Abstract
Although somatic mutations and overexpression of the tyrosine kinase Fibroblast Growth Factor Receptor 3 (FGFR3) are strongly associated with bladder cancer, evidence for their functional involvement in the pathogenesis remains elusive. Previously we showed that activation of Fgfr3 alone is not sufficient to initiate urothelial tumourigenesis in mice. Here we hypothesise that cooperating mutations are required for Fgfr3-dependent tumourigenesis in the urothelium and analyse a mouse model in which an inhibitor of Pi3k-Akt signalling, Pten, is deleted in concert with Fgfr3 activation (UroIICreFgfr3+/K644EPtenflox/flox). Two main phonotypical characteristics observed in the urothelium were increased urothelial thickness and abnormal cellular histopathology, including vacuolisation, condensed cellular appearance, enlargement of cells and nuclei, and loss of polarity. These changes were not observed when either mutation was present individually. Expression patterns of known urothelial proteins indicated the abnormal cellular differentiation. Furthermore, quantitative analysis showed that Fgfr3 and Pten mutations cooperatively caused cellular enlargement, while Pten contributed to an increased cell proliferation. Finally, FGFR3 overexpression was analysed along the level of phosphorylated mTOR in sixty-six T1 urothelial tumours in tissue microarray, which supported the occurrence of functional association of these two signalling pathways in urothelial pathogenesis. Taken together, this study provides evidence supporting a functional role of FGFR3 in the process of pathogenesis in urothelial neoplasm. Given the wide availability of inhibitors specific to FGF signalling pathways, our model may open the avenue for FGFR3-targeted translation in urothelial disease.
Keywords: transitional cell carcinoma, transgenic mouse model, Fibroblast Growth Factors, prognostic marker, personalised therapy, comparative pathology
Introduction
The majority of bladder cancers are urothelial carcinomas (UC) that occur in the urothelial lining and present as either non-muscle invasive (NMIBC) or muscle-invasive bladder cancers (MIBC). Clinically operable MIBC are treated with radical cystectomy or radiotherapy, however up to 50% of the patients relapse [1]. NMIBC tends to recur frequently and it is estimated that 10-20% of NMIBC potentially progress MIBC [2,3]. A number of genetic and epigenetic alterations have been identified in MIBC, including amplification of ERBB2 and loss of TP53, Retinoblastoma (RB) and the phosphatase and tensin homolog (PTEN) [4]. NMIBC is strongly associated with activating mutations in FGFR3 with a frequency between 60-80% [5-8], followed by chromosome 9 deletion (36-66%) and mutations in RAS family genes and in Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA) [4]. FGFR3 protein is also overexpressed in 42% of bladder tumours without FGFR3 mutations [49].
FGFR3 is a tyrosine kinase receptor that mediates the effects of Fibroblast Growth Factors (FGFs) [9]. FGFR3 mainly stimulates the RAS-Mitogen-Activated Protein Kinase (MAPK) and Phosphatidylinositide-3 Kinase (PI3K)-AKT pathway and triggers a range of cellular processes such as cell proliferation and differentiation. When activating mutations occur in the germ line, FGFR3 causes several forms of dwarfism: hypochondroplasia, achondroplasia and thanatophoric dysplasia, and malformation of the cerebral cortex [10-13]. FGFR3 mutations are identified also in several cancer types, including multiple myeloma, cervical, prostate cancer and spermatocytic seminomas [14]. The functional role of FGFR3 mutation in tumour formation was first demonstrated in multiple myeloma [15], skin hyperplasia in transgenic mice overexpressing mutant FGFR3 [16] and in xenograft models [17]. However the mechanistic role of FGFR3 activation caused by mutations and overexpression in bladder tumourigenesis remains to be elucidated.
We have previously used a mouse line in which K644E, a highly activating mutation in the Fgfr3 kinase domain, is conditionally expressed in the urothelium (UroIICreFgfr3+/K644E) to investigate the functional role of Fgfr3 activation in bladder tumourigenesis [18]. These mice did not show any urothelial phenotype, indicating that Fgfr3 activation alone is not sufficient to drive tumourigenesis in the urothelium. However, in combination with K-Ras or β-Catenin, Fgfr3 activation caused tumours in the skin and in the lungs, respectively, suggesting that Fgfr3 is able to induce tumourigenesis in the presence of cooperating mutations [18]. We found that the Pi3k-Akt pathway was highly up-regulated in lung tumours which formed in the presence of Fgfr3 and β-Catenin mutations, while in the urothelium this up-regulation was not observed. This has led us to hypothesise that urothelial tumourigenesis may require increased PI3K-AKT signalling. In humans, activating PIK3CA mutations, including those at the hotspots (E542K, E545K in the helical domain and H1047R in the kinase domain), are found in 13-25% of bladder cancer [31,41-45]. Interestingly, FGFR3 and PIK3CA mutations are shown to co-occur [31,41-45]. Therefore it was proposed that activation of PI3K-AKT pathway may enhance the effects of FGFR3 mutation [30,31].
In this study, we wished to test the synergistic effects of Fgfr3 and Pi3k-Akt signalling activation. PTEN is a well-known inhibitor of PI3K-AKT signalling and previous mouse models demonstrated that the loss of Pten leads to urothelial hyperplasia [19,20]. Therefore we used the mouse line with Pten deletion as an experimental tool to assess the effects of Pi3k-Akt activation in the presence of Fgfr3 mutation in the urothelium. The urothelium of double mutant mice was thickened and showed abnormal cellular features including enlarged cells and loss of polarity. The mechanisms leading to this phenotype was further investigated for cell differentiation, cell proliferation and apoptosis. Finally, the synergistic effects of mutations in downstream signalling were characterised in the mouse urothelium, and evaluated along the level of FGFR3 overexpression in clinical specimens in the tissue microarray (TMA) platform.
