(T) composite schemes shows that the two sets well agree with each another, thus supporting the idea beyond the “cheap” scheme, i.e., that the different effects/corrections can be reliably evaluated at the less expensive MP2 level without losing the accuracy that characterizes the CCSD(T) method.
TABLE V.
Harmonic IR intensities (in km mol−1) of trans-2,3-dideuterooxirane. Mean and maximum absolute deviations for methyloxirane and dimethyloxirane are also reported.
| Mode | Symmetry | B3LYP/ SNSD |
B3LYP/ aug-cc-pVTZ |
CAM/ SNSD |
B2PLYP/ aug-cc-pVTZ |
MP2/ aug-cc-pVTZ |
CCSD(T)/ cc-pVTZ |
CCSD(T)/ cc-pVQZ |
besta cheap |
bestCC b | Assignment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| trans-2,3-dideuterooxirane | |||||||||||
| 1 | A | 7.96 | 7.64 | 7.09 | 7.26 | 6.20 | 5.70 | 5.98 | 7.08 | 6.99 | CH sym stretch |
| 2 | A | 6.93 | 6.50 | 6.42 | 6.13 | 5.33 | 4.87 | 5.12 | 6.02 | 5.93 | CD sym stretch |
| 3 | A | 6.00 | 5.16 | 7.34 | 4.12 | 3.46 | 3.88 | 3.92 | 3.96 | 3.92 | CHD sym scissor |
| 4 | A | 9.38 | 9.33 | 9.78 | 7.70 | 6.40 | 6.68 | 6.99 | 7.50 | 7.68 | ring breathing |
| 5 | A | 0.08 | 0.08 | 0.00 | 0.21 | 0.51 | 0.03 | 0.05 | 0.33 | 0.11 | CH sym twisting |
| 6 | A | 19.00 | 18.21 | 27.25 | 15.99 | 17.18 | 17.83 | 19.20 | 19.91 | 21.55 | CHD wagging |
| 7 | A | 28.65 | 28.25 | 23.79 | 30.02 | 28.39 | 21.74 | 23.18 | 30.83 | 28.32 | ring CC stretching |
| 8 | A | 21.41 | 22.25 | 20.46 | 22.35 | 19.90 | 18.12 | 18.93 | 21.61 | 21.20 | CD sym twisting |
| 9 | B | 39.72 | 38.91 | 32.05 | 33.63 | 23.22 | 37.91 | 33.19 | 23.12 | 22.13 | CH asym stretch |
| 10 | B | 25.01 | 24.53 | 20.74 | 21.50 | 15.36 | 23.59 | 21.08 | 15.49 | 15.07 | CD asym stretch |
| 11 | B | 0.23 | 0.24 | 0.45 | 0.21 | 0.22 | 0.34 | 0.24 | 0.27 | 0.04 | CHD asym scissor |
| 12 | B | 1.42 | 1.51 | 1.72 | 1.50 | 1.57 | 1.60 | 1.59 | 1.38 | 1.66 | CH asym twisting |
| 13 | B | 2.78 | 2.90 | 3.39 | 2.80 | 2.60 | 3.09 | 2.94 | 2.41 | 2.60 | CD asym twisting |
| 14 | B | 10.67 | 10.31 | 10.37 | 9.24 | 7.81 | 7.85 | 8.24 | 8.24 | 8.18 | CO asym stretching |
| 15 | B | 0.11 | 0.15 | 0.16 | 0.17 | 0.22 | 0.01 | 0.06 | 0.31 | 0.24 | CHD rocking |
| MAEc | 2.6 | 2.5 | 2.4 | 1.9 | 0.8 | 2.8 | 2.0 | 0.5 | |||
| ∣MAX∣c | 17.6 | 16.8 | 9.9 | 11.5 | 4.4 | 15.8 | 11.1 | 2.5 | |||
| MAEd | 2.6 | 2.5 | 2.7 | 1.6 | 0.8 | 2.9 | 2.0 | ||||
| ∣MAX∣d | 16.6 | 15.8 | 8.9 | 10.5 | 2.7 | 14.8 | 10.1 | ||||
| methyloxirane | B3LYP/SNSD | CAM/SNSD | B2PLYP/aug-cc-pVTZ | MP2/aug-cc-pVTZ | CCSD(T)/cc-pVTZ | CCSD(T)/aug-cc-pVTZ | |||||
| MAEd | 2.2 | -.- | 1.2 | 1.1 | 1.2 | 1.6 | 0.9 | ||||
| ∣MAX∣d | 11.7 | -.- | 4.2 | 5.5 | 8.3 | 8.6 | 3.9 | ||||
| dimethyloxirane | |||||||||||
| MAEd | 1.8 | -.- | 1.9 | 1.0 | 1.8 | 1.4 | . | ||||
| ∣MAX∣d | 11.7 | -.- | 9.4 | 4.7 | 19.6 | 10.7 | . | ||||
Best-estimated (best) harmonic IR intensities obtained by means of the “cheap” scheme according to Eq. (7).
Best-estimated (bestCC) harmonic IR intensities obtained at the CCSD(T) level according to Eq. (6).
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (bestCC) harmonic IR intensities.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best-estimated (“cheap”) harmonic IR intensities.
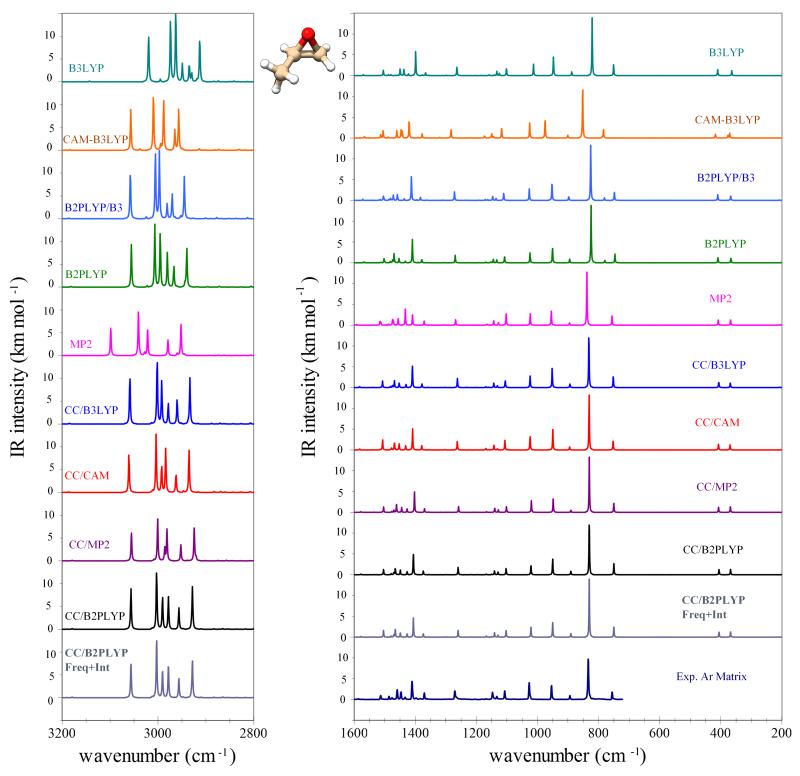
C. IR spectra
For an accurate comparison with experimental data, it is necessary to go beyond the double-harmonic approximation, which involves the inclusion of mechanical anharmonic effects on vibrational energies, i.e., anharmonic shifts, as well as electric anharmonic effects on intensities, thus allowing for the consideration of overtones and combination bands74, which have null intensities at the harmonic level. As an example, the simulation of fully anharmonic spectra gives the possibility to distinguish between low-intensity features related to non-fundamental transitions of the most abundant conformer and the fundamental transitions of the less abundant ones42. This strategy is particularly effective if complementary vibrational spectroscopies, e.g., IR and Raman50,104, are combined, together with VCD and Vibrational Raman Optical Activity (VROA) for chiral molecules104,105.
