Abstract
Adipose tissue inflammation has been linked to age-related metabolic diseases. However, the underlying mechanisms are poorly understood. Adipose tissue inflammation and insulin resistance in diet associated obesity has been correlated with aberrant endoplasmic reticulum (ER) stress. This study was undertaken to test our hypothesis that increased ER stress response contributes to age-associated adipose tissue inflammation. We found elevated ER stress response in adipose tissue of old (18–20 months) compared to young (4–6 months) mice. Elevated ER stress markers BIP (GRP78), CHOP, cleaved-ATF-6, phospho-IRE1α, and XBP-1 were observed in old compared to young adipose tissue stromal cells. Additionally, old adipose tissue stromal cells were more sensitive to an ER stress inducer, thapsigargin. Similar experiments with adipose tissue macrophages showed elevated Chop and Bip expression in old adipose tissue macrophages when induced with thapsigargin. Treatment of chemical chaperone 4-phenyle-butyric acid alleviated ER stress in adipose tissue stromal cells and adipose tissue macrophages and attenuated the production of IL-6 and MCP-1 by adipose tissue stromal cells, and TNF-α by adipose tissue macrophages from both young and old mice. Finally, old mice fed with 4-phenyle-butyric acid have reduced expression of ER stress and inflammatory cytokine genes. Our data suggests that an exaggerated ER stress response in aging adipose tissue contributes to age-associated inflammation that can be mitigated by treatment with chemical chaperones.
Key Words: Aging, Adipose tissue, ER stress response, Chemical chaperones.
Aging is associated with the redistribution of adipose tissue in the visceral organs (1,2). Visceral adipose tissue has been recognized as a major source of chronic circulatory inflammatory cytokines in aging (inflamm-aging) due to its abundance of inflammatory mediators and M1 proinflammatory macrophages (3,4).
A central role of adipose tissue in inflammation and in insulin resistance has been derived mostly from obesity research (5–7). Other studies demonstrated that age-associated insulin resistance develops in adipose tissue before the liver and skeletal muscle (8). Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) have been shown to affect inflammation and insulin resistance in murine obesity models (9,10). Obesity-related ER stress leads to hyper-activation of c-Jun N-terminal kinase, and the subsequent serine phosphorylation of insulin receptor substrate-1 (IRS-1) leads to insulin signaling inhibition (10). Recently, the UPR pathways have been linked to other aging-associated diseases such as neurodegenerative diseases (11,12), nephropathy (13), and macular degeneration (14). In this study, we proposed that elevated ER stress in adipose tissue contributes to the chronic inflammatory state found in aging.
Adipose tissue is composed of a diverse cell population. Aside from the adipocytes that contribute to fat storage, there are nonadipocytes that are derived after collagenase digestion ex vivo, and known as the stromal vascular fraction (SVF). The majority of cells in the SVF consist of leukocytes and adipose tissue stromal cells (ATSCs), which primarily include preadipocytes and fibroblasts. Adipose tissue macrophages (ATMs) are the major leukocyte population found in adipose tissue and are believed to play a key role in promoting inflammation in obesity and metabolic dysfunction (15). On the other hand, a comparison via ex vivo analysis between ATMs (CD11b+), ATSCs (CD11b− SVFs), and adipocytes from young and old mice revealed that ATSCs were the predominant source of inflammatory cytokines (eg, IL-6, MCP1). Furthermore, the levels of proinflammatory cytokines were significantly higher in old compared to young mice (4). An age-associated increase in TNF-α production by preadipocytes has also been reported (16). Interestingly, peritoneal macrophages from young mice produced significantly higher levels of IL-6 and TNFα when they were cultured in the presence of ATSC-conditioned media from old mice as compared to ATSC-conditioned media from young mice (4). These studies strongly suggest that ATSCs, a component of visceral adipose tissue (which also contains preadipocytes), are the main contributor in age-associated adipose tissue inflammation. Moreover, increased TNFα levels in old adipose tissue has been reported to impede adipogenesis and has been correlated with an increase in CHOP expression CCAAT enhancer-binding protein homologous protein), a downstream target of the ER stress response pathway (16).
The ER is primarily recognized as the site of synthesis and folding of secreted, membrane-bound, and certain organelle-targeted proteins. Factors that perturb ER function and contribute to the development of ER stress include: increases in protein synthesis or protein misfolding rates that exceed the capacity of protein chaperones (client load), alteration of calcium stores of the ER lumen, oxidative stress, and disturbances to the redox balance (17). Cells respond to ER stress through three upstream signaling proteins—PKR-like ER kinase (PERK), Inositol-requiring enzyme 1 (IRE1α), and activating transcription factor 6 (ATF6). The activity of these three pathways collectively leads to an ER-specific UPR. When activated, the UPR begins a cascade of corrective actions (18): (a) suppression of protein translation to prevent the generation of more unfolded proteins (19), (b) induction of genes encoding ER molecular chaperones to facilitate protein folding (20), and (c) activation of ER-associated degradation to reduce unfolded protein accumulation in the ER (21,22) or modulate autophagy activity (23). Although ER stress is critical for cell survival, chronic or unresolved ER stress can also lead to apoptosis (24,25).