Materials and Methods
Mice
UroIICre transgenic mice [23] were intercrossed with Fgfr3+/K644Eneo [11] and Ptenflox/flox mice [24] to generate UroIICreFgfr3+/K644EPtenflox/flox. Except for UroIICre (FVB/N), background of mice was C57Bl/6. The Z/EG reporter line is from Novak et al., 2000 [25]. Genotype was performed by Transnetyx, USA. The Control were C57Bl/6 (n=7) (Charles River, UK) and mice that carries inactive 'T2/Onc3' transposon allele [26] (n=4) which do not lead to any phenotype. All experiments were carried out in accordance with the Project Licence under Home Office Animal (Scientific Procedures) Act 1986 in the UK.
Histology
Bladders were gently emptied of urine and placed in formalin for overnight fixation. Haematoxylin and eosin (H&E) and PAS staining (Leica Microsystems staining solutions #3803812 and #03800E) was performed on 4 μm-thick paraffin sections. Formalin-fixed OCT-embedded frozen sections were used in X-Gal staining (K1465-01, Invitrogen Life Technologies, UK).
Immunohistochemistry (IHC)
Antigen retrieval was performed with 0.01M citric acid (pH 6) unless specified otherwise. Samples were incubated in 0.3% H2O2 in distilled water for 20 min. Following the blocking, incubation with primary antibody was performed overnight at 4°C, with secondary antibody for 2 hours at room temperature. Antibody binding was visualised by ABC Elite Standard kit (Vector Labs, PK-6100) and 3,3′-Diaminobenzidine (DAB; K3468, Dako), counterstained with haematoxylin. For fluorescent visualisation, sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000) and mounted with Vectashield (Vector Labs, H-1000). Pictures were taken by Axio Imager (Zeiss A1) and Zeiss upright confocal microscope (Zeiss 710) for light and fluorescent microscopy, respectively. The slides were scanned by Nanozoomer slide scanner (Hamamastu) and analysed by SlidePath Digital Image Hub (Leica Biosystems). IHC staining was examined in the minimum of n=3 per genotype. Antibodies used were: Caspase-3 (R&D Systems, AF835; 1:200), CK5 (Abcam, ab24647, 1:500), CK18 (Progen, #61028, 1:10), GFP (Abgent, AM1009a; 1:25), E-cadherin (BD Transduction Laboratories Clone 36; 1:1000), pERK1/2 (Cell Signaling, #9101 and #4370; 1:100), FGFR3 (Santa Cruz Biotechnology, C-15; 1:40, no antigen retrieval), Ki67 (Vector Labs Burlingame USA, VP-RM04; 1:100), Pten (Cell Signaling, #9559; 1:100), UroII (Santa Cruz, #sc15178, 1:50), pAkt1/2 (Ser473) (Cell Signalling, #3787; 1:50), p21 (Santa Cruz, M19; 1:500), p63 (Santa Cruz, sc-8431, 1:100). Biotinylated goat secondary antibodies from Vector Labs, Anti-Rabbit IgG (BA-1000), Anti-Rat IgG (BA-9401), and Anti-Mouse IgG (BA-9200) were used for chromatogenic signals, Alexa Fluor 488 Goat Anti-Mouse IgG1 (Invitrogen, A-21121) and Alexa Fluor 594 Goat Anti-Rabbit IgG (Invitrogen, A-11012) were used for fluorescent.
Quantitative analysis
Three representative photos were taken for each H&E stained section at 40x magnification. Urothelial thickness was measured in 25-30 μm intervals at random fashion using ImageJ software (NIH, Bethesda, USA). Each picture contained 20-50 measurements. The mean value of the thickness was initially calculated for each sample, and subsequently the mean value in each genotype was determined as presented in the results. For cell size, each cell was marked around the cell membrane in E-cadherin stained sections and the areas were quantified using ImageJ. The sizes of fifteen cells each layer were measured per sample. For quantitative analysis, the umbrella layer was defined as the outermost single layer, basal layer the innermost, and the intermediate cell layer between those two in the urothelium. Cells positive with Ki67 or Caspase-3 staining, as well as total cell number in the urothelium were counted. The percentage of Ki67 or Caspase-3-positive cells within the total cell number was calculated per sample and the mean value was determined in each genotype. Statistics were performed using the Mann-Whitney test for non-parametric distribution of data (SPSS Version 19, IBM).
Tissue microarray (TMA) analysis of clinical specimens
With the ethical approval under the medical-ethical committee of the University Health Network, Toronto, T1 urothelial tumours on TMA were stained with FGFR3 (Santa Cruz, B-9; 1:300, overnight incubation) and p-mTOR(S2448) (Cell Signaling, #2976; 1:30). Positive and negative controls were included in each run. Slides were assessed by the pathologists. Both protein levels were scored based on membrane staining according to the 4-point scale (0=negative, 1=faint, 2=intermediate, 3=strong) (Figure S5). Correlation was statistically evaluated by Pearson Chi-square statistics (SPSS).
Results
Together with Pten loss, the activating Fgfr3 mutation increases the thickness of the urothelium in mice
In order to see whether Fgfr3 activation is able to form UC in the absence of Pten, we generated a cohort of UroIICreFgfr3+/K644EPtenflox/flox mice. In these mice, the heterozygous Fgfr3 K644E knock-in mutation [11] and deletion of both Pten alleles [24] occurs concomitantly in the urothelium [27]. Cohorts of UroIICreFgfr3+/K644E, UroIICrePtenflox/flox and UroIICreFgfr3+/K644EPtenflox/flox were examined at 5-18 months (Table 1). Cre-dependent recombination in the urothelium was assessed using Z/EG reporter mice. In the presence of the UroIICre allele, cells with a GFP-positive nucleus were observed in the majority of urothelial cells (Figure S1h) while little X-gal staining remained in the urothelium (Figure S1i), confirming successful recombination. We detected similar levels and patterns of Fgfr3 protein expression in the urothelium of Control and UroIICreFgfr3+/K644E (Figure S1a, b) as we reported previously [18], and in UroIICrePtenflox/flox and UroIICreFgfr3+/K644EPtenflox/flox (Figure S1c, d), indicating that Fgfr3 expression was neither influenced by the Fgfr3 mutation nor Pten deletion. An increased FGFR3 protein level observed in 85% of bladder tumours with FGFR3 mutations in humans [49] was not apparent. Lack of Pten expression was confirmed in UroIICrePtenflox/flox urothelium (Figure S1g). Levels of Pten protein were similarly low in Control and UroIICreFgfr3+/K644E (Figure S1e, f), indicating that Pten expression is unaltered in the presence of Fgfr3 mutation.