Let us first consider the accuracy of anharmonic contributions to the vibrational wavenumbers and IR intensities for the fundamental transitions of trans-2,3-dideuterooxirane, which are reported in Tables VI and VII, respectively. Since all computations were performed with hybrid models using the same harmonic part (i.e., the best-estimated harmonic wavenumbers), the differences are only related to the anharmonic part of the force field. For both vibrational wavenumbers and IR intensities, anharmonic corrections computed at the CCSD(T)/cc-pVTZ level are taken as reference. For wavenumbers, rather accurate anharmonic corrections can be computed at the DFT level, with MAE of 4-6 cm−1 and maximum deviations smaller than 15 cm−1 for fundamental transitions when functionals B3LYP and CAM-B3LYP are used in conjunction with basis set SNSD. If all transitions up to two quanta are considered, the mean discrepancy is still about 10 cm−1, and maximum errors do not exceed 40 cm−1. The accuracy can be improved by inclusion of correlation effects at either B2PLYP or MP2 levels; in both cases this leads to an excellent agreement with the CCSD(T) results: MAE of about 2.5 cm−1 and 5 cm−1 and maximum deviations of 6-7 cm−1 and 15 cm−1 are observed for fundamentals and all transitions, respectively. Concerning the intensities, it is interesting to note that essentially all methods perform very well; in particular, the largest absolute errors, on average about 0.5 km mol−1, are associated to fundamental transitions, while overtones and combination bands present mean absolute errors of 0.1 km mol−1, which are therefore largely sufficient to describe correctly these less-intense transitions.
TABLE VI.
Anharmonic corrections to the vibrational wavenumbers (in cm−1) of trans-2,3-dideuterooxirane within a hybrid scheme using the best-estimated (bestCC) harmonic wavenumbers defined in Eq. (4).
| Mode | Symmetry | B3LYP/ SNSD |
B3LYP/ aug-cc-pVTZ |
CAM-B3LYP/ SNSD |
B2PLYP/ aug-cc-pVTZ |
MP2/ aug-cc-pVTZ |
CCSD(T)/ cc-pVTZ |
Assignment |
|---|---|---|---|---|---|---|---|---|
| 1 | A | −134.1 | −132.1 | −136.6 | −138.6 | −139.9 | −143.1 | CH sym stretch |
| 2 | A | −78.6 | −77.8 | −82.3 | −81.9 | −83.9 | −84.3 | CD sym stretch |
| 3 | A | −35.2 | −34.8 | −36.7 | −37.7 | −39.9 | −39.2 | CHD sym scissor |
| 4 | A | −24.7 | −25.0 | −25.4 | −27.0 | −27.0 | −28.2 | ring breathing |
| 5 | A | −26.3 | −26.0 | −28.9 | −29.3 | −29.0 | −30.6 | CH sym twisting |
| 6 | A | −18.8 | −18.8 | −21.5 | −19.6 | −19.8 | −21.0 | CHD wagging |
| 7 | A | −17.4 | −17.5 | −16.5 | −19.7 | −18.0 | −19.6 | ring CC stretching |
| 8 | A | −12.5 | −12.4 | −13.4 | −14.2 | −13.7 | −15.6 | CD sym twisting |
| 9 | B | −135.4 | −133.3 | −137.6 | −139.8 | −141.0 | −146.8 | CH asym stretch |
| 10 | B | −77.9 | −77.2 | −79.4 | −81.2 | −82.0 | −85.5 | CD asym stretch |
| 11 | B | −28.5 | −28.3 | −28.6 | −30.6 | −31.7 | −36.0 | CHD asym scissor |
| 12 | B | −22.2 | −22.8 | −23.8 | −25.4 | −23.9 | −29.0 | CH asym twisting |
| 13 | B | −14.2 | −14.7 | −16.1 | −16.0 | −15.0 | −20.2 | CD asym twisting |
| 14 | B | −25.3 | −24.5 | −26.5 | −26.3 | −25.8 | −26.1 | CO asym stretching |
| 15 | B | −7.1 | −7.3 | −7.9 | −9.0 | −8.7 | −11.4 | CHD rocking |
| MAE Funda | 5.2 | 5.6 | 3.8 | 2.7 | 2.6 | |||
| ∣MAX∣ Funda | 11.4 | 13.5 | 9.1 | 7.0 | 5.8 | |||
| MAE Allb | 10.2 | 11.1 | 7.5 | 5.3 | 5.0 | |||
| ∣MAX∣ Allb | 28.5 | 35.5 | 20.6 | 14.4 | 14.6 |
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣), with respect to the hybrid force field with the anharmonic part at the CCSD(T)/cc-pVTZ level, computed for fundamental transitions.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣), with respect to the hybrid force field with the anharmonic part at the CCSD(T)/cc-pVTZ level, computed for fundamental transitions, overtones and combinational bands.
TABLE VII.
Anharmonic corrections to the IR intensities (in km mol−1) of trans-2,3-dideuteriooxirane within a hybrid scheme using the best-estimated (bestCC) harmonic wavenumbers defined in Eq. (4)
| Mode | Symmetry | B3LYP/ SNSD |
B3LYP/ aug-cc-pVTZ |
CAM-B3LYP/ SNSD |
B2PLYP/ aug-cc-pVTZ |
MP2/ aug-cc-pVTZ |
CCSD(T)/ cc-pVTZ |
Assignment |
|---|---|---|---|---|---|---|---|---|
| 1 | A | 0.70 | 0.70 | 0.76 | 0.67 | 0.70 | – a | CH sym stretch |
| 2 | A | −0.47 | −0.42 | −0.45 | −0.37 | −0.18 | – a | CD sym stretch |
| 3 | A | −1.24 | −1.07 | −1.46 | −1.00 | −0.90 | −0.90 | CHD sym scissor |
| 4 | A | 0.52 | 0.57 | 0.40 | 0.45 | 0.41 | 0.12 | ring breathing |
| 5 | A | 0.23 | 0.23 | 0.05 | 0.41 | 0.66 | 0.21 | CH sym twisting |
| 6 | A | −4.60 | −4.47 | −4.99 | −4.63 | −4.80 | −4.22 | CHD wagging |
| 7 | A | 2.86 | 2.64 | 3.79 | 2.67 | 2.58 | 2.56 | ring CC stretching |
| 8 | A | 0.19 | 0.15 | 0.03 | −0.09 | 0.20 | −0.45 | CD sym twisting |
| 9 | B | 8.95 | 8.79 | 7.60 | 7.73 | 5.66 | 4.33 | CH asym stretch |
| 10 | B | −2.17 | −2.31 | −2.46 | −1.93 | −1.22 | −2.00 | CD asym stretch |
| 11 | B | −0.03 | −0.03 | −0.02 | −0.02 | 0.01 | −0.07 | CHD asym scissor |
| 12 | B | −0.11 | −0.13 | −0.13 | −0.15 | −0.13 | −0.21 | CH asym twisting |
| 13 | B | −0.09 | −0.12 | −0.16 | −0.13 | −0.08 | −0.21 | CD asym twisting |
| 14 | B | 0.91 | 0.90 | 0.96 | 0.67 | 0.55 | 0.34 | CO asym stretching |
| 15 | B | −0.05 | −0.06 | −0.06 | −0.06 | −0.07 | −0.01 | CHD rocking |
| MAE Fundb | 0.6 | 0.6 | 0.6 | 0.4 | 0.4 | |||
| ∣MAX Fund∣b | 4.6 | 4.5 | 3.3 | 3.4 | 1.3 | |||
| MAE Allc | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||
| ∣MAX All∣c | 4.6 | 4.5 | 3.3 | 3.4 | 1.3 |
Bands involved in Fermi resonances, for which CCSD(T)/cc-pVTZ computations with the CFour code report unreliable anharmonic corrections, were excluded from the analysis.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣), with respect to the hybrid force field with the anharmonic part at the CCSD(T)/cc-pVTZ level, computed for IR intensities of fundamental bands.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣), with respect to the hybrid force field with the anharmonic part at the CCSD(T)/cc-pVTZ level, computed for IR intensities of fundamental bands, overtones and combinational bands.