The present study was undertaken to test our hypothesis that ER stress contributes to age-associated adipose tissue inflammation. Using real-time polymerase chain reaction (PCR) analysis, we examined mRNA expression of ER stress response genes on the adipose tissue derived from old (18–20 months) and young (4–6 months) mice. We analyzed relative protein expression of ER stress markers BIP (GRP78), CHOP, cleaved-ATF-6, phospho-IRE1α and XBP-1 in young and old ATSCs and mRNA expression of Bip and Chop in ATMs at rest and in the presence of an ER stress inducer, thapsigargin—a Ca2+-ATPase inhibitor. Finally, in order to rescue ER stress response in old adipose tissue, we examined the role of chemical chaperone, 4-phenyle-butyric acid (4-PBA) in both young and old mice in vivo and in ATSCs and ATMs ex vivo.
Methods
Mice
Young (4–6 months) and old (18–22 months) male C57BL/6 mice were obtained from the National Institute on Aging through Harlan Sprague Dawley (Indianapolis, IN). Mice were maintained in a pathogen-free environment provided by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor, MI) until they were used. All the experimental research in the current study has been approved by the University of Michigan University Committee on Use and Care of Animals.
Reagents
Thapsigargin (Tg), 4-PBA, and collagenase were obtained from Sigma–Aldrich (St. Louis, MO). All the chemicals were dissolved in the appropriate media solution or dimethyl sulfoxide and then used at indicated concentration.
Isolation of Adipose Tissue
Careful inspection was done to exclude aged animals with cancer or lymphoma. Gonadal/epididymal fat pads from mice were excised under sterile condition. Five hundred microliters of radioimmunoprecipitation assay buffer (supplemented with protease inhibitors and phosphatase inhibitor) was added to adipose tissue (~300mg) and sonicated to obtain the cell lysates for Western blotting. Total RNA was isolated from 100mg of adipose tissue using a Qiagen lipid isolation kit.
Epididymal adipose tissue was fractionated into adipocyte and SVF portions, as previously described (26). Briefly, adipose tissue (7–10 mice for young, and 3–5 for old) were weighed and minced into smaller pieces. ATSCs (CD11b−) were purified from SVFs after collagenase digestion followed by CD11b+ magnetic beads using the MACS micro beads technology (Miltenyi Biotec, Bergisch-Gladbach, Germany), according to the manufacturer’s instructions (4).
RNA Extraction and Real-Time Reverse Transcription-PCR
Total adipose tissue or ATMs, were placed directly in RNA lysate buffer, and RNA was extracted using the RNeasy kit (Qiagen). The adipocytes were added to the QIAzol lysis reagent (Qiagen), and RNA was purified using RNeasy Lipid Tissue Midi Kit (Qiagen). Real-time PCR experiments were performed using QuantiTect SYBR green RT-PCR kit (Qiagen) using RNA samples with Corbett Rotor Gene 6000 Series (Qiagen). Data analysis was performed by the comparative 2( −ddCT) method. Both forward and reverse primers for different genes are listed in Supplementary Table 1.
Cell Culture and Treatment
ATSC pellets were resuspended in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum and plated in 25cm2 flask at the density of 1 × 105/cm2. After 12 hours of incubation in 37°C/5% CO2 incubator, floating cells were discarded, adherent cells were washed, trypsinized and replated at a density of 5 × 104 cells/cm2 in 12-well plate. Cells were then either treated with vehicle (dimethyl sulfoxide) or thapsigargin (Tg, 330 nM) and culture supernatant were collected at 4 hours or 16 hours following the treatments. Cells were washed with phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer for protein analysis. ATMs (CD11b+) were resuspended in complete DMEM media replated at a density of 0.5 × 106 in a 12 well dish overnight and harvested for total RNA extraction after 4 hours of Tg treatment. For rescue experiments, both ATSCs and ATMs were pretreated with 4-phenylbutyric acid (4-PBA, 1 mM) for 12 hours. To test the in vivo effect, 4-PBA (500mg/Kg body weight/day) was fed to the mice daily for 10 days with oral gavage (9).
Cytokine Bead Assay
Supernatants of cell culture media were assayed for IL-6, IL-10, IL12-p70, MCP-1, and TNF-α, and IFN-γ cytokine levels using the mouse inflammatory Cytometric Bead Array kit (BD Biosciences), following the manufacturer’s instructions. This assay kit provides a mixture of six micro beads with distinct fluorescence intensity that is precoated with capturing antibodies specific for the inflammatory cytokines. When the beads were incubated with the corresponding PE-conjugated detection antibodies and the test sample, sandwich complexes are formed. Fluorescence produced by the beads was measured using a FACS Caliber flow cytometer.
Enzyme-Linked Immunosorbent Assay
TNF-α was performed on the culture supernatants from treated ATMs using Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN), according to the manufacturer’s protocol.