Table 1. Summary of mouse cohorts and bladder phenotype.
| Genotype | Cohort size (n) |
Age at time of analysis |
Non-bladder related deaths (n) |
Increased urothelial thickness (n) |
Cellular abnormalities (n) |
|---|---|---|---|---|---|
| Control | 11 | 10-18 months | None | None | None |
|
UroIICre Fgfr3+/K644E |
25 | 5-15 months | 2 (8%) | None | None |
|
UroIICre Ptenflox/flox |
20 | 9-18 months | 4 (20%) | 12 (60%) mild | None |
|
UroIICre Fgfr3+/K644E Ptenflox/flox |
24 | 11-18 months | 4 (17%) | 4 (17%) mild, 19 (79%) severe, 23 (96%) total |
20 (83%) |
|
UroIICre Fgfr3K644E/K644E |
12 | 7-13 month | 10 (83%)* | 11 (92%) mild | None |
|
UroIICre Fgfr3K644E/K644E Ptenflox/flox |
3 | 10-12 months | None | 2 (66%) severe | 2 (66%) |
The mouse cohorts analysed in this study are summarised. Cellular abnormalities observed include vacuolisation, enlarged cells, and loss of cell orientation within the urothelium. Causes of non-bladder related deaths include infection and lymphoma, and termination due to skin rash on the back owing to Cre-lox recombination occurred in the epidermis [18].
UroIICreFgfr3K644E/K644E mice were sacrificed at the time when kyphosis became prevalent. This phenotype is due to a low-level of Fgfr3 expression in the presence of homozygous Fgfr3K644Eneo allele [11].
We observed a severe increase in thickness of the urothelium in UroIICreFgfr3+/K644EPtenflox/flox mice compared to Control (Table 1, Figure 1). A mild increase was also observed in 60% of UroIICrePtenflox/flox, while the thickness of UroIICreFgfr3+/K644E urothelium was normal. The urothelium of mice with homozygous Fgfr3 mutation (UroIICreFgfr3K644E/K644E) showed a mild thickening (Table 1, Figure S1k, l). In contrast, mice with homozygous mutations in both Fgfr3 and Pten (UroIICreFgfr3K644E/K644EPtenflox/flox) revealed a similar phenotype to that of UroIICreFgfr3+/K644EPtenflox/flox (Figure S1m, n).
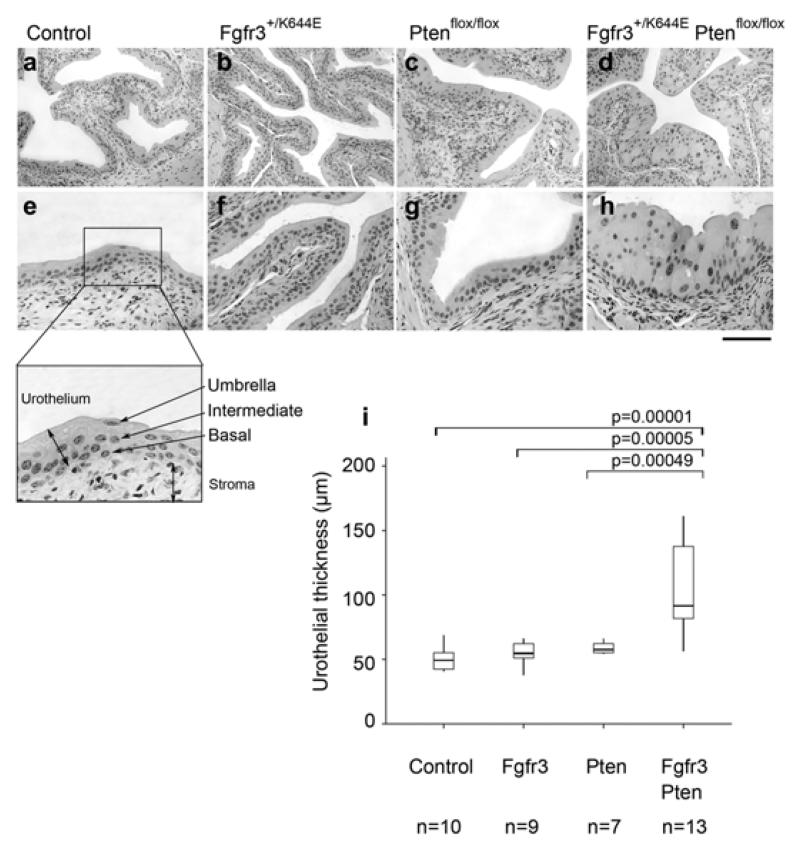
Figure 1. Increased thickness of the UroIICreFgfr3+/K644EPtenflox/flox urothelium.
Representative images of H&E stained bladder sections of Control (a, e), UroIICreFgfr3+/K644E (b, f), UroIICrePtenflox/flox (c, g) and UroIICreFgfr3+/K644E Ptenflox/flox (d, h) at low (a-d) and high magnification (e-h). The murine urothelium consists of three layers, namely umbrella, intermediate, and basal cells and borders with connective tissue and the stroma (e, insert). Scale bar represents 200 μm in panel a-d and 100 μm in panel e-h. Thickness of the urothelium was quantified in Control, UroIICreFgfr3+/K644E (Fgfr3), UroIICrePtenflox/flox (Pten) and UroIICreFgfr3+/K644EPtenflox/flox (Fgfr3Pten) in the number of animals indicated (i). The error bars indicate the standard deviations.
We have quantified these changes in mice aged between 11-18 months. Consistent with our previous observations [18,21], urothelial thickness of UroIICreFgfr3+/K644E and UroIICrePtenflox/flox were similar to Control (Figure 1i). However, thickness was significantly increased when both mutations were present in UroIICreFgfr3+/K644EPtenflox/flox compared to either UroIICreFgfr3+/K644E (p=0.00005) or UroIICrePtenflox/flox (p=0.00049), suggesting that this increase is generated by the cooperation of Fgfr3 and Pten mutations.