The overall accuracy of the anharmonic wavenumbers computed at different levels of theory is further assessed by the comparison with experiment and the best theoretical estimates for trans-2,3-dideuterooxirane, methyloxirane and trans-2,3-dimethyloxirane (Tables VIII, IX and X, respectively), while for IR intensities, the experimental values are not adequate for the validation of the theoretical results. In fact, the values reported for D2Ox9 were derived from integrated intensities of solution-phase sample for all bands except ν3, for which the experimental value was estimated from the relative intensities in the gas-phase spectrum. We thus limit our comparison with experiment to wavenumbers. The DFT computations with functionals B3LYP and CAM-B3LYP already perform fairly well, with MAEs of 10-12 cm−1 and maximum discrepancies of about 30-40 cm−1. It is interesting to note that, while for B3LYP the largest deviations are observed for high-frequency vibrations, the opposite is true for CAM-B3LYP, thus suggesting a sort of recipe to select the most suitable functional to improve the accuracy, if a specific spectral range is under investigation. At variance, an overall improvement in the accuracy can be obtained with ab initio methods and hybrid models with the harmonic part computed at least at the B2PLYP/aug-cc-pVTZ level. As expected, the most accurate results (MAE = 5-8 cm−1 and maximum discrepancies < 20 cm−1) are obtained when best-estimated harmonic wavenumbers are considered. In that respect, as already noted in the previous section, essentially equivalent results are obtained by means of a composite scheme entirely relying on CCSD(T) calculations (Eq. 4) and in the frame of the so-called “cheap” scheme (Eq. 5). With respect to the anharmonic part, all methods provide mean absolute errors within 2 cm−1, with the most accurate results obtained in conjunction with the B2PLYP and MP2 anharmonic corrections that lead to a reduction of the maximum discrepancies within 14-17 cm−1. It is noteworthy that among the various anharmonic computations performed, the full MP2/aug-cc-pVTZ results yield the largest errors, which in turn should be mainly attributed to the harmonic part. On the contrary, B2PLYP/aug-cc-pVTZ computations perform very well at a comparable computational cost, and can be also recommended as an alternative to the best-estimated harmonic wavenumbers within hybrid schemes. Indeed, B2PLYP/B3LYP values show significant improvements over B3LYP (MAE reduced to 6 cm−1), with an accuracy similar to that of full B2PLYP computations.
TABLE VIII.
Anharmonic fundamental vibrational wavenumbers (in cm−1) of trans-2,3-dideuteriooxirane computed with hybrid schemes and the best-estimated anharmonic IR intensities (in km mol −1).
| Mode | Symmetry | B3LYPa | CAM-B3LYPa | B2PLYPb/ |
MP2b |
best cheapc/ |
bestCCd/ |
Exp.e | Best IRf | Assignment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP | B2PLYP | B3LYP | B2PLYP | CCg | B3LYP | CAM | B2PLYP | MP2 | CCf | ||||||||
| 1 | A | 2995 | 3027 | 3034 | 3029 | 3068 | 3036 | 3031 | 3027 | 3036 | 3033 | 3031 | 3030 | 3027 | 3015 | 6.32 | CH sym stretch |
| 2 | A | 2235 | 2261 | 2259 | 2256 | 2285 | 2255 | 2252 | 2250 | 2257 | 2253 | 2254 | 2252 | 2251 | 2254 | 6.30 | CD sym stretch |
| 3 | A | 1391 | 1415 | 1399 | 1397 | 1397 | 1391 | 1389 | 1387 | 1392 | 1390 | 1389 | 1387 | 1388 | 1397 | 4.93 | CHD sym scissor |
| 4 | A | 1233 | 1254 | 1237 | 1235 | 1238 | 1229 | 1227 | 1226 | 1231 | 1231 | 1229 | 1229 | 1228 | 1235 | 7.23 | ring breathing |
| 5 | A | 1104 | 1120 | 1116 | 1113 | 1111 | 1111 | 1108 | 1107 | 1112 | 1109 | 1109 | 1109 | 1108 | 1112 | 0.30 | CH sym twisting |
| 6 | A | 955 | 975 | 958 | 957 | 955 | 953 | 953 | 951 | 958 | 955 | 957 | 957 | 955 | 961 | 26.18 | CHD wagging |
| 7 | A | 877 | 895 | 882 | 879 | 886 | 879 | 877 | 877 | 882 | 883 | 879 | 881 | 879 | 885 | 25.65 | Ring deformation |
| 8 | A | 747 | 756 | 755 | 753 | 757 | 755 | 754 | 752 | 756 | 755 | 755 | 755 | 753 | 754 | 21.29 | CD sym twisting |
| 9 | B | 2999 | 3031 | 3039 | 3035 | 3073 | 3041 | 3036 | 3029 | 3039 | 3037 | 3035 | 3034 | 3028 | 3028 | 14.39 | CH asym stretch |
| 10 | B | 2220 | 2244 | 2245 | 2241 | 2266 | 2244 | 2241 | 2237 | 2245 | 2243 | 2242 | 2241 | 2237 | 2240 | 17.01 | CD asym stretch |
| 11 | B | 1331 | 1340 | 1344 | 1342 | 1341 | 1332 | 1329 | 1324 | 1331 | 1331 | 1329 | 1328 | 1323 | 1339 | 0.07 | CHD asym scissor |
| 12 | B | 1099 | 1113 | 1113 | 1109 | 1107 | 1111 | 1107 | 1104 | 1111 | 1109 | 1108 | 1109 | 1104 | 1106 | 1.81 | CH asym twisting |
| 13 | B | 911 | 924 | 918 | 916 | 913 | 913 | 912 | 907 | 914 | 912 | 913 | 914 | 908 | 914 | 2.73 | CD asym twisting |
| 14 | B | 813 | 858 | 806 | 805 | 815 | 807 | 806 | 807 | 814 | 813 | 813 | 814 | 814 | 817 | 7.51 | Ring deformation |
| 15 | B | 655 | 661 | 661 | 660 | 660 | 658 | 656 | 654 | 658 | 657 | 656 | 656 | 654 | 673 | 0.30 | CHD rocking |
| MAEh | 10.9 | 11.2 | 6.2 | 4.6 | 12.6 | 7.0 | 7.1 | 7.8 | 6.0 | 5.9 | 5.8 | 5.8 | 6.4 | ||||
| ∣MAX∣h | 28.6 | 40.6 | 18.6 | 14.1 | 53.4 | 20.7 | 17.0 | 19.4 | 20.9 | 18.4 | 16.9 | 16.6 | 19.3 | ||||
| MAEi | 8.9 | 14.4 | 8.2 | 5.8 | 14.3 | 5.0 | 3.1 | 1.6 | 5.2 | 3.8 | 2.7 | 2.6 | |||||
| ∣MAX∣i | 32.0 | 43.9 | 21.0 | 18.9 | 45.3 | 12.7 | 8.2 | 7.1 | 11.4 | 9.1 | 7.0 | 5.8 | |||||
Computed with the SNSD basis set.
Computed with the aug-cc-pVTZ basis set.
Best-estimated (best) harmonic wavenumbers obtained by means of the “cheap” scheme according to Eq. (5).
Best-estimated (bestCC) harmonic wavenumbers obtained at the CCSD(T) level according to Eq. (4).
Experimental gas-phase data from Ref.9
Best-estimated anharmonic IR intensities computed at the bestCC/B2PLYP level.
Anharmonic corrections computed at the CCSD(T)/cc-pVTZ level.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to experiment.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best theoretical estimates (bestCC//CCSD(T)/VTZ).
TABLE IX.
