Abstract
Nerve conduction velocity (NCV), the speed at which electrical signals propagate along peripheral nerves, is used in the clinic to evaluate nerve function in humans. A decline in peripheral nerve function is associated with a number of age-related pathologies. While several studies have shown that NCV declines with age in humans, there is little information on the effect of age on NCV in peripheral nerves in mice. In this study, we evaluated NCV in male and female C57Bl/6 mice ranging from 4 to 32 months of age. We observed a decline in NCV in both male and female mice after 20 months of age. Sex differences were detected in sensory NCV as well as the rate of decline during aging in motor nerves; female mice had slower sensory NCV and a slower age-related decline in motor nerves compared with male mice. We also tested the effect of dietary restriction on NCV in 30-month-old female mice. Dietary restriction prevented the age-related decline in sciatic NCV but not other nerves. Because NCV is clinically relevant to the assessment of nerve function, we recommend that NCV be used to evaluate healthspan in assessing genetic and pharmacological interventions that increase the life span of mice.
Key Words: Peripheral nerves, Dietary restriction, Nerve conduction.
Over the past two decades, great strides have been made in identifying experimental manipulations, largely genetic, that increase the life span of animal models ranging from yeast, nematodes, and flies to rodents. It is generally assumed that a manipulation which increases the mean and maximum life span of an organism also delays aging. While life span gives an accurate measure of how long an animal will live in response to a specific manipulation, it does not provide information on the effect of the manipulation on physiological functions of the animal. Because long life in the presence of increased morbidity and dysfunction is not a desirable endpoint, we need to evaluate whether manipulations that increase life span also increase healthspan. Thus, healthspan data are very important when assessing the effect of an experimental manipulation on aging and determining the potential of translating the experimental intervention to humans.
In this study, we employed a method used in humans for assessing the physiological function of peripheral nerves, nerve conduction velocity (NCV). The peripheral nervous system is the main intermediary between the brain and peripheral tissues. Unlike the central nervous system, which is protected by bone and the blood brain barrier, the peripheral nervous system is more exposed to environmental toxins and can easily undergo trauma. Dysfunction in the peripheral nervous system can result from aging, injury, or disease, including infection and diabetes. Peripheral neuropathy is relatively common and affects 2%–3% of the population. Interestingly, this prevalence increases over threefold with age (1). In addition, peripheral nerve dysfunction is associated with skeletal muscle loss and sensory disorders, depending on the nerves affected. For example, motor nerve dysfunction can result in skeletal muscle atrophy in various neuromuscular diseases and sensory nerve dysfunction can result in altered nociception (2) or thermosensation (3). Oxidative stress, altered myelin protein and lipid composition and reduced cholesterol synthesis have been proposed to contribute to peripheral neuropathy associated with aging (4–6).
Nerve conduction assays are commonly used in humans to measure the functional status of both the neurons and myelin sheath and to diagnose diseases of both the nervous system and skeletal muscle, including amyotrophic lateral sclerosis and myasthenia gravis (7). NCV, one measurement derived from a nerve conduction study, is a property of both the axons and myelin sheath (8). Large, myelinated nerve fibers have faster NCV than small, unmyelinated nerves, and reduced NCV can arise from degeneration of the myelin sheath as well as axonal dysfunction. Therefore, NCV is a good measurement of overall peripheral nerve health. In humans, aging has been shown to be associated with reduced NCV in peripheral nerves (9–11) with the reduction occurring after 55 years of age in males and after 70 years of age in females (10). Peripheral nerve function is associated with changes in physical performance, including balance and walking speed in diabetic patients (12) and old adults (11) as well as low bone mineral density (13). Therefore, changes in nerve function have important implications for quality of life.