Abnormal histopathology of the UroIICreFgfr3+/K644EPtenflox/flox urothelium
In addition to increased urothelial thickness, several abnormal cellular morphologies were observed in UroIICreFgfr3+/K644EPtenflox/flox, including vacuolisation, condensed cellular appearance, enlargement of cells and nuclei, and loss of polarity (Figure 2A). High glycogen levels were detected in vacuoles by Periodic acid-Schiff (PAS) staining (n=3) (Figure 2Ag). At least one of these cellular features were observed in the majority of UroIICreFgfr3+/K644EPtenflox/flox (83%), while none of these abnormal features were present in Control (Figure 2Aa, d), UroIICreFgfr3+/K644E or UroIICrePtenflox/flox urothelium (Table 1).
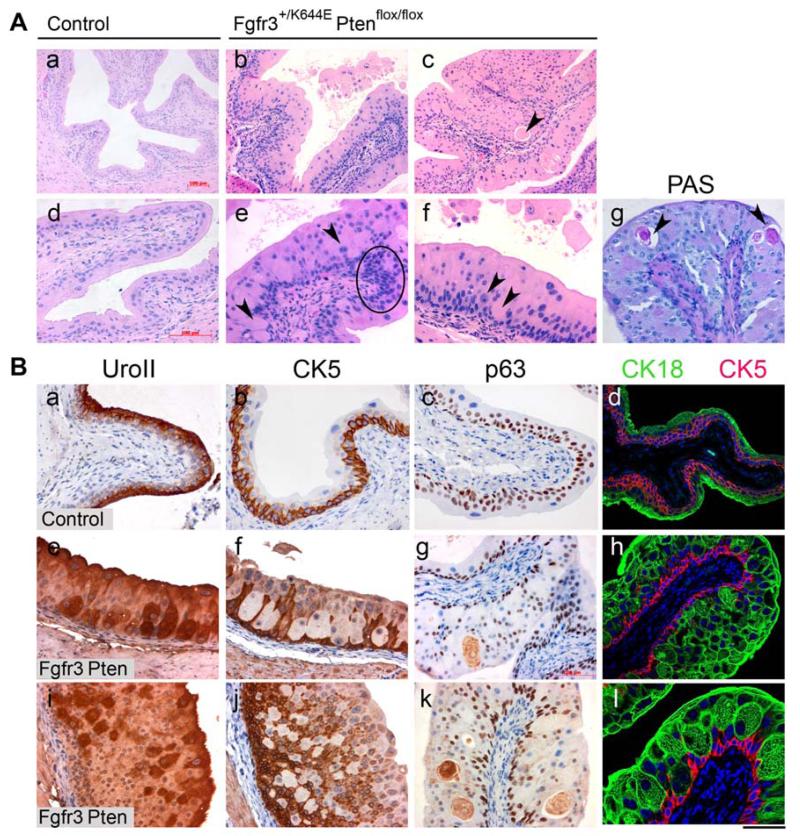
Figure 2. Abnormal cellular morphology in the UroIICreFgfr3+/K644EPtenflox/flox urothelium.
A, Representative images of H&E-stained Control (a, d) and UroIICreFgfr3+/K644EPtenflox/flox urothelia (b, c, e, f) at low (a-c) and high magnification (d-f). Vacuolisation (arrow in c), condensed cellular appearance (circled in e), enlargement of cells and nuclei (arrow heads in e, f), and loss of polarity were observed. Periodic acid-Schiff (PAS) staining marked glycogen-rich vacuoles in UroIICreFgfr3+/K644EPtenflox/flox (g). B, Expression of UroplakinII (a, e, i), CK5 (b, f, j and d, h, l), p63 (c, g, k), CK18 (d, h, l) was used to assess differentiation of umbrella (UroII and CK18), intermediate (p63) and basal cells (p63 and CK5) in Control (a-d) and UroIICreFgfr3+/K644EPtenflox/flox (e-l) (immunohistochemistry). Double staining with CK18 (green) and CK5 (red) (d, h, l) shows the abnormal localisation of CK18-positive cells deeper in the urothelium of UroIICreFgfr3+/K644EPtenflox/flox. Scale bar represents 200 μm in A (a-c) and 100 μm in A (d g), B (a-l).
In order to see whether these abnormal cellular appearances are caused by a change in urothelial cell identities, staining was performed with urothelial markers well-established in mice, UroII (umbrella and some intermediate cells [28]), Cytokeratin 5 (CK5) (basal cells [29]) and p63 (basal and intermediate cells [30]) (Figure 2Ba-c). In UroIICreFgfr3+/K644EPtenflox/flox, UroII-positive cells were present in deeper layers close to the submucosa, while CK5 expression was absent in some parts of the innermost layers of the urothelium, showing an inverse expression pattern (Figure 2Be, f, i, j). Furthermore, p63 showed a disorganised expression pattern (Figure 2Bg, k). Double staining of CK18, an alternative marker of umbrella cells in mice [30], together with CK5 clearly showed the abnormal localisation of CK18-positive cells deep in the urothelium of UroIICreFgfr3+/K644EPtenflox/flox (Figure 2Bh, l). Taken together, the results indicated abnormal differentiation of urothelial cells in UroIICreFgfr3+/K644EPtenflox/flox.
Urothelial cell size and proliferation is differently regulated by Fgfr3 and Pten mutations
The increase in urothelial thickness can be caused by deregulation of cell size and/or cell number. No significant difference in cell size was observed in the innermost basal and outermost umbrella layers among the cohorts (Figure 3a, c). In contrast, in the intermediate (between the inner and outermost) urothelial layers, while Fgfr3 mutation alone did not influence the cell size, Pten loss alone increased the cell size compared to Control (p=0.017) (Figure 3b). Furthermore, the presence of both Fgfr3 and Pten mutations further increased the cell size comparing to Fgfr3 mutation alone (p=0.001), as well as to Pten alone (p=0.016), suggesting that the Fgfr3 mutation cooperates with the Pten loss to further increase the cell size.