Anharmonic fundamental vibrational wavenumbers (in cm−1) of methyloxirane computed with hybrid schemes and the best-estimated anharmonic IR intensities (in km mol −1).
| Mode | B3LYPa | CAMa | B2PLYPb/ |
MP2b |
best cheapc/ |
Exp.d | Best IRe | Assignment | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP | B2PLYP | B3LYP | CAM | B2PLYP | MP2 | |||||||
| 1 | 3019 | 3057 | 3058 | 3055 | 3098 | 3058 | 3060 | 3056 | 3055 | 3051 | 24.51 | CH2 asym stretch |
| 2 | 2974 | 3009 | 3005 | 3006 | 3041 | 3002 | 3004 | 3003 | 3000 | 3001 | 40.62 | CH stretch |
| 3 | 2963 | 2988 | 2997 | 2995 | 3021 | 2992 | 2984 | 2990 | 2981 | 2995 | 18.66 | CH3 asym stretch |
| 4 | 2949 | 2994 | 2981 | 2980 | 3028 | 2978 | 2992 | 2978 | 2986 | 2974 | 22.75 | CH3 asym stretch |
| 5 | 2913 | 2957 | 2945 | 2940 | 2952 | 2933 | 2935 | 2928 | 2924 | 25.55 | CH2 sym stretch | |
| 6 | 2935 | 2965 | 2970 | 2967 | 2979 | 2960 | 2962 | 2956 | 2952 | 2942 | 13.73 | CH3 sym stretch |
| 7 | 1505 | 1505 | 1504 | 1502 | 1516 | 1507 | 1507 | 1504 | 1504 | 1514 | 5.11 | CH2 scisor |
| 8 | 1450 | 1460 | 1472 | 1470 | 1472 | 1468 | 1468 | 1466 | 1462 | 1459 | 6.02 | CH3 asym def |
| 9 | 1437 | 1447 | 1459 | 1453 | 1456 | 1453 | 1453 | 1449 | 1445 | 1447 | 3.40 | CH3 asym def |
| 10 | 1399 | 1420 | 1413 | 1410 | 1409 | 1409 | 1409 | 1406 | 1403 | 1411 | 14.55 | CHx bending |
| 11 | 1366 | 1378 | 1383 | 1379 | 1371 | 1378 | 1379 | 1374 | 1370 | 1371 | 2.67 | CH3 umbrella |
| 12 | 1264 | 1283 | 1272 | 1269 | 1268 | 1262 | 1263 | 1260 | 1258 | 1271 | 5.15 | ring breathing |
| 13 | 1158 | 1172 | 1171 | 1170 | 1168 | 1169 | 1169 | 1168 | 1167 | 1170 | 0.88 | CH2 rocking |
| 14 | 1133 | 1150 | 1147 | 1145 | 1143 | 1143 | 1142 | 1141 | 1140 | 1147 | 3.45 | CH bending |
| 15 | 1125 | 1144 | 1136 | 1133 | 1128 | 1131 | 1131 | 1129 | 1128 | 1133 | 1.52 | CH2 wagging |
| 16 | 1102 | 1117 | 1111 | 1108 | 1105 | 1106 | 1107 | 1103 | 1102 | 1108 | 5.14 | CH2 twist |
| 17 | 1013 | 1026 | 1027 | 1024 | 1024 | 1024 | 1024 | 1021 | 1020 | 1027 | 7.81 | CH2,CH3 rocking |
| 18 | 948 | 975 | 952 | 951 | 955 | 952 | 950 | 950 | 949 | 954 | 11.50 | ring deformation |
| 19 | 888 | 901 | 898 | 895 | 895 | 894 | 895 | 891 | 891 | 894 | 2.71 | CH2, CH3 rocking |
| 20 | 821 | 852 | 826 | 825 | 838 | 832 | 831 | 831 | 830 | 834 | 44.18 | ring CC stretching |
| 21 | 751 | 784 | 748 | 746 | 756 | 752 | 752 | 750 | 750 | 756 | 7.76 | CO asym stretching |
| 22 | 410 | 417 | 410 | 409 | 408 | 407 | 407 | 406 | 407 | 409 | 3.97 | CH3 bend |
| 23 | 364 | 371 | 368 | 367 | 368 | 369 | 370 | 368 | 369 | 375 | 4.06 | CH3 bend |
| 24 | 193 | 195 | 208 | 200 | 206 | 208 | 200 | 198 | 191 | 200 | 0.35 | CH3 torsion |
| MAEf | 12.0 | 9.6 | 6.2 | 5.1 | 11.7 | 4.8 | 5.7 | 5.2 | 6.1 | |||
| ∣MAX∣f | 32.4 | 28.2 | 28.1 | 24.6 | 53.3 | 17.8 | 20.2 | 14.2 | 13.9 | |||
| MAEg | 11.1 | 11.2 | 6.1 | 4.1 | 12.0 | 2.7 | 3.5 | 2.6 | ||||
| ∣MAX∣g | 36.8 | 34.1 | 16.9 | 11.7 | 49.6 | 9.1 | 14.1 | 9.3 | ||||
Computed with the SNSD basis set.
Computed with the aug-cc-pVTZ basis set.
Best-estimated (best) harmonic wavenumbers obtained by means of the “cheap” scheme according to Eq. (5).
Experimental results. The 2900-3100 cm−1 wavenumbers range: high-resolution jet-cooled IR spectrum from Ref.106. The 200-1600 cm−1 wavenumbers range: low-temperature matrix data from Refs.3,27.
Best-estimated anharmonic IR intensities computed at the best cheap/B2PLYP level.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to experiment.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best theoretical estimates (best cheap/B2PLYP).
TABLE X.
Anharmonic fundamental vibrational wavenumbers (in cm−1) of trans-2,3-dimethyloxirane computed with hybrid schemes and the best estimated anharmonic IR intensities (in km mol −1).
| Mode | Symmetry | B3LYPa | CAMa | B2PLYPb/ |
best cheapc/ |
Best IRd | Assignmente | |||
|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP | B2PLYP | B3LYP | CAM | B2PLYP | ||||||
| 1 | A | 2968 | 3003 | 2998 | 2994 | 2993 | 2996 | 2989 | 3.92 | CH3 ∥ bend |
| 2 | A | 2944 | 2983 | 2976 | 2974 | 2975 | 2978 | 2973 | 29.65 | CH3 ⊥ bend |
| 3 | A | 2914 | 2957 | 2946 | 2943 | 2944 | 2934 | 2936 | 2.97 | asym C-CH3 str |
| 4 | A | 2930 | 2958 | 2962 | 2959 | 2958 | 2960 | 2951 | 3.28 | CH3 rocking |
| 5 | A | 1482 | 1496 | 1492 | 1490 | 1494 | 1493 | 1492 | 6.79 | ∥CH |
| 6 | A | 1441 | 1454 | 1465 | 1461 | 1462 | 1462 | 1454 | 12.53 | CH3 asym def |
| 7 | A | 1424 | 1441 | 1436 | 1433 | 1438 | 1441 | 1434 | 0.76 | CH3 sym stretch |
| 8 | A | 1375 | 1384 | 1392 | 1388 | 1386 | 1386 | 1383 | 0.19 | CH3 asym stretch +CH str |
| 9 | A | 1249 | 1268 | 1259 | 1258 | 1253 | 1253 | 1253 | 1.25 | CH3 torsion |
| 10 | A | 1156 | 1175 | 1164 | 1165 | 1161 | 1167 | 1171 | 0.57 | CH3 ⊥ bend |
| 11 | A | 1108 | 1126 | 1121 | 1121 | 1118 | 1118 | 1118 | 6.39 | ring CC stretching |