Western Blotting
Protein levels were analyzed by Western blotting. A total of 25–50 µg total proteins lysates were separated on Mini PROTEAN Precast Gels with appropriate amounts of 2 × Laemmli sample buffer (with 2.5% β-mercaptoethanol) to a final 30 µL volume. The proteins were transferred onto a polyvinylidene flouride membrane and blocked with superblock solution and probed with anti-Grp78 (1:1000, ENZO Life Sciences), anti-CHOP (1:500, Santa Cruz Biotechnology), Anti-ATF6 (1:1000, abcam), anti-IRE1α (1:1000, Novus Biologicals), anti-XBP1s (1:1000, Santa Cruz Biotechnology), anti-GADD34 (1:1000, Santa Cruz Biotechnology) and anti-α tubulin (1:5000, abcam) for overnight at 4°C. Anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) was used at a dilution of 1:5000 for 1 hour at room temperature. The binding of specific antibodies was visualized via exposure to a photographic film after treating with enhanced chemiluminescence system reagents (Fisher Scientific). The film was scanned and quantified using the quantification software (ImageJ, NIH). For the quantification of specific bands, the same size square was drawn around each band to measure the intensity, and then the value was adjusted by the intensity of the background near the band. The results were expressed as a relative ratio of the target protein to reference protein.
Statistical Analysis
Results are expressed as mean ± SD. The significance of difference between means with single variable was analyzed by Student’s t test and two-way analysis of variance was performed with data involving more than two variables to find out the interaction between them. p < .05 was considered to be statistically significant. All the statistical analyses were performed with GraphPad Prism software.
Results
Elevated ER Stress Response Correlates With Proinflammatory Cytokines Profile in Aging Adipose Tissue
To examine whether aging is accompanied by a higher level of ER stress in adipose tissue, we compared the expression of Bip, Chop, and Atf4 mRNA derived from epididymal fat pads of young (4–6 months) and old (20 months) mice. We observed a significant increase in mRNA expression of Bip and Chop in adipose tissue samples from the old mice. However, no change in Atf4 mRNA between young and old adipose tissue was observed. We also determined the mRNA expression levels of other downstream ER stress markers, including protein disulphide-isomerase3a (Pdia3a), ER degradation manosidase 1 (Edem1) and ER Dnaj 4 (Erdj4) in the adipose tissue from young and old mice. The expression level of all these ER stress markers were significantly unregulated in the old mice compared to the young mice (Figure 1A).
Figure 1.
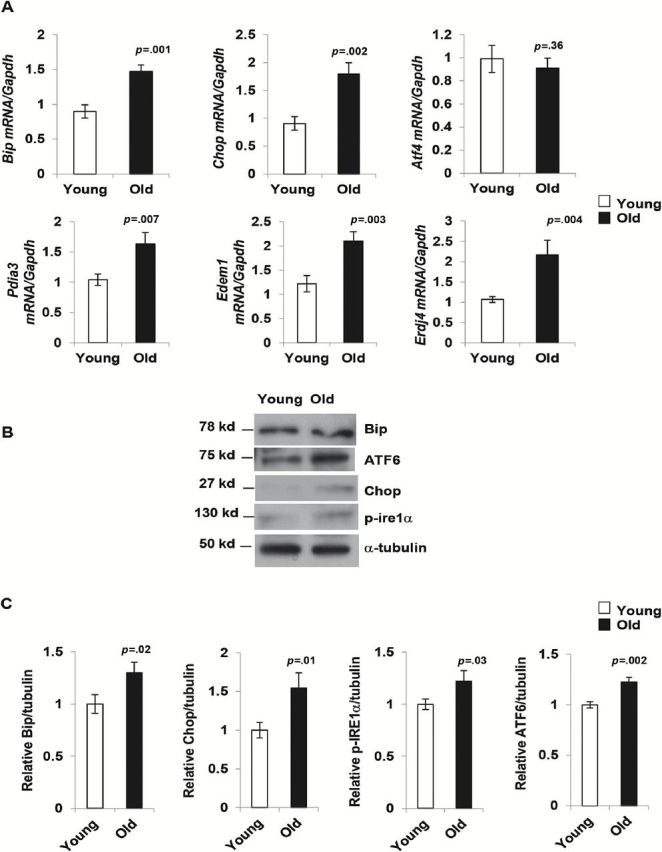
Endoplasmic stress-response pathway is elevated in aging adipose tissue. (A) mRNA expression of endoplasmic reticulum (ER) stress markers Bip, Chop, Atf4, Pdia3, Edem1, and Erdj4 in adipose tissue of young (4 m) and old mice (20 m). Expression of mRNA of all genes mentioned except Atf4 were significantly up-regulated in old mice. Values are mean ± SD (n = 10), *p < .05 (unpaired Student’s t test). (B) ER stress response proteins BIP, chopped-ATF6, CHOP, p-IRE1α are elevated in old mice. Data presented here are representative image of three independent experiments from pooled samples from five young and three old mice each time. The relative density of protein bands were plotted in (C). Values were presented as mean ± SD and p < .05 in Student’s t test was considered significant.