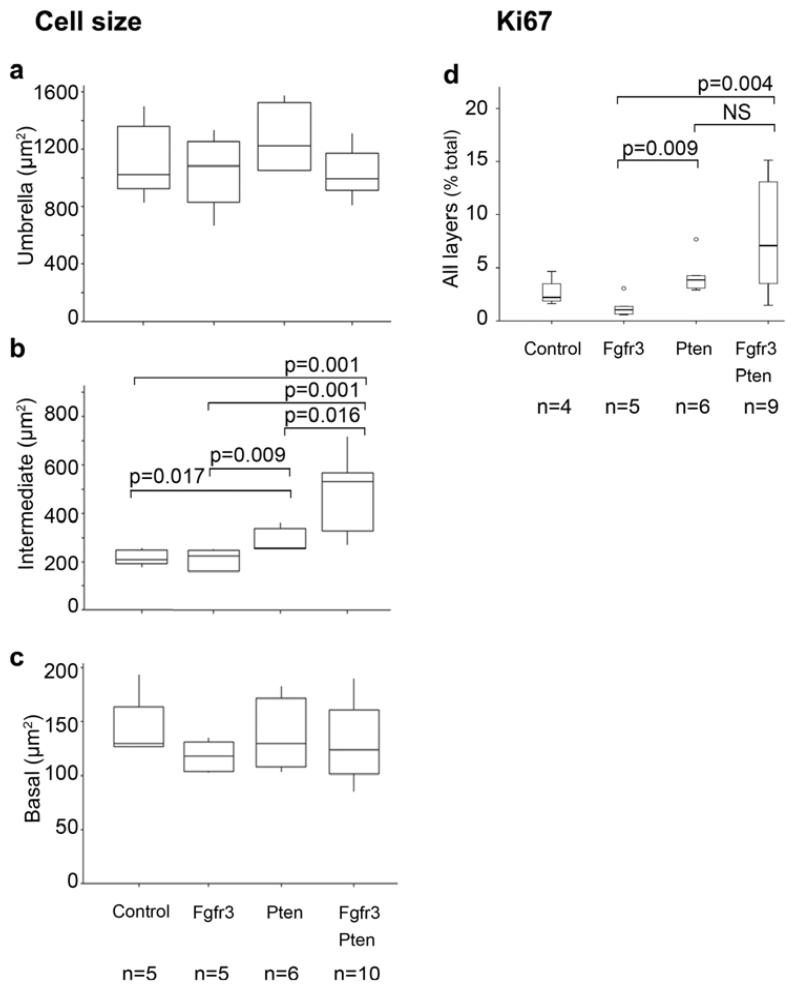
Figure 3. Differential effects of Fgfr3 and Pten mutations in regulation of urothelial cell size and proliferation.
The cell size of was analysed comparing cells in each layer using immunohistochemistry with E-cadherin. The sizes of cells in the outermost umbrella (a) and innermost basal (c) cell layers did not show any significant differences among the cohorts. However increase in cell size was observed in the intermediate (between the inner and outmost) layer, in particular in UroIICrePtenflox/flox and in UroIICre Fgfr3+/K644EPtenflox/flox (b). Ki67-positive cells were increased in the urothelium of UroIICrePtenflox/flox as well as UroIICreFgfr3+/K644EPtenflox/flox mice, comparing to other cohorts (d). The error bars indicate the standard deviations.
Albeit in a relatively small number of cells, Ki67 positivity was identified in all layers of the urothelium, including umbrella cells (Figure S2a, b) [30]. Taken all layers together, a significant increase in Ki67-positive cells was seen in the presence of both Fgfr3 and Pten mutations (p=0.004), as well as in the presence of Pten loss only (p=0.009), compared to Fgfr3 mutation only (Figure 3d). This indicates that cell proliferation was increased due to Pten loss. Similar effects were observed in the outermost and intermediate layers, while no significant changes were seen in the innermost basal layer (Figure S2). Very few apoptotic events were observed in all cohorts (Figure S3).
In summary, these results indicate that Fgfr3 and Pten mutations cooperatively caused urothelial hypertrophy, while Pten contributed to hyperplasia in the UroIICreFgfr3+/K644EPtenflox/flox urothelium.
Changes in downstream signalling associates with abnormal differentiation
We addressed how Fgfr3 and Pten mutations altered their downstream signalling cascades using antibodies for the phosphorylated forms of Erk1/2 (pErk1/2) and Akt(Ser473) (pAkt). In Controls, pErk1/2 was observed in a patchy fashion in the urothelium, not limited to a particular cell or cell type (n=9) (Figure 4a). Staining was similar in UroIICreFgfr3+/K644E (n=9) and UroIICrePtenflox/flox (n=6/9) (Figure 4d, g). In contrast, in UroIICreFgfr3+/K644EPtenflox/flox samples (n=9/12, 75%) pErk1/2 was seen more in a cell-specific fashion (Figure 4j), which resembled the overall pattern of UroII and CK5 staining (Figure 2B). As this staining was rather unusual, an alternative pErk antibody was also used to confirm this pattern (Figure S4). This observation may suggest the involvement of local MapK signalling dysregulation in pathogenesis of the urothelium through regulation of cell differentiation. However this requires further investigation. The staining of pAkt was absent in Control (n=3), UroIICreFgfr3+/K644E (n=6) and UroIICrePtenflox/flox (n=7) (Figure 4b, e, h), but found to be up-regulated in 55% of UroIICreFgfr3+/K644EPtenflox/flox samples (n=6/11, Figure 4k). Cyclin-dependent kinase inhibitor and tumour suppressor p21 was previously shown to be up-regulated in the mouse urothelium in which Pten is deleted [20]. p21 was present most abundantly in the outermost umbrella layer in Control (n=3) (Figure 4c), and this remained similar in UroIICreFgfr3+/K644E (n=3) and UroIICrePtenflox/flox (n=3) in this study (Figure 4f, i). However, in UroIICreFgfr3+/K644EPtenflox/flox urothelium (n=4), p21 was expressed throughout the urothelium (Figure 4l). This p21 up-regulation appeared to have coincided with the overall up-regulation of Pi3k-Akt signalling (Figure 4k), which is in accordance with the evidence in vitro that activation of PI3K-AKT pathway up-regulates p21 [48]. Altogether, these results provide the evidence to support the activation of Pi3k-Akt pathway in the current mouse model, as a result of cooperation between Fgfr3 and Pten mutations together, but not when either mutation was present individually.