| 12 | A | 1015 | 1031 | 1027 | 1030 | 1024 | 1022 | 1027 | 13.44 | ⊥ CH + CH3 rock |
| 13 | A | 886 | 909 | 888 | 887 | 890 | 889 | 889 | 17.37 | ∥CH + ring bretching |
| 14 | A | 801 | 820 | 811 | 809 | 813 | 812 | 811 | 19.56 | CH3 umbrella sym |
| 15 | A | 458 | 463 | 459 | 460 | 459 | 458 | 459 | 0.01 | Ring breathing |
| 16 | A | 245 | 249 | 250 | 248 | 250 | 249 | 249 | 2.14 | CH sym stretch |
| 17 | A | 173 | 174 | 189 | 185 | 188 | 178 | 183 | 0.32 | CH3 asym stretch +CH str |
| 18 | B | 2971 | 3006 | 3001 | 2994 | 2996 | 2998 | 2989 | 54.01 | CH3 torsion |
| 19 | B | 2944 | 2983 | 2976 | 2973 | 2974 | 2977 | 2971 | 4.72 | CO asym stretching |
| 20 | B | 2953 | 2991 | 2987 | 2967 | 2980 | 2982 | 2960 | 25.02 | CH3 rocking + ⊥ CH |
| 21 | B | 2933 | 2954 | 2967 | 2966 | 2963 | 2962 | 2959 | 30.46 | ⊥ CH + CH3 rock |
| 22 | B | 1451 | 1459 | 1470 | 1467 | 1468 | 1468 | 1465 | 8.86 | CH3 umbrella asym |
| 23 | B | 1434 | 1443 | 1456 | 1453 | 1453 | 1451 | 1449 | 5.75 | CH3 asym def |
| 24 | B | 1376 | 1390 | 1392 | 1389 | 1391 | 1390 | 1388 | 14.43 | CH asym stretch |
| 25 | B | 1333 | 1344 | 1346 | 1345 | 1334 | 1334 | 1334 | 8.69 | CH3 asym stretch +CH str |
| 26 | B | 1146 | 1163 | 1158 | 1176 | 1154 | 1154 | 1171 | 1.33 | CH3 ∥ bend |
| 27 | B | 1095 | 1114 | 1111 | 1109 | 1115 | 1115 | 1113 | 10.08 | ring def + C-CH3 str |
| 28 | B | 1004 | 1025 | 1012 | 1008 | 1015 | 1021 | 1012 | 10.75 | CH3 rocking |
| 29 | B | 951 | 960 | 965 | 966 | 959 | 959 | 960 | 1.87 | ⊥ CH |
| 30 | B | 730 | 766 | 727 | 727 | 731 | 730 | 731 | 11.52 | CH3 asym def |
| 31 | B | 472 | 480 | 469 | 472 | 468 | 468 | 468 | 5.50 | CH3 asym def |
| 32 | B | 281 | 283 | 282 | 283 | 281 | 281 | 282 | 0.46 | CH3 sym stretch |
| 33 | B | 191 | 193 | 208 | 203 | 209 | 200 | 204 | 0.27 | CH3 asym stretch |
| MAEf | 12.3 | 9.3 | 5.8 | 3.9 | 4.0 | 4.3 | ||||
| ∣MAX∣f | 28.8 | 34.7 | 27.8 | 11.5 | 20.5 | 22.5 | ||||
Computed with the SNSD basis set.
Computed with the aug-cc-pVTZ basis set.
Best-estimated (best) harmonic wavenumbers obtained by means of the “cheap” scheme according to Eq. (5).
Best estimated anharmonic IR intensities computed at the best cheap/B2PLYP level.
Notation adopted from Ref.11; ⊥: perpendicular and ∥= parallel to the C2C3C5/7 plane (Figure 1), respectively.
Mean absolute error (MAE) and maximum absolute deviations (∣MAX∣) with respect to the best theoretical estimate (best cheap/B2PLYP).
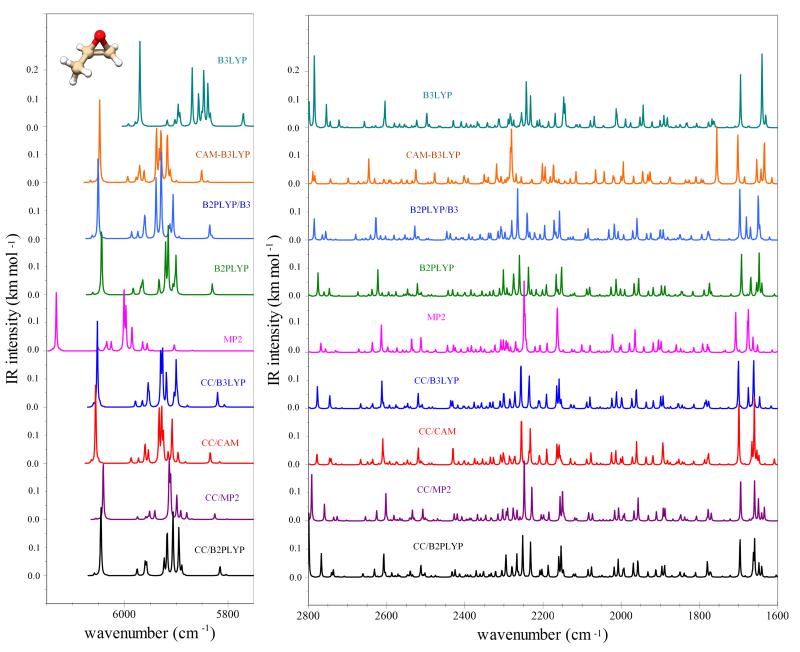
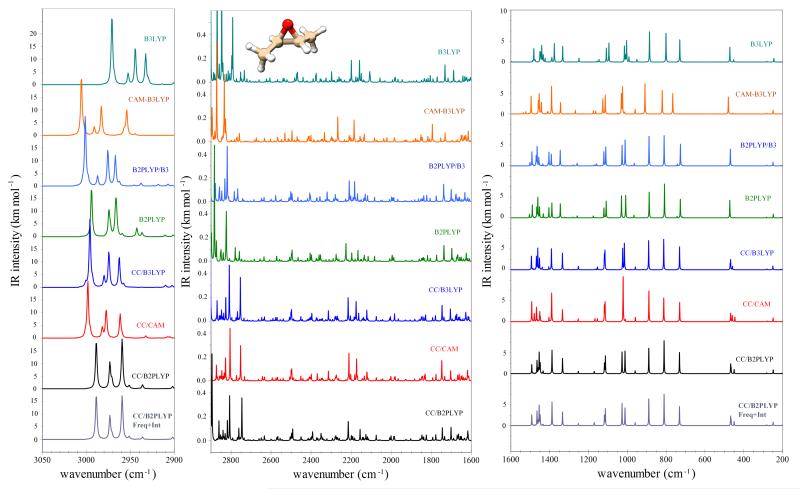
Moving to the issue of simulating IR spectra, in most cases reliable relative intensities along with accurate band positions are sufficient to obtain a correct spectral line-shape/intensity pattern, which in turn is required for the analysis of experimental results. For this reason, we focus on the convergence of simulated IR spectra of methyloxirane and trans-2,3-dimethyloxirane with respect to different methods and hybrid models. These are presented in Figures 2 and 3 for the former, and in Figure 4 for the latter. First of all, we note that the two spectra simulated with vibrational wavenumbers computed at the CC/B2PLYP level are very similar, irrespective of the correction for IR intensities at the harmonic level with best estimates (CC/B2PLYP Freq+Int) or not (CC/B2PLYP). The good accuracy of CC/B2PLYP results is confirmed, whenever feasible, by the comparison with experimental spectra, i.e., for methyloxirane in the 700-1600 cm−1 spectral range27. For this reason, all other spectra are reported by applying the hybrid scheme only for the wavenumbers, with the same level of theory used for harmonic and anharmonic contributions to the IR intensities. At first glance, we observe that in the 200-2800 cm−1 range a similar intensity pattern is obtained in most cases, even in the regions related to overtones and combination bands (1600-2800 cm−1). The largest discrepancies are observed for CAM-B3LYP and MP2, while all hybrid schemes based on the best-estimated harmonic wavenumbers lead to similar spectra. Concerning the C-H stretching vibrations and their overtones, we note more significant differences, with the fully anharmonic MP2 force field leading to a different energy pattern and B3LYP giving spectral features shifted to lower wavenumbers. At variance, all hybrid models with the harmonic part computed at least at the B2PLYP level reproduce well the results obtained with the best-estimated harmonic force field. In conclusion, we can point out that the B2PLYP/B3LYP model provides fairly good estimates of IR spectra up to 6000 cm−1, while for less expensive computations, B3LYP or CAM-B3LYP can be considered for the 200-2800 cm−1 and 2800-3600 cm−1 spectral ranges, respectively. Improved accuracy can be obtained by means of hybrid CC/DFT schemes with the harmonic part based on composite schemes and anharmonic corrections at the B2PLYP level.