To validate the reverse transcription-PCR results, we performed Western blot analysis of BIP, CHOP, ATF-6, and p-IRE1α on adipose tissue lysates from young and old mice. Densitometry analysis and normalization of protein bands with α-tubulin band reveal that there was a significant increase in both the expression of CHOP and cleaved-ATF6. We observed a smaller, but significant, increase in BIP and p-IRE1α expression in the old mice (Figure1B and C). We also performed Western blot analysis of p-PERK, p-eIF2a, and ATF4 but were unable to detect the bands for neither p-PERK nor p-eIF2a (data not shown). Additionally, we observed no notable difference in ATF4 levels in the adipose tissue lysates derived from young and old mice (data not shown).
To examine the cause-and-effect of age-associated ER stress and inflammation in visceral adipose tissue, we determined the mRNA expression of selected inflammatory cytokines (Il-6, Tnf-α, and Mcp-1) in the adipose tissue from young and old mice by reverse transcription-PCR. In agreement with published reports (3,4), mRNA expression levels of all three inflammatory cytokines were elevated in old mice (Supplementary Figure 1A). These observations provided a potential link between ER stress and the presence of elevated inflammatory mediators in aged-adipose tissue.
Both Basal and Thapsigargin-induced ER Stress Responses Are Elevated in Aged ATSC
Although aging is associated with an increase in the number of proinflammatory ATMs, we have shown that ATSCs, rather than ATMs, are the main source of inflammation in adipose tissue in aging (4). We therefore tested the contribution of ER stress responses from the ATSCs in the context of age-associated adipose tissue inflammation. Our results indicated that the basal levels of BIP CHOP, p-IRE1α, GADD34, and cleaved-ATF6 are elevated in old ATSCs in comparison to the young (Figure 2A and 2B). We also observed that, compared to young ATSCs, old ATSCs were more sensitive to the ER stress inducer Tg, as BIP, CHOP, Gadd34, p-IRE1α, XBP1 and cleaved-ATF-6 levels were significantly higher at 4 hour posttreatment (Figure 2A–C). The levels of CHOP and GADD34 remained significantly higher in old-ATSCs even after 16 hours treatment with Tg (Figure 2A–C).
Figure 2.
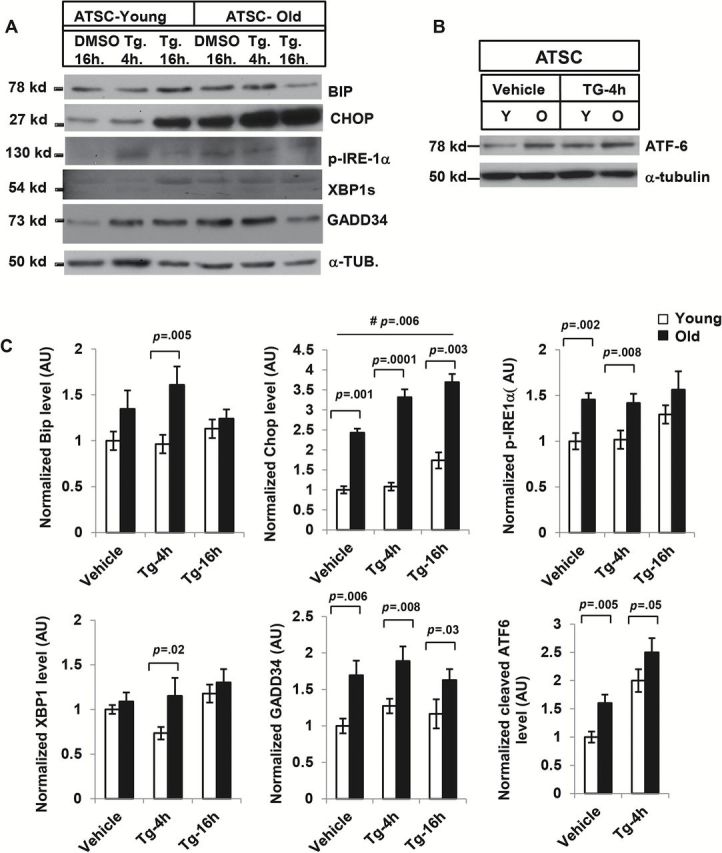
Effect of thapsigargin (Tg) on adipose tissue derived stromal cells from young and old mice. (A) Western blot analysis of endoplasmic reticulum (ER) stress response pathway components: Adipose tissue stromal cells (ATSCs) from young (5 mice) and old mice (3 mice) were treated with Tg (330nM) and the lysates were subjected to Western blot analysis with BIP, CHOP, p-IRE1α, XBP-1, GADD34, and ATF-6 antibodies (B). Elevated expression of these ER stress responses was observed in old ATSC both in resting and Tg-induced condition. (C) The relative density of protein bands for BIP, CHOP, p-IRE-1a, XBP-1, GADD34, and ATF-6 were plotted after normalization with γ-tubulin intensity. Values were presented as mean ± SD and p < .05 in Student’s t test was considered significant. # p value indicates the significance level of two-way analysis of variance for the interaction between treatment (Tg) and age factor (y vs o).