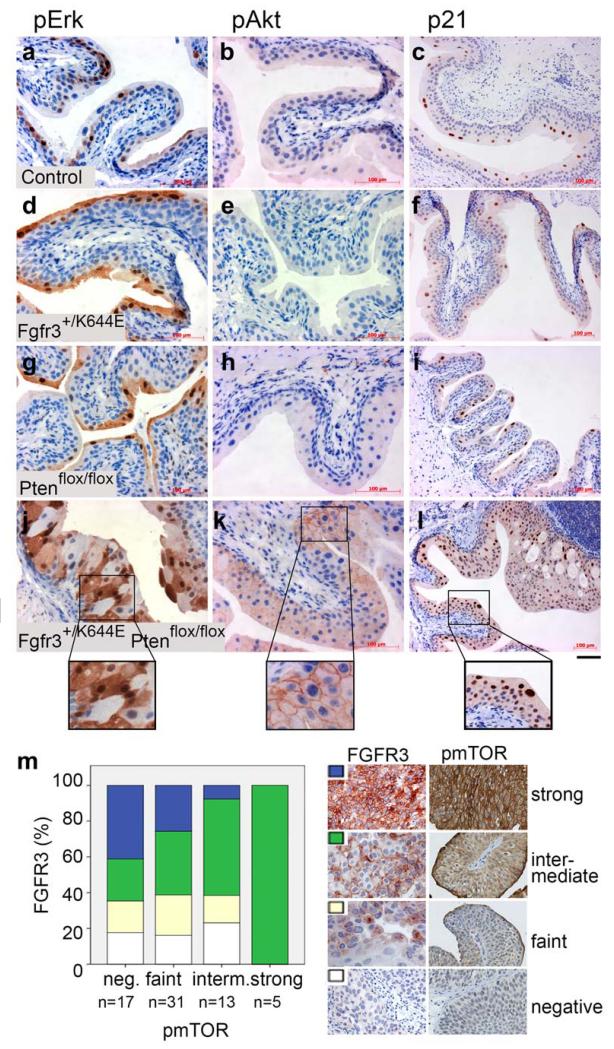
Figure 4. Deregulation of downstream signalling and cell cycle arrest in the UroIICreFgfr3+/K644EPtenflox/flox urothelium.
Immunohistochemistry was performed in Control (a-c), UroIICreFgfr3+/K644E (d-f), UroIICrePtenflox/flox (g-i), and UroIICreFgfr3+/K644EPtenflox/flox urothelia (j-l) with antibodies against phosphorylated Erk1/2 (pErk) (a, d, g, j), phosphorylated Akt (pAkt) (b, e, h, k) and p21 (c, f, i, l). (m) Levels of FGFR3 expression and p-mTOR were semi-quantitatively evaluated in TMA containing T1 urothelial tumours using the 4-point scale (negative, faint, intermediate, strong). Scale bar represents 50 μm in a, b, d, e, g, h, j, k, 100 μm in c, f, i, l, 25 μm in inserts of j-k and 50 μm in insert l.
Previous studies have investigated mutations in FGFR3 and PI3K-AKT pathway genes [31, 41-47] and their activation, using antibodies specific to the phosphorylated form of the protein, such as pAKT [43-45,47]. Mammalian Target of Rapamycin (mTOR) is downstream of PI3K-AKT signalling, and a candidate for effective therapeutic target [21,22]. We evaluated the levels of FGFR3 protein and p-mTOR(S2448) in 66 T1 urothelial tumours in TMA (Figure 4m, Figure S5). While the decrease in strong FGFR3 was statistically not significant (p=0.101), an increase in intermediate FGFR3 expression was correlated with an increased level of p-mTOR (p=0.014), supporting the presence of functional synergy of FGFR3 and PI3K-AKT pathways.
Discussion
FGFR3 mutations are highly associated with NMIBC [5,32]. A series of studies have shown the frequent co-occurrences of FGFR3 and PIK3CA mutations and evaluated gene alterations in PI3K-AKT pathway in clinical cohorts of bladder cancer [31,41-47]. However functional cooperation between Fgfr3 and Pi3k-Akt activation has never been demonstrated before. As both mutations and overexpression of FGFR3 is also highly associated with bladder cancer [49], our approach was to address the role of Fgfr3 activation using the existing mouse line with Fgfr3 K644E mutation [11,18]. Although the kinase domain mutations are less common in contrast to S249C in humans [5,32], this mouse line offers several advantages as an experimental model, namely because the Cre-Lox construct enables the expression of mutant Fgfr3 in the urothelium-specific fashion and the mutation is highly activating, thus maximising the chance of detectable phenotype. In the absence of available mouse lines with PI3KCA mutations, we turned to a model that enables conditional Pten deletion [19,20]. Pten is a well-known inhibitor of Pi3k-Akt signalling and it was shown that its deletion resulted in pAkt up-regulation in bladder tumours in mice [21].
Significantly, while the papillary structures with fibro-vascular cores seen in human NMIBC were not observed, the urothelium of UroIICreFgfr3+/K644EPtenflox/flox did demonstrate several morphological abnormalities that may reflect pathogenesis in humans. Two main phenotypes were increased urothelial thickness (Figure 1) and abnormal urothelial cellular histopathology, including vacuolisation, condensed cellular appearance, enlargement of cells and nuclei, and loss of polarity (Figure 2). Fgfr3 and Pten mutations cooperatively caused urothelial hypertrophy through regulation of cell size, while increased cell proliferation was mainly an effect of Pten deletion (Figure 3). The pErk staining in the double mutant urothelium showed an unusual cell-specific pattern (Figure 4j) and may suggest the local involvement of MapK pathway in pathogenesis through regulation of cell differentiation. These histopathological features are comparable to hyperplasia and dysplasia, regarded as early stages in the putative model of bladder cancer pathogenesis in humans. To our knowledge, this is a unique observation that has not been reported in any other genetic models of urothelial abnormalities.