FIG. 2.
Anharmonic IR spectra of methyloxirane in the 2800-3200 cm−1 and 200–1600 cm−1 ranges (related to the fundamental bands) computed with hybrid schemes. Spectra line-shapes were convoluted with Lorentzian distribution functions with a HWHM of 1 cm−1. Experimental spectrum measured in argon matrix at 10 K from Ref.27.
FIG. 3.
Anharmonic IR spectra of methyloxirane in the 5750–6150 cm−1 and 1600–2800 cm−1 ranges (related to the overtones and combination bands) computed with hybrid schemes. Spectra line-shapes were convoluted with Lorentzian distribution functions with a HWHM of 1 cm−1.
FIG. 4.
Anharmonic IR spectra of trans-2,3-dimethyloxirane in the 200–1600 cm−1, 1600–2900 cm−1 and 2900–3050 cm−1 ranges computed with hybrid schemes. Spectra line-shapes were convoluted with Lorentzian distribution functions with a HWHM of 1 cm−1.
IV. CONCLUDING REMARKS
In this paper we investigate the equilibrium structures and infrared spectra of prototypical chiral oxiranes by mean of accurate quantum-chemical methods within composite approaches and, for IR spectra the CC/DFT hybrid schemes, thus providing benchmarks for computational approaches applicable also to medium-sized systems. The reported results show that equilibrium geometries accurate to 0.001 Å for bond lengths and 0.1 degrees for angles are obtainable together with vibrational wavenumbers and IR intensities accurate to 10 cm−1 and 0.5 km mol−1, respectively. This systematic analysis paves the route toward reliable studies of larger molecular systems possibly taking into account also solvent effects. Moreover, the validation of a reliable computational protocol for the unequivocal determination of band positions and IR intensities in vibrational spectra of chiral prototypes, like substituted oxiranes, can represent, in our opinion, a robust background toward the development and validation of effective computational strategies for more demanding spectroscopies like VCD, which require not only the values, but also the relative orientations of the derivatives of electric and magnetic moments with respect to normal modes.
Supplementary Material
FIG. 1.
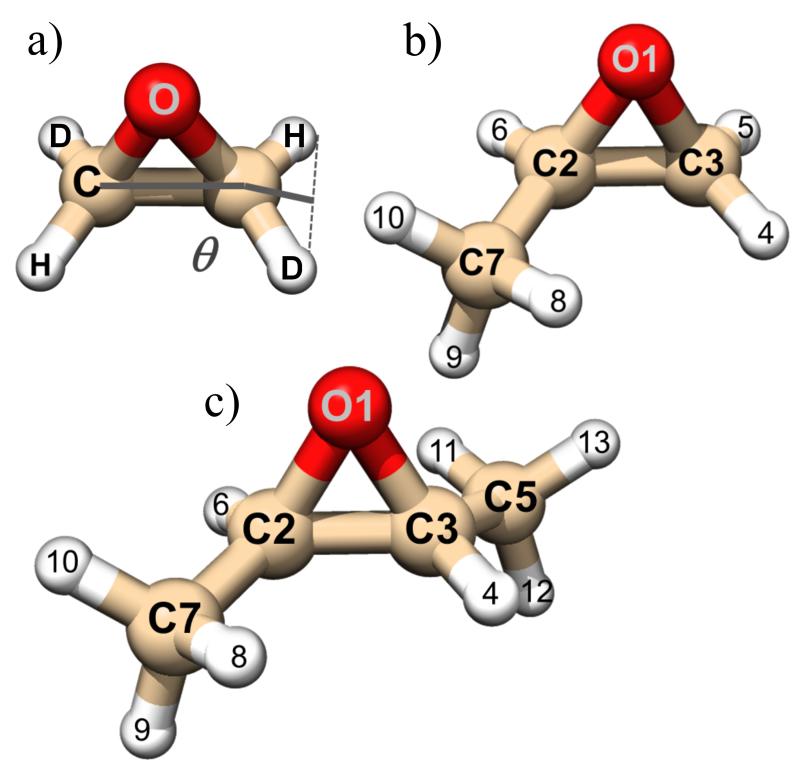
Molecular structure of trans-2,3-dideuteriooxirane (a), methyloxirane (b), and trans-2,3-dimethyloxirane (c). The atom labeling is given.
ACKNOWLEDGMENT
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement Nº ERC-2012-AdG-320951-DREAMS. This work was also supported by Italian MIUR (PRIN 2012 “STAR: Spectroscopic and computational Techniques for Astrophysical and atmospheric Research” and PON01-01078/8) and by the University of Bologna (RFO funds). The high performance computer facilities of the DREAMS center (http://dreamshpc.sns.it) are acknowledged for providing computer resources. The authors thank Profs. Yunjie Xu and Christian Merten for providing the experimental data of IR spectroscopic measurements of methyloxirane. The support of COST CMTS-Action CM1002 “COnvergent Distributed Environment for Computational Spectroscopy (CODECS)” is also acknowledged.
REFERENCES
- 1.Berova N, Polavarapu PL, Nakanishi K, Woody RW, editors. Comprehensive Chiroptical Spectroscopy: Instrumentation, Methodologies, and Theoretical Simulations. Vol. 1. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2012. [Google Scholar]
- 2.Yang G, Xu Y. In: Electronic and Magnetic Properties of Chiral Molecules and Supramolecular Architectures, Topics in Current Chemistry. Naaman R, Beratan DN, Waldeck DH, editors. Vol. 298. 2011. pp. 189–236. [DOI] [PubMed] [Google Scholar]
- 3.Polavarapu PL, Hess BA, Schaad LJ. J. Chem. Phys. 1985;82:1705. [Google Scholar]
- 4.Freedman TB, Paterlini MG, Lee NS, Nafie LA, Schwab JM, Ray T. J. Am. Chem. Soc. 1987;109:4727. [Google Scholar]
- 5.Jalkanen KJ, Stephens PJ, Amos RD, Handy NC. J. Am. Chem. Soc. 1988;110:2012. [Google Scholar]
- 6.Dutler R, Rauk A. J. Am. Chem. Soc. 1989;111:6957. [Google Scholar]
- 7.Polavarapu PL, Bose PK. J. Chem. Phys. 1990;93:7524. [Google Scholar]
- 8.Stephens PJ, Jalkanen KJ, Kawiecki RW. J. Am. Chem. Soc. 1990;112:6518. [Google Scholar]
- 9.Freedman TB, Spencer KM, Ragunathan N, Nafie LA, Moore JA, Schwab JM. Can. J Chem. 1991;69:1619. [Google Scholar]
- 10.Carnell M, Peyerimhoff SD, Breest A, Gödderz KH, Ochmann P, Hormes J. Chem. Phys. Lett. 1991;180:477. [Google Scholar]
- 11.Rauk A, Yang D. J. Phys. Chem. 1992;96:437. [Google Scholar]
- 12.Stephens PJ, Jalkanen KJ, Devlin FJ, Chabalowski CF. J. Phys. Chem. 1993;97:6107. [Google Scholar]
- 13.Bak KL, Jørgensen P, Helgaker T, Ruud K. Faraday Discussions. 1994;99:121. [Google Scholar]
- 14.Yang D, Rauk A. J. Chem. Phys. 1994;100:7995. [Google Scholar]
- 15.Carnell M, Grimme S, Peyerimhoff S. Chem. Phys. 1994;179:385. [Google Scholar]
- 16.Breest A, Ochmann P, Pulm F, Gödderz KH, Carnell M, Hormes J. Mol. Phys. 1994;82:539. [Google Scholar]
- 17.Bak KL, Bludský O, Jørgensen P. J. Chem. Phys. 1995;103:10548. [Google Scholar]
- 18.Bludský O, Bak KL, Jørgensen P, Spirko V. J. Chem. Phys. 1995;103:10110. [Google Scholar]
- 19.Devlin FJ, Finley JW, Stephens PJ, Frisch MJ. J. Phys. Chem. 1995;99:16883. [Google Scholar]
- 20.Tam MC, Russ NJ, Crawford TD. J. Chem. Phys. 2004;121:3550. doi: 10.1063/1.1772352. [DOI] [PubMed] [Google Scholar]
- 21.Turchini S, Zema N, Contini G, Alberti G, Alagia M, Stranges S, Fronzoni G, Stener M, Decleva P, Prosperi T. Physical Review A. 2004;70:014502. doi: 10.1063/1.1940632. [DOI] [PubMed] [Google Scholar]