Nerve conduction studies are less invasive than many other measurements of healthspan and can be easily incorporated into longitudinal studies. Because NCV is sensitive to both myelin sheath and axonal changes, it is an excellent measure of the function of the peripheral nervous system. While performing the nerve conduction assay, action potential amplitude can also be measured, which gives insight into axonal loss. The summation of the amplitudes of muscle fiber action potentials, known as a compound muscle action potential (CMAP) and sensory neuron action potential (SNAP) amplitudes can also be derived from the nerve conduction assay. These measurements are indicative of axonal atrophy, and both CMAP and SNAP decline during aging in humans (9). Although NCV is associated with a number of age-associated conditions [eg diabetes (11), central nervous system dysfunction (10), inflammation (14,15) and oxidative stress (4,6)], there is very little information on the age-associated changes in NCV in mice. In this study, we characterize the effect of age on NCV in male and female mice fed ad libitum over a wide range of ages. In addition, we assess the effect of dietary restriction (DR) on NCV. Our data show that NCV declines significantly after 20 months of age in male and female mice and that some age-related changes in NCV are prevented in female mice, similar to what has been observed in humans. Therefore, NCV is a valuable parameter to measure when assessing the healthspan of mice.
Methods
Animals
Male and female C57Bl/6 mice were used for the initial characterization of the effect of age on NCV. Only female C57Bl/6 mice were used for the DR study. Mice used in the initial experiments at ages 4, 20, 28 and 32 months (n = 12–24) were obtained from the aging colony maintained by the National Institute on Aging. The mice in the DR study (12 and 30 months old; n = 6–16) were obtained from Jackson Labs and were fed either ad libitum or restricted by 40% relative to ad-libitum fed mice as previously described (16). The DR regimen was started at 4 months of age. Mice were multiply housed (4–5/cage) under specific pathogen free conditions on a 12-hour light/dark cycle. All animal protocols were approved by the University of Texas Health Science Center at San Antonio IACUC.
Nerve Conduction Studies
To measure NCV, mice were anesthetized with constant flow of isoflurane, and external body temperature was maintained at 34 °C with a heating lamp. Stainless steel subdermal needle electrodes were used to deliver supramaximal stimulation with 0.02 millisecond impulses using the Nicolet Viking Quest portable EMG apparatus (CareFusion, San Diego, CA) as previously described (6). Low frequency filters were set to 1 Hz and high frequency filters were set to 10kHz. These settings were used for all measurements. We measured NCV in four nerves; sensory and motor nerves of the tail and sensory (sural) and motor (sciatic) nerves in the hind limb. For tail measurements, stimulating electrodes and recording electrodes were inserted in the tail 3cm apart. The take-off latency of the tail motor action potential was measured by stimulating proximally and recording distally, and the tail sensory NCV was measured by stimulating distally and recording proximally. Tail distal motor latency (TDML) represents the time it takes for an electrical impulse to travel 3cm along the tail motor nerve, while all other measurements are velocities (distance/latency). Thus, TDML includes the time it takes an electrical impulse to travel down the nerve, across the neuromuscular junction and elicit a CMAP. Sural NCV was measured by stimulating at the ankle and recording over the fourth and fifth digit dorsally. For sciatic NCV, initial-ankle foot latency was measured using stimulating electrodes placed at the ankle and recording electrodes were placed dorsally over all five digits. The latency and distance between electrodes was measured, and then the stimulating electrodes were moved to the sciatic notch. The nerve was again stimulated and the resulting latency was subtracted from the initial ankle–foot latency. This difference was divided between the distance between the notch and ankle to determine velocity. The distance was determined by stretching the foot so that a linear distance could be measured between stimulating and recording electrodes. An image of the needle placement for sciatic NCV has been previously published (17), and video recording of similar methods are also available (18). All procedures were performed according to the methods outlined by the Animal Models of Diabetic Complications Consortium. CMAP or SNAP amplitudes were measured for each evoked response with electrode placement identical to the NCV measurements. Thus, the caudal and plantar muscles contribute to the tail and sciatic CMAP, respectively. Figure 1A shows a typical CMAP used to measure motor NCV; latency is the time it takes to activate the rising phase and is used to calculate NCV while amplitude is the height of the peak. To determine if NCV was reproducible in the same mice over a short timeframe, we measured sciatic NCV at baseline and again one week later. As shown in Figure 1B, variation from repeated analysis showed little change (an average of 3%) over a short timeframe.
Figure 1.