Furthermore, up-regulation of pAkt was observed when both Fgfr3 and Pten mutations together, and not in single mutants (Figure 4). In humans, higher pAKT level was found in 50% bladder tumours independent of stage/grades and is associated with the presence of mutations, including PIK3CA, FGFR3, and both together [43]. Activation of PI3K-AKT downstream protein, mTOR, is confirmed to be strongly associated with pAKT [47]. Relationship between FGFR3 overexpression and p-mTOR is relatively unstudied and one report showed no statistical association [47]. However, in the current study, although the number of samples analysed was small, we were able to show association of the intermediate level of FGFR3 overexpression with increased p-mTOR (Figure 4m). In humans, PIK3CA mutations were also found in normal urothelium, indicating that it is an early event [44]. Altogether, this study provide functional evidence that supports that up-regulation of FGFR3 signalling together with that of PI3K-AKT signalling plays a role in the initiation of urothelial tumourigenesis.
In contrast, the current model with Fgfr3 and Pten mutations did not produce tumours in the life time of the animal models up to 18 months. This indicates that pathogenesis caused by FGFR3 and PI3K-AKT signalling pathway mutations are unlikely to progress unless further mutations occur. Our data in mice show that Fgfr3 and Pten mutations cooperatively promote morphological changes of the urothelium, while not when mutated individually. Previously it was reported that loss of Pten alone leads to urothelial hyperplasia [19,20]. However, hyperplasia was not observed by Pten loss in the studies by our group [21] and others [22]. In the current study, although mild urothelial thickening was observed in 60% of the UroIICrePtenflox/flox mice (Table 1), this increase was statistically not significant upon quantification (Figure 1i). An in vitro study using Normal Human Urothelial Cells stably expressing the hotspot PI3KCA mutations showed a large, vacuolated and flattened morphology [33]. Cell proliferation was also increased in cells with helical domain mutant. Despite the difference that these effects were singly resulted by PIK3CA activation in vitro, the findings are supportive of our observations in mice. This may reflect the complexity of signalling events leading to tumourigenesis in vivo in which multiple gene mutations and epigenetic events are likely to be required.
There are several differences between the natures of our mouse model in relationship to human bladder cancer that may limit the direct interpretation. Firstly, although we used Pten loss as an experimental tool in this study, in humans, occurrence of FGFR3 mutations are associated with NMIBC while PTEN loss with MIBC, and therefore little overlap is expected [4,41]. FGFR3 mutations and loss of heterozygosity in PTEN are found together only in a small number of cases of TaG1, T1G2, and T2G3 (Table S5 in ([41]). Statistically, PTEN loss was not associated with up-regulation of pAKT in clinical bladder cancer specimens [46]. Secondly, a study of clinical specimens reported that 85% of tumours with FGFR3 mutations also overexpressed FGFR3 protein [49]. In contrast, changes in Fgfr3 protein level were not apparent in our model (Figure S1a-d). This could be due to the expected low level of endogenous protein expressed in the mouse urothelium. The mechanism of FGFR3 mutations leading to an increased protein level is unclear, however the in vitro studies showed that impaired lysosomal degradation of FGFR3 protein was caused by mutations, increasing the stability of FGFR3 mutant protein in the plasma membrane [40].
FGF signalling inhibitors have been developed and applied in many cancer types [34]. Inhibition of FGFR had been suggested as a therapeutic option of UC [35]. Several novel drugs against FGFRs, including R3Mab [17], BGJ398 [36] and AZD4547 [37], are shown to be effective in cell lines and xenograft models. A recent attempt to re-classify UCs primarily based on molecular features has revealed that FGFR3 mutations and overexpression are associated with a subgroup of MIBC with significantly poor prognosis [38]. Although at a low frequency (3-4%), FGFR3 and AKT1 mutations were found to occur together in high-grade UC [43,46]. Overexpression of FGFR1 is also found in UC across all stages and grades [39]. Further functional studies of FGF and FGFR3 signalling pathway is therefore essential in both NMIBC and MIBC, firstly to allow stratification according to risk of progression and/or recurrence, and secondly to aid in patient selection for potential combination therapies.
Supplementary Material
Acknowledgements
This work is funded by School of Medicine, College of MVLS, University of Glasgow (TI), Cancer Research UK (OS), and University of Glasgow MRC Centenary Award (MF). We would like to thank Dr Mohammad Derakhshan for his assistance in statistical analysis of TMA results, Iain McPherson, Dionysios Theofilopoulos, Despoina Natsiou, Biological and Histology Services at Beatson Institute for Cancer Research for their technical support.
Footnotes
No conflicts of interest were declared.