Treatment of ER Stress Inhibitor Suppresses Proinflammatory Cytokine Production by ATSCs From Both Young and Old Mice
We next tested if elevated ER stress in ATSCs from old mice could contribute to chronic inflammation in aging. To examine whether chemical chaperones can suppress ER stress in aging ATSCs, we incubated the cells with 4-PBA overnight. Treatment with 4-PBA significantly reduced the level of ER stress markers CHOP and cleaved-ATF6 in both young and old ATSCs (Figure 3A and 3B), indicating that ER stress can be inhibited by 4-PBA. We treated both young and old ATSCs with 4-PBA as indicated earlier and measured the amount of proinflammatory cytokines (IL-6, IL-10, MCP-1, IFN-α, TNF-α, and IL-12p) in the supernatant using cytometric bead arrays. Our data indicated that both IL-6 and MCP-1 production was significantly higher in aged murine adipose tissue (Figure 3C), and treatment of chemical chaperone 4-PBA reduced the level of IL-6 and MCP-1 in both young and old ATSCs (Figure 3C). This was consistent with our previous study (4), demonstrating that IL-6 and MCP-1 are the major contributors of ATSC-mediated inflammation.
Figure 3.
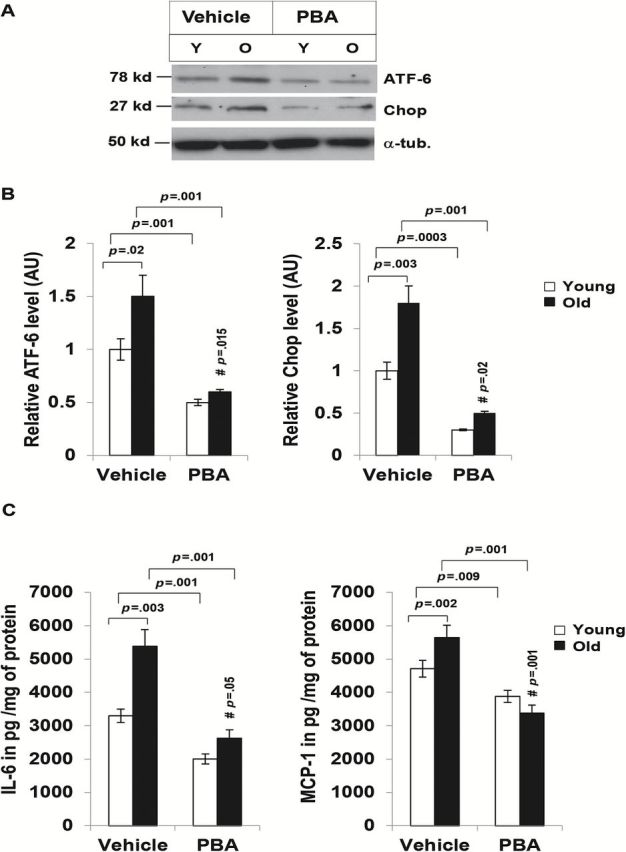
Endoplasmic reticulum stress response is inhibited by chemical chaperone 4-phenyle-butyric acid (4-PBA). Adipose tissue stromal cells (ATSCs) from five young and three old mice were treated with either 4-PBA (2mM) or water vehicle controls for 16 hours and the lysates were subjected to Western blot analysis with anti-Chop and ATF-6 antibodies. (A) PBA treatment reduced the levels of Chop and cleaved-ATF6 expression both young and old ATSC lysates. Data presented here are representative of three independent experiments. (B) Densitometry analysis of relative expression of Chop and cleaved-ATF6 in ATSC from young and old mice. (C) ATSCs from five young and three old mice were treated with either vehicle or 4-PBA (2mM) for 16 hours ex vivo. The level of proinflammatory cytokines (IL-6, IL-10, MCP-1, IFN-γ, TNF-α, and IL-12p70) in the culture supernatants were analyzed by cytometric bead array. Among six different cytokines only IL-6 and MCP-1 were detectable, and they were plotted after normalization with the protein amount in the cell lysates. Values were presented as mean ± SD and p < .05 in Student’s t test was considered significant. # p value indicates the significance level of two-way analysis of variance for the interaction between treatment (4-PBA) and age factor (y vs o).
ER Stress Markers Are Also Elevated in Aging ATMs
Since ATMs are the one of the major contributors of adipose tissue inflammation, we tested the expression of ER stress markers Bip and Chop in CD11b+ cells derived from young and old mice under resting or with ER stress inducer (Tg). Our data indicate significant difference in Chop but not Bip expression between young and old ATMs under basal condition (Figure 4A). When induced with Tg, significant increase of Bip and Chop expression was observed in old ATMs and significant elevation of Chop but not Bip was observed even in young ATMs. Notably, significantly higher expression of both Bip and Chop was observed in old ATMs compared to the young ATMs (Figure 4A). This data indicated that old ATMs are relatively more sensitive to ER stress compared to young ATMs.