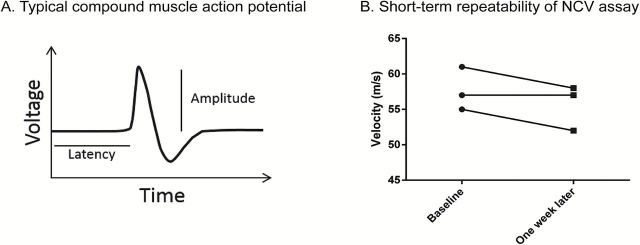
(A) Typical compound muscle action potential generated that is used to measure motor nerve conduction velocity (NCV). Latency is the time it takes to initiate the rising phase. Latency and distance traveled are used to calculate nerve conduction velocity. Amplitude is the distance from the baseline to the peak. (B) Sciatic NCV was measured in three mice at baseline and again one week later (No significant change was detected; p > .05 by paired t-test).
Statistical Analysis
Data were analyzed using IBM SPSS Statistics. Two-way ANOVA was used to determine the effect of age and sex or age and diet. Tukey’s post-test was used to for multiple comparisons. Statistical significance was considered when p < .05. Pairwise comparisons (α < 0.05) with Bonferroni correction for multiple comparisons was used to determine significant effect of sex at specific ages.
Results
Effect of Sex on NCV
We measured NCV in male and female C57Bl/6 mice from four age groups: 4, 20, 28, and 32 months of age. Reference values for means, ranges and confidence intervals are reported for NCV in Supplementary Table 1 and CMAP and SNAP amplitude in Supplementary Table 2. We determined the effect of sex and age on NCV using two-way ANOVA. Significant sex differences were detected in sensory nerve conduction measurements. Pairwise analysis revealed that female tail sensory NCV was slower than male tail sensory NCV at 4, 20, and 28 months of age and that female sural NCV was slower than male sural NCV at all ages. Although the sex factor was not significant in TDML or sciatic NCV, an age–sex interaction factor was significant or trended to significance in these motor NCV (TDML p < .01, sciatic NCV p = .062). Pairwise analysis revealed that females had lower TDML at 28 and 32 months of age relative to male mice and faster sciatic NCV at 28 months of age, suggesting females are partially protected from the age-related decline in NCV in motor nerves. We analyzed the effect of age on NCV and CMAP/SNAP in male and female mice in the following sections.
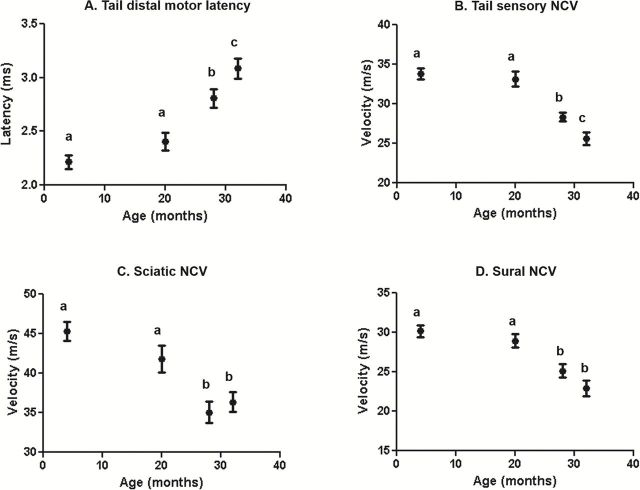
Effect of Age on Nerve Conduction Velocity in Male Mice
The mean life span reported for male and female C57BL/6 mice in the NIA colony is 26.2 and 24.8 months, respectively. At 28 months of age, 55% of males and 70% of females have died, and at 32 months of age 87% of males and 91% of females have died (19). Figure 2 shows the NCV in motor and sensory nerves in the tail and hind limb of male C57BL/6 mice. The distal motor latency (TDML), which is the time it takes for an impulse to travel a specific length of the nerve, showed no significant difference in 4- and 20-month-old mice. However, TDML increased significantly in 28-month-old mice compared to 4- and 20-month old mice (27% and 16%, respectively) and continued to increase significantly (11%) from 28 to 32 months of age (Figure 2A). The same pattern was seen with the tail sensory nerve conduction velocity (TSNCV). TSNCV was slower in 28-month-old mice when compared to 4- and 20-month-old mice (16% and 15%, respectively), and a further 10% reduction was observed in 32-month-old mice relative to 28-month-old mice (Figure 2B).