References
- 1.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–825. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Zieger K, Dyrskjot L, Wiuf C, et al. Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. Clin Cancer Res. 2005;11:7709–7719. doi: 10.1158/1078-0432.CCR-05-1130. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163:60–61. discussion 61-62. [PubMed] [Google Scholar]
- 4.Knowles MA. Molecular pathogenesis of bladder cancer. International Journal of Clinical Oncology. 2008;13:287–297. doi: 10.1007/s10147-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 5.Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 7.Lamy A, Gobet F, Laurent M, et al. Molecular profiling of bladder tumors based on the detection of FGFR3 and TP53 mutations. J Urol. 2006;176:2686–2689. doi: 10.1016/j.juro.2006.07.132. [DOI] [PubMed] [Google Scholar]
- 8.Lindgren D, Liedberg F, Andersson A, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene. 2006;25:2685–2696. doi: 10.1038/sj.onc.1209249. [DOI] [PubMed] [Google Scholar]
- 9.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laederich MB, Horton WA. FGFR3 targeting strategies for achondroplasia. Expert Rev Mol Med. 2012;14:e11. doi: 10.1017/erm.2012.4. [DOI] [PubMed] [Google Scholar]
- 11.Iwata T, Chen L, Li C, et al. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:1603–1613. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- 12.Iwata T, Li CL, Deng CX, et al. Highly activated Fgfr3 with the K644M mutation causes prolonged survival in severe dwarf mice. Hum Mol Genet. 2001;10:1255–1264. doi: 10.1093/hmg/10.12.1255. [DOI] [PubMed] [Google Scholar]
- 13.Iwata T, Hevner RF. Fibroblast growth factor signaling in development of the cerebral cortex. Dev Growth Differ. 2009;51:299–323. doi: 10.1111/j.1440-169X.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta. 2012;1823:850–860. doi: 10.1016/j.bbamcr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Chesi M. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma. Blood. 2001;97:729–736. doi: 10.1182/blood.v97.3.729. [DOI] [PubMed] [Google Scholar]
- 16.Logie A. Activating mutations of the tyrosine kinase receptor FGFR3 are associated with benign skin tumors in mice and humans. Human Molecular Genetics. 2005;14:1153–1160. doi: 10.1093/hmg/ddi127. [DOI] [PubMed] [Google Scholar]
- 17.Qing J, Du X, Chen Y, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad I, Singh LB, Foth M, et al. K-Ras and {beta}-catenin mutations cooperate with Fgfr3 mutations in mice to promote tumorigenesis in the skin and lung, but not in the bladder. Dis Model Mech. 2011;4:548–555. doi: 10.1242/dmm.006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuruta H. Hyperplasia and Carcinomas in Pten-Deficient Mice and Reduced PTEN Protein in Human Bladder Cancer Patients. Cancer Research. 2006;66:8389–8396. doi: 10.1158/0008-5472.CAN-05-4627. [DOI] [PubMed] [Google Scholar]
- 20.Yoo LI, Liu DW, Le Vu S, et al. Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res. 2006;66:1929–1939. doi: 10.1158/0008-5472.CAN-05-1986. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad I, Morton JP, Singh LB, et al. beta-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene. 2011;30:178–189. doi: 10.1038/onc.2010.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo L, Cheng J, Lee EY, et al. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol. 2005;289:F562–568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- 24.Lesche R, Groszer M, Gao J, et al. Cre/loxP-mediated inactivation of the murinePten tumor suppressor gene. genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 25.Novak A, Guo C, Yang W, et al. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 26.Dupuy AJ, Rogers LM, Kim J, et al. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res. 2009;69:8150–8156. doi: 10.1158/0008-5472.CAN-09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZT, Pak J, Shapiro E, et al. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999;59:3512–3517. [PubMed] [Google Scholar]
- 28.Kong XT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. The Journal of Cell Biology. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin K, Lee J, Guo N, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, et al. Molecular pathways of urothelial development and bladder tumorigenesis. Urologic Oncology: Seminars and Original Investigations. 2010;28:401–408. doi: 10.1016/j.urolonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Knowles E, Hernandez S, Malats N, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401–7404. doi: 10.1158/0008-5472.CAN-06-1182. [DOI] [PubMed] [Google Scholar]
- 32.van Rhijn BW, Montironi R, Zwarthoff EC, et al. Frequent FGFR3 mutations in urothelial papilloma. J Pathol. 2002;198:245–251. doi: 10.1002/path.1202. [DOI] [PubMed] [Google Scholar]
- 33.Ross RL, Askham JM, Knowles MA. PIK3CA mutation spectrum in urothelial carcinoma reflects cell context-dependent signaling and phenotypic outputs. Oncogene. 2013;32:768–776. doi: 10.1038/onc.2012.87. [DOI] [PubMed] [Google Scholar]
- 34.Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 35.Lamont FR, Tomlinson DC, Cooper PA, et al. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011;104:75–82. doi: 10.1038/sj.bjc.6606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guagnano V, Furet P, Spanka C, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamin o]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem. 2011;54:7066–7083. doi: 10.1021/jm2006222. [DOI] [PubMed] [Google Scholar]
- 37.Gavine PR, Mooney L, Kilgour E, et al. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 38.Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson DC, Lamont FR, Shnyder SD, et al. Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer. Cancer Res. 2009;69:4613–4620. doi: 10.1158/0008-5472.CAN-08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho JY, Guo C, Torello M, et al. Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci U S A. 2004;101:609–614. doi: 10.1073/pnas.2237184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platt FM, Hurst CD, Taylor CF, et al. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 42.Kompier LC, Lurkin I, van der Aa MN, et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juanpere N, Agell L, Lorenzo M, et al. Mutations in FGFR3 and PIK3CA, singly or combined with RAS and AKT1, are associated with AKT but not with MAPK pathway activation in urothelial bladder cancer. Hum Pathol. 2012;43:1573–1582. doi: 10.1016/j.humpath.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Duenas M, Martinez-Fernandez M, Garcia-Escudero R, et al. PIK3CA gene alterations in bladder cancer are frequent and associate with reduced recurrence in non-muscle invasive tumors. Mol Carcinog. 2013 doi: 10.1002/mc.22125. doi: 10.1002/mc.22125. [DOI] [PubMed] [Google Scholar]
- 45.Calderaro J, Rebouissou S, de Koning L, et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer. 2013 doi: 10.1002/ijc.28518. doi: 10.1002/ijc.28518. [DOI] [PubMed] [Google Scholar]
- 46.Askham JM, Platt F, Chambers PA, et al. AKT1 mutations in bladder cancer: identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene. 2010;29:150–155. doi: 10.1038/onc.2009.315. [DOI] [PubMed] [Google Scholar]
- 47.Korkolopoulou P, Levidou G, Trigka EA, et al. A comprehensive immunohistochemical and molecular approach to the PI3K/AKT/mTOR (phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin) pathway in bladder urothelial carcinoma. BJU Int. 2012;110:E1237–1248. doi: 10.1111/j.1464-410X.2012.11569.x. [DOI] [PubMed] [Google Scholar]
- 48.Yohn NL, Bingaman CN, DuMont AL, et al. Phosphatidylinositol 3′-kinase, mTOR, and glycogen synthase kinase-3beta mediated regulation of p21 in human urothelial carcinoma cells. BMC Urol. 2011;11:19. doi: 10.1186/1471-2490-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomlinson DC, Blado O, Hamden P, et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213:91–98. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.