Figure 4.

Endoplasmic reticulum (ER) stress response and inflammatory response in young and old adipose tissue macrophages (ATMs). mRNA expression of ER stress markers Bip, Chop, in ATMs treated with either vehicle (dimethyl sulfoxide) or thapsigargin (Tg) (A) and 4-phenyle-butyric acid (4-PBA) (B) derived from young (4 m) and old mice (20 m). (A) mRNA analysis by reverse transcription-polymerase chain reaction indicated significant increase in the expression of Bip, Chop in vehicle or Tg treated old ATMs in compare to the young ATMs. (B) Treatment of chemical chaperone 4-PBA significantly diminished the mRNA expression of Bip and Chop both in young and old ATMs. (C) ELISA analysis on the culture media of ATMs (CD11b+) from young and old mice was performed after 4-PBA (2mM) treatment overnight. Data indicated that 4-PBA treatment decreased the release of TNF-α in the culture media by young and old ATMs. Data presented here are representative of three independent experiments. Values were presented as mean ± SD and p < .05 in Student’s t test was considered significant. # p value indicates the significance level of two-way analysis of variance for the interaction between treatment (Tg or 4-PBA) and age factor (y vs o).
ER Stress Inhibitor Treatment Alleviates ER Stress and Suppresses TNF-α Production by ATMs From Both Young and Old Mice
We also analyzed the expression of Bip and Chop after treatment with 4-PBA for overnight in ATMs. We observed significantly diminished expression of Bip and Chop in both young and old ATMs (Figure 4B). Since ATMs are the main source of TNF-α (4), we analyzed the release in the culture medium with or without PBA treatment in young and old ATMs. Significant decrease of TNF-α release was observed in the PBA treated ATMs from both young and old mice compared to the vehicle treated ATMs (Figure 4C). These data suggested that inhibition of ER stress by chemical chaperone reduced the production of TNF-α by ATMs.
Chemical Chaperone Suppresses the Expression of ER Stress Response Genes and Inflammatory Cytokines in the Adipose Tissue of Old Mice
We analyzed the mRNA expression of ER stress response genes (Bip, Chop, and Pdia3) in adipose tissue from old mice fed with 4-PBA (500mg/Kg body weight/day in water) or vehicle (water) for 10 days. We found a significant reduction in the levels of Bip, Chop, and Pdia3 in the 4-PBA fed mice in comparison to the vehicle-fed mice (Figure 5). PBA fed mice also showed lower levels of Il-6, Tnf-α and Mcp1 in the adipose tissue. Our data showed that a chemical chaperone that inhibited ER stress response also mitigated inflammatory cytokine production in aging adipose tissue.
Figure 5.
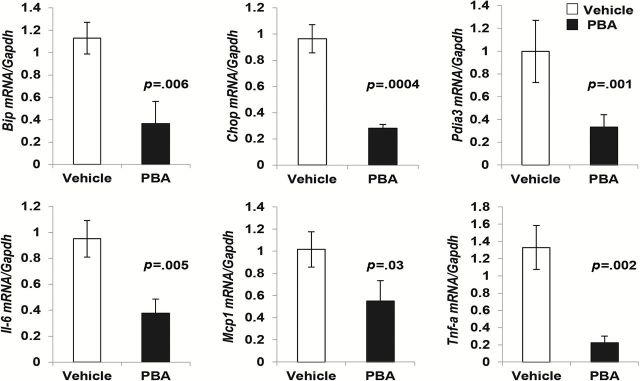
Diminished expression of endoplasmic reticulum (ER) stress response genes and proinflammatory cytokine genes upon treatment with 4-phenyle-butyric acid (4-PBA) in aging adipose tissue derived stromal cells (ATSCs). Mice were treated with either vehicle or 4-PBA for 10 days and ATSCs were harvested for mRNA analysis for ER stress markers Bip, Chop, and Pdia3 and also for proinflammatory markers Il-6, Mcp1, and Tnf-α. Diminished expression of Bip, Chop, and Pdia3 was observed in the 4-PBA treated group compared to the vehicle treated group (Figure 4). Expression of proinflammatory cytokine genes Il-6, Mcp1, and Tnf-α was also significantly diminished in the 4-PBA treated group. Values were presented as mean ± SD (n = 3) and p < .05 in Student’s t test was considered significant.