- 22.Begue D, Gohaud N, Pouchan C, Cassam-Chenai P, Lievin J. J. Chem. Phys. 2007;127:164115. doi: 10.1063/1.2795711. [DOI] [PubMed] [Google Scholar]
- 23.Autschbach J. Chirality. 2009;21:E116. doi: 10.1002/chir.20789. [DOI] [PubMed] [Google Scholar]
- 24.Egidi F, Barone V, Bloino J, Cappelli C. J. Chem. Theory Comput. 2012;8:585. doi: 10.1021/ct2008473. [DOI] [PubMed] [Google Scholar]
- 25.Lipparini F, Egidi F, Cappelli C, Barone V. J. Chem. Theory Comput. 2013;9:1880. doi: 10.1021/ct400061z. [DOI] [PubMed] [Google Scholar]
- 26.Baiardi A, Bloino J, Barone V. J. Chem. Theory Comput. 2013;9:4097. doi: 10.1021/ct400450k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merten C, Bloino J, Barone V, Xu Y. J. Phys. Chem. Lett. 2013;4:3424. [Google Scholar]
- 28.Barone V, editor. Computational Strategies for Spectroscopy: from Small Molecules to Nano Systems. John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 29.Pulay P, Meyer W, Boggs JE. J. Chem. Phys. 1978;68:5077. [Google Scholar]
- 30.Puzzarini C, Stanton JF, Gauss J. Int. Rev. Phys. Chem. 2010;29:273. [Google Scholar]
- 31.Pawlowski F, Jørgensen P, Olsen J, Hegelund F, Helgaker T, Gauss J, Bak KL, Stanton JF. J. Chem. Phys. 2002;116:6482. [Google Scholar]
- 32.Demaison J. Mol. Phys. 2007;105:3109. [Google Scholar]
- 33.Puzzarini C. Phys. Chem. Chem. Phys. 2013;15:6595. doi: 10.1039/c3cp44301a. [DOI] [PubMed] [Google Scholar]
- 34.Jaeger HM, Schaefer HHF, III, Demaison J, Császár AG, Allen WD. J. Chem. Theory Comput. 2010;6:3066. doi: 10.1021/ct1000236. [DOI] [PubMed] [Google Scholar]
- 35.Demaison J, Craig NC, Cocinero EJ, Grabow J-U, Lesarri A, Rudolph HD. J. Phys. Chem. A. 2012;116:8684. doi: 10.1021/jp304178n. [DOI] [PubMed] [Google Scholar]
- 36.Puzzarini C, Barone V. Phys. Chem. Chem. Phys. 2011;13:7189. doi: 10.1039/c0cp02636k. [DOI] [PubMed] [Google Scholar]
- 37.Hirose C. Bulletin of the Chemical Society of Japan. 1974;47:1311. [Google Scholar]
- 38.Hamer E, Sutter DH. Z. Naturforsch. A. 1976;31:265. [Google Scholar]
- 39.Demaison J, Császár AG, Margulés LD, Rudolph HD. J. Phys. Chem. A. 2011;115:14078. doi: 10.1021/jp2063595. [DOI] [PubMed] [Google Scholar]
- 40.Imachi M, Kuczkowski R. J. Molec. Structure. 1982;96:55. [Google Scholar]
- 41.Raghavachari K, Trucks GW, Pople JA, Head-Gordon M. Chem. Phys. Lett. 1989;157:479. [Google Scholar]
- 42.Barone V, Biczysko M, Bloino J, Puzzarini C. Phys. Chem. Chem. Phys. 2013;15:10094. doi: 10.1039/c3cp50439e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckert M, Kállay M, Gauss J. Mol. Phys. 2005;103:2109. [Google Scholar]
- 44.Heckert M, Kállay M, Tew DP, Klopper W, Gauss J. J. Chem. Phys. 2006;125:044108. doi: 10.1063/1.2217732. [DOI] [PubMed] [Google Scholar]
- 45.Puzzarini C, Cazzoli G, López JC, Alonso JL, Baldacci A, Baldan A, Stopkowicz S, Cheng L, Gauss J. J. Chem. Phys. 2012;137:024310. doi: 10.1063/1.4731284. [DOI] [PubMed] [Google Scholar]
- 46.Harding ME, Metzroth T, Gauss J, Auer AA. J. Chem. Theory Comput. 2008;4:64. doi: 10.1021/ct700152c. [DOI] [PubMed] [Google Scholar]
- 47.Harding ME, Vázquez J, Gauss J, Stanton JF, Kállay M. J. Chem. Phys. 2011;135:044513. doi: 10.1063/1.3609250. [DOI] [PubMed] [Google Scholar]
- 48.Barone V, Biczysko M, Bloino J, Egidi F, Puzzarini C. J. Chem. Phys. 2013;138:234303. doi: 10.1063/1.4810863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Møller C, Plesset MS. Phys. Rev. 1934;46:618. [Google Scholar]
- 50.Barone V, Biczysko M, Bloino J. Phys. Chem. Chem. Phys. 2014;16:1759. doi: 10.1039/c3cp53413h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen HH. Reviews of Modern Physics. 1951;23:90. [Google Scholar]
- 52.Mills IM. Chap. Vibration-Rotation Structure in Asymmetric- and Symmetric-Top Molecules. Academic; New York: 1972. Molecular spectroscopy: Modern research; p. 115. [Google Scholar]
- 53.Isaacson AD, Truhlar DG, Scanlon K, Overend J. J. Chem. Phys. 1981;75:3017. [Google Scholar]
- 54.Schneider W, Thiel W. Chem. Phys. Lett. 1989;157:367. [Google Scholar]
- 55.Barone V. J. Chem. Phys. 2005;122:014108. doi: 10.1063/1.1824881. [DOI] [PubMed] [Google Scholar]
- 56.Clabo DA, Jr., Allen WD, Remington RB, Yamaguchi Y, Schaefer HF., III Chem. Phys. 1988;123:187. [Google Scholar]
- 57.Allen WD, Yamaguchi Y, Császár AG, Clabo DA, Jr., Remington RB, Schaefer HF., III Chem. Phys. 1990;145:427. [Google Scholar]
- 58.Amos RD, Handy NC, Green WH, Jayatilaka D, Willets A, Palmieri P. J. Chem. Phys. 1991;95:8323. [Google Scholar]
- 59.Vázquez J, Stanton JF. Mol. Phys. 2006;104:377. [Google Scholar]
- 60.Gaw F, Willetts A, Handy N, Green W. Spectro - a program for derivation of spectroscopic constants from provided quartic force fields and cubic dipole fields. JAI Press; 1991. pp. 169–185. [Google Scholar]
- 61.Hermes MR, Hirata S. J. Chem. Phys. 2013;139:034111. doi: 10.1063/1.4813123. [DOI] [PubMed] [Google Scholar]
- 62.Carbonniere P, Lucca T, Pouchan C, Rega N, Barone V. J. Comput. Chem. 2005;26:384. doi: 10.1002/jcc.20170. [DOI] [PubMed] [Google Scholar]
- 63.Miani A, Cane E, Palmieri P, Trombetti A, Handy NC. J. Chem. Phys. 2000;112:248. [Google Scholar]
- 64.Barone V. J. Phys. Chem. A. 2004;108:4146. [Google Scholar]
- 65.Burcl R, Handy NC, Carter S. Spectrochim. Acta A. 2003;59:1881. doi: 10.1016/s1386-1425(02)00421-3. [DOI] [PubMed] [Google Scholar]
- 66.Burcl R, Carter S, Handy NC. Phys. Chem. Chem. Phys. 2004;6:340. [Google Scholar]
- 67.Boese AD, Martin J. J. Phys. Chem. A. 2004;108:3085. [Google Scholar]
- 68.Cane E, Miani A, Trombetti A. J. Phys. Chem. A. 2007;111:8218. doi: 10.1021/jp071610p. [DOI] [PubMed] [Google Scholar]