Discussion
This study demonstrated that the ER stress response is elevated in aged adipose tissue, which in turn contributed to the increased production of proinflammatory cytokine by the adipose tissue. Our study focused on ATSCs and ATMs, since these cells are shown to be the primary source of proinflammatory cytokines in aging adipose tissue (4). An elevated ER stress response was observed in aged ATSCs. The basal expression of ER stress markers such as CHOP, cleaved ATF-6, GADD34, and p-IRE1α was higher in aged ATSCs in comparison to the younger mice (Figure 2). ATSCs derived from old mice were more sensitive to the ER stress inducer Tg, as this treatment caused an increased in ER stress markers expression (Figure 2). Treatment with the chemical chaperone 4-PBA alleviated ER stress in adipose tissue, as evidenced by the level of CHOP and ATF-6 (Figure 3A and 3B), and at the same time the production of IL-6 and MCP-1 was attenuated in both young and old mice (Figure 3C). Elevated expression of Chop in old ATMs under basal condition and Tg treated condition (Figure 4A) suggested that hyper-responsive ER stress prevailed in the old ATMs. Elevated Bip expression in old ATMs under Tg treated condition but not basal condition suggested that old ATMs are more sensitive to ER stress compared to young ATMs. Noninduction of Bip mRNA upon Tg treatment might reflect efficient chaperoning capacity of young ATMs (Figure 4A). Treatment of old mice with 4-PBA also reduced the expression of ER stress response genes and inflammatory cytokines in ATSCs (Figure 3), in ATMs (Figure 4), and in total adipose tissue (Figure 5).
Our data also revealed that resting levels of GRP78 and CHOP (the most common ER stress response effectors) and the activation of ER stress sensors ATF-6 and p-IRE1α levels were significantly higher in adipose tissue from aged mice. Up-regulation of Edem1, ER Dnaj4, and Pdia3 in aged adipose tissue further confirmed the higher levels of basal ER stress in aged adipose tissue. We then observed an increase of ER stress response proteins in isolated ATSCs ex vivo, suggesting that the intrinsic chaperoning capacity of ER is compromised with aging, in agreement with similar observations in other chronic inflammatory states (13). Consistent with our hypothesis, the levels of these key ER stress markers were found at even higher levels when ATSCs and ATMs were treated with Tg, which induces ER stress by blocking Ca2+-ATPase in the ER membrane. Induced ER stress further aggravated the already compromised chaperoning system in the aged ATSCs and ATMs. Among the three key sensors (PERK, ATF-6, and IRE1α) of the UPR pathway (Figure 6), we were able to detect the activation of both ATF6 and IRE1α. We could not detect p-PERK or its downstream effector p-eIF2a in our system, although the total eIF2a remained unchanged between young and old adipose tissue samples (data not shown). Given that there was no significant difference in ATF4 levels in young and old adipose tissue, involvement of the PERK-eIF2a-ATF4 pathway needs further clarification.
Figure 6.

Endoplasmic reticulum (ER) stress response pathway in aging adipose tissue: Compromised chaperoning system in the ER lumen, lipolysis and free fatty acids, hypoxia, proteasome dysfunction or autophagy defects might lead to ER stress in old adipose tissue. Cells respond to ER stress through three upstream signaling proteins—PKR-like ER kinase (PERK), Inositol-requiring enzyme 1 (IRE1α) and activating transcription factor 6 (ATF6). Upon activation PERK phosphorylates e-IF2α which inhibits protein translation to avoid further ER stress. Activated p-e-IF2a also activates ATF4 via noncanonical fashion, which leads to expression of gene of redox reactions, stress response, autophagy, and Chop. Cleaved ATF-6 activates transcription of uXBP1 which is cleaved by p-IRE1 to XBP1s. XBP1s induces the transcription of Chop, other chaperones and enzymes for protein folding such as disulphide-isomerase3a (Pdia3a), ER degradation manosidase 1 (Edem1) and ER Dnaj 4 (Erdj4). Activated p-IRE1 activated c-Jun N-terminal kinase (JNK) which activates transcription factors NF-κB and AP1 and induces the production of proinflammatory cytokines. Inflammatory cytokines might results in insulin resistance via JNK activation.
Compromised chaperoning system in the ER lumen along with lipolysis and free fatty acids, hypoxia, proteasome dysfunction or autophagy defects might feed into the ER stress in old adipose tissue (Figure 6). Treatment with Tg further challenges the already compromised UPR responses in aging, resulting aggravated ER stress signaling. On the other hand, treatment of chemical chaperone 4-PBA effectively rescued the ER stress response and reduced the production of inflammatory mediators in the aging adipose tissue.
Compromised ER-chaperoning capacity has been reported with a variety of age-associated diseases, including neurodegenerative diseases (11,12), macular degeneration (14), and other inflammatory diseases (27,28). Recent studies also support the idea that compromised proteasome function is associated with aging (29). Therefore, it is plausible that compromised ER-chaperoning capacity along with reduced proteasome function may both contribute to the increased ER stress in aged adipose tissue. Compromised autophagy activity has also been reported to enhance ER stress and inflammation in the liver of hepatocyte specific atg7 (autophagy associated gene 7) knockout mice (30). We cannot rule out the possibility of compromised age-associated autophagy activity that may in turn contribute to the elevated ER stress response in aging adipose tissue.
The chemical chaperones have been used to examine disease models involving the UPR. These include obesity (9), neurodegenerative diseases (31,32), and sleeping disorders (33). The chemical chaperone (4-PBA) is a potent ER stress inhibitor and a nonselective chaperone that binds to the exposed hydrophobic regions of misfolded proteins and has been shown to stabilize protein conformation, to improve the folding capacity of ER, and to facilitate the trafficking of mutant proteins (34). Our data suggested that 4-PBA markedly reduced CHOP and cleaved-ATF-6 levels in both young and old ATSCs and expression of ER stress response genes in ATMs, confirming nonselective chaperoning capacity. Chemical chaperone 4-PBA treatment mitigated proinflammatory cytokine production by both young and old ATSCs and ATMs. Oral feeding of 4-PBA reduced the ER stress gene expression and also diminished production of proinflammatory cytokines gene expression in the old adipose tissue. Therefore, intervening ER stress by chemical chaperone and modulating signaling components of the UPR could be a promising step in the therapeutic intervention of inflammation in aging adipose tissue and age-associated metabolic diseases.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This project has been funded in part by National Institutes of Health grants (AG020628, AG028268, HL58984 to R.Y.), University of Michigan Claude D. Pepper Older American Independence Center (AG024824 to R.Y.), Nathan Shock Center for the Basic Biology of Aging (AG013283 to A.K.G., R.Y., and S.G.) and Aging Rodent Core (F034237 to A.K.G.), Center for the Study of Lifestage Exposures and Adult Disease (ES017885 to R.Y.), and Geriatrics Research, Education and Clinical Care Center (GRECC) of the VA Ann Arbor Healthcare System (R.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
References
- 1. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. :10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. J Am Geriatr Soc. 1997;45:959–967. [DOI] [PubMed] [Google Scholar]
- 3. Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. [DOI] [PubMed] [Google Scholar]
- 4. Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. :10.4049/jimmunol.1102188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 6. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. :10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. [DOI] [PubMed] [Google Scholar]
- 8. Serrano R, Villar M, Gallardo N, Carrascosa JM, Martinez C, Andrés A. The effect of aging on insulin signalling pathway is tissue dependent: central role of adipose tissue in the insulin resistance of aging. Mech Ageing Dev. 2009;130:189–197. :10.1016/j.mad.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 9. Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. :10.1038/srep00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. [DOI] [PubMed] [Google Scholar]
- 11. Lu M, Sun XL, Qiao C, Liu Y, Ding JH, Hu G. Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol Aging. 2014;35:421–430. :10.1016/j.neurobiolaging.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 12. Fonseca AC, Oliveira CR, Pereira CF, Cardoso SM. Loss of proteostasis induced by amyloid beta peptide in brain endothelial cells. Biochim Biophys Acta. 2014;1843:1150–1161. :10.1016/j.bbamcr.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 13. Wu J, Zhang R, Torreggiani M, et al. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol. 2010;176:2163–2176. :10.2353/ajpath.2010.090386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–542. :10.2119/molmed.2010.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. :10.1146/annurev-physiol-021909-135846 [DOI] [PubMed] [Google Scholar]
- 16. Tchkonia T, Pirtskhalava T, Thomou T, et al. Increased TNFalpha and CCAAT/enhancer-binding protein homologous protein with aging predispose preadipocytes to resist adipogenesis. Am J Physiol Endocrinol Metab. 2007;293:E1810–E1819. [DOI] [PubMed] [Google Scholar]
- 17. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. :10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- 18. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- 19. Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. [DOI] [PubMed] [Google Scholar]
- 20. Li M, Baumeister P, Roy B, et al. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol. 2000;20:5096–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng DT, Spear ED, Walter P. The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J Cell Biol. 2000;150:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. [DOI] [PubMed] [Google Scholar]
- 23. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. :10.1038/nrm2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. [DOI] [PubMed] [Google Scholar]
- 25. Lipson KL, Fonseca SG, Urano F. Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr Mol Med. 2006;6:71–77. [DOI] [PubMed] [Google Scholar]
- 26. Westcott DJ, Delproposto JB, Geletka LM, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. :10.1084/jem.20091333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289:1203–1211. :10.1074/jbc.R113.534743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naidoo N. ER and aging-protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. [DOI] [PubMed] [Google Scholar]
- 29. Dasuri K, Zhang L, Ebenezer P, et al. Proteasome alterations during adipose differentiation and aging: links to impaired adipocyte differentiation and development of oxidative stress. Free Radic Biol Med. 2011;51:1727–1735. :10.1016/j.freeradbiomed.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. :10.1038/nature07976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiley JC, Pettan-Brewer C, Ladiges WC. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell. 2011;10:418–428. :10.1111/j.1474-9726.2011.00680.x [DOI] [PubMed] [Google Scholar]
- 32. Ricobaraza A, Cuadrado-Tejedor M, Marco S, Pérez-Otaño I, García-Osta A. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus. 2012;22:1040–1050. :10.1002/hipo.20883 [DOI] [PubMed] [Google Scholar]
- 33. Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE, Naidoo N. Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging. 2013;35:1431–1441. doi:10.1016/j.neurobiolaging.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