- 69.Puzzarini C, Biczysko M, Barone V. J. Chem. Theory Comput. 2010;6:828. doi: 10.1021/ct900594h. [DOI] [PubMed] [Google Scholar]
- 70.Bloino J, Biczysko M, Barone V. J. Chem. Theory Comput. 2012;8:1015. doi: 10.1021/ct200814m. [DOI] [PubMed] [Google Scholar]
- 71.Begue D, Carbonniere P, Pouchan C. J. Phys. Chem. A. 2005;109:4611. doi: 10.1021/jp0406114. [DOI] [PubMed] [Google Scholar]
- 72.Begue D, Benidar A, Pouchan C. Chem. Phys. Lett. 2006;430:215. [Google Scholar]
- 73.Puzzarini C, Biczysko M, Barone V. J. Chem. Theory Comput. 2011;7:3702. doi: 10.1021/ct200552m. [DOI] [PubMed] [Google Scholar]
- 74.Bloino J, Barone V. J. Chem. Phys. 2012;136:124108. doi: 10.1063/1.3695210. [DOI] [PubMed] [Google Scholar]
- 75.Carnimeo I, Puzzarini C, Tasinato N, Stoppa P, Charmet AP, Biczysko M, Cappelli C, Barone V. J. Chem. Phys. 2013;139:074310. doi: 10.1063/1.4817401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biczysko M, Panek P, Scalmani G, Bloino J, Barone V. J. Chem. Theory Comput. 2010;6:2115. doi: 10.1021/ct100212p. [DOI] [PubMed] [Google Scholar]
- 77.Dunning TH., Jr. J. Chem. Phys. 1989;90:1007. [Google Scholar]
- 78.Kendall A, Dunning TH, Jr., Harrison RJ. J. Chem. Phys. 1992;96:6796. [Google Scholar]
- 79.Woon DE, Dunning TH., Jr. J. Chem. Phys. 1995;103:4572. [Google Scholar]
- 80.Stanton JF, Gauss J, Harding ME, Szalay PG, Auer AA, Bartlett RJ, Benedikt U, Berger C, Bernholdt DE, Bomble YJ, Christiansen O, Heckert M, Heun O, Huber C, Jagau T-C, Jonsson D, Jusélius J, Klein K, Lauderdale WJ, Matthews D, Metzroth T, Mueck LA, O’Neill DP, Price DR, Prochnow E, Puzzarini C, Ruud K, Schiffmann F, Schwalbach W, Stopkowicz S, Tajti A, Vázquez J, Wang F, Watts JD. CFour a quantum chemical program package. 2011; and the integral packages MOLECULE (; Almloef J, Taylor PR. ; ), PROPS (; Taylor PR. ; ), ABACUS (; Helgaker T, Jensen H. J. Aa., Jørgensen P, Olsen J. ; ), and ECP routines by ; Mitin AV, van Wuellen C. [accessed September 13, 2012]; For the current version, see http://www.cfour.de.
- 81.Barone V, Cimino P, Stendardo E. J. Chem. Theory Comput. 2008;4:751. doi: 10.1021/ct800034c. [DOI] [PubMed] [Google Scholar]
- 82.Bloino J, Biczysko M, Santoro F, Barone V. J. Chem. Theory Comput. 2010;6:1256. doi: 10.1021/ct8004744. [DOI] [PubMed] [Google Scholar]
- 83.Becke D. J. Chem. Phys. 1993;98:5648. [Google Scholar]
- 84. [accessed February 1, 2014];Double and triple-ζ basis sets of sns and n07 families, are available for download. 2012 visit http://compchem.sns.it.
- 85.Yanai T, Tew DP, Handy NC. Chem. Phys. Lett. 2004;393:51. [Google Scholar]
- 86.Rizzo A, Coriani S, Ruud K. Chap. Response Function Theory Computational Approaches to Linear and Nonlinear Optical Spectroscopy. John Wiley & Sons, Inc.; 2011. Computational strategies for spectroscopy, from small molecules to nano systems; pp. 77–135. [Google Scholar]
- 87.Grimme S. J. Chem. Phys. 2006;124:034108. doi: 10.1063/1.2148954. [DOI] [PubMed] [Google Scholar]
- 88.Schwabe T, Grimme S. Phys. Chem. Chem. Phys. 2007;9:3397. doi: 10.1039/b704725h. [DOI] [PubMed] [Google Scholar]
- 89.Kozuch S, Gruzman D, Martin JML. J. Phys. Chem. C. 2010;114:20801. [Google Scholar]
- 90.Biczysko M, Bloino J, Carnimeo I, Panek P, Barone V. J. Molec. Structure. 2012;1009:74. [Google Scholar]
- 91.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HR, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JR, Jr., Peralta JA, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin R, A. J., Cammi, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09 Revision D.01. gaussian Inc.; Wallingford CT: 2013. 2009. [Google Scholar]
- 92.Feller D. J. Chem. Phys. 1993;98:7059. [Google Scholar]
- 93.Helgaker T, Klopper W, Koch H, Noga J. J. Chem. Phys. 1997;106:9639. [Google Scholar]
- 94.Puzzarini C, Biczysko M, Barone V, Pena I, Cabezas C, Alonso JL. Phys. Chem. Chem. Phys. 2013;15:16965. doi: 10.1039/c3cp52347k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Puzzarini C. J. Phys. Chem. A. 2009;113:14530. doi: 10.1021/jp9040327. [DOI] [PubMed] [Google Scholar]
- 96.Tew DP, Klopper W, Heckert M, Gauss J. J. Phys. Chem. A. 2007;111:11242. doi: 10.1021/jp070851u. [DOI] [PubMed] [Google Scholar]
- 97.Gauss J, Stanton J. Chem. Phys. Lett. 1997;276:70. [Google Scholar]
- 98.Stanton JF, Lopreore CL, Gauss J. J. Chem. Phys. 1998;108:7190. [Google Scholar]
- 99.Stanton JF, Gauss J. Int. Rev. Phys. Chem. 2000;19:61. [Google Scholar]
- 100.Thiel W, Scuseria G, Schaefer HF, Allen WD. J. Chem. Phys. 1988;89:4965. [Google Scholar]
- 101.Schneider W, Thiel W. Chem. Phys. Lett. 1989;157:367. [Google Scholar]
- 102.Martin JML, Lee TJ, Taylor PM, François J-P. J. Chem. Phys. 1995;103:2589. [Google Scholar]
- 103.See Supplementary Material Document No. for (i) Harmonic vibrational wavenumbers and IR intensities of methyloxirane and 2,3-trans-dimethyloxirane computed by DFT and CC approaches (ii) Various contributions to the best-estimated geometries, harmonic wavenumbers and IR intensities for 2,3-trans-dideuterooxirane, methyloxirane and 2,3-trans-methyloxirane.
- 104.Barone V, Baiardi A, Biczysko M, Bloino J, Cappelli C, Lipparini F. Phys. Chem. Chem. Phys. 2012;14:12404. doi: 10.1039/c2cp41006k. [DOI] [PubMed] [Google Scholar]
- 105.Cappelli C, Bloino J, Lipparini F, Barone V. J. Phys. Chem. Lett. 2012;3:1766. doi: 10.1021/jz3006139. [DOI] [PubMed] [Google Scholar]
- 106.Sunahori FX, Su Z, Kang C, Xu Y. Chem. Phys. Lett. 2010;494:14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.