Figure 2.
Effect of age on nerve conduction velocity (NCV) in male C57Bl/6 mice. The motor latency (A) and sensory nerve conduction velocity (B) for the tail and the sciatic nerve conduction velocity (C), and sural nerve conduction velocity (D) is shown. Data were obtained with 12–14 mice per age group and expressed as the mean with the standard error. Tukey’s post-test was used to determine significant differences between age groups after two-way ANOVA determined a significant effect of age. Values that are statistically different are denoted with different letters.
Next, we measured NCV in the nerves that innervate the hind limbs. Again, no significant difference was found between 4 and 20 months of age in sciatic NCV. However, sciatic NCV was significantly slower in 28- and 32-month-old mice compared to 4-month-old mice (23% and 20%, respectively) and 20-month-old mice (by 16% and 13%, respectively). Furthermore, a significant difference was observed between 28 and 32 months of age (Figure 2C). Similarly, sural NCV was slower in 28- and 32-month-old mice relative to 4- and 20-month-old mice (by 17% and 13% in 28-month-old mice, respectively, and 24% and 21% in 32-month-old mice, respectively), and no further reduction in NCV was observed after 28 months (Figure 2D).
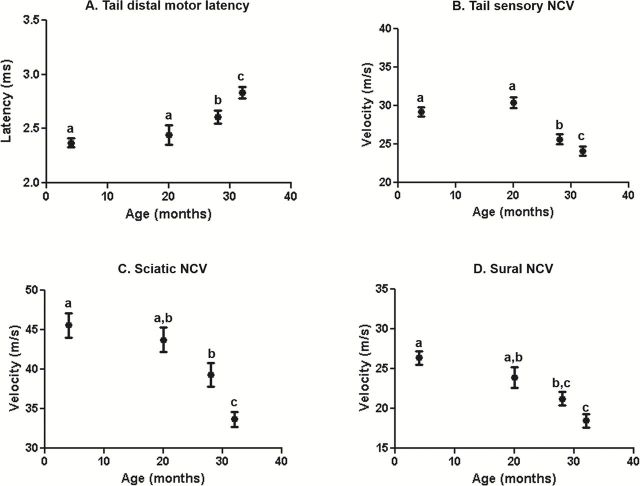
Effect of Aging on the Nerve Conduction Velocity of Female Mice
Figure 3A and B show NCV in tail motor and sensory nerves of female C57BL/6 mice. TDML was not altered between 4 and 20 months of age; however it was significantly slower in the 28- to 32-month-old mice compared with the 4- and 20-month-old mice. Additionally, there was a significant increase in latency in 32-month-old group compared to the 28-month-old female mice (Figure 3A). No change in TSNCV was detected between 4 and 20 months of age, but 28- and 32-month-old female mice had significantly slower NCV compared with 4- and 20-month-old female mice and 32 month-old females shows reduced NCV compared with 28-month-old females (Figure 3B). Figure 3C and D shows hind limb NCV in the sciatic and sural nerves of female C57BL/6 mice. No change was observed in sciatic NCV between 4 and 20 months of age. However, there was a significant difference in sciatic NCV in 28- and 32-month-old mice compared with 4-month-old mice. While sciatic NCV did not differ between 20- and 28-month-old mice, there was a significant difference between these two age groups and 32-month-old mice (Figure 3C). Sural NCV was significantly slower in 32-month-old female mice compared with 4- and 20-month-old females (Figure 3D).
Figure 3.
Effect of age on nerve conduction velocity (NCV) in female mice. The tail motor latency (A), tail sensory nerve conduction velocity (B), sciatic nerve conduction velocity (C), and sural nerve conduction velocity (D) is shown for female C57Bl/6 mice. Data were obtained with 18–24 mice per age and expressed as the mean with the standard error. Tukey’s post-test was used to determine significant differences between age groups after two-way ANOVA determined a significant effect of age. Values that are statistically different are denoted with different letters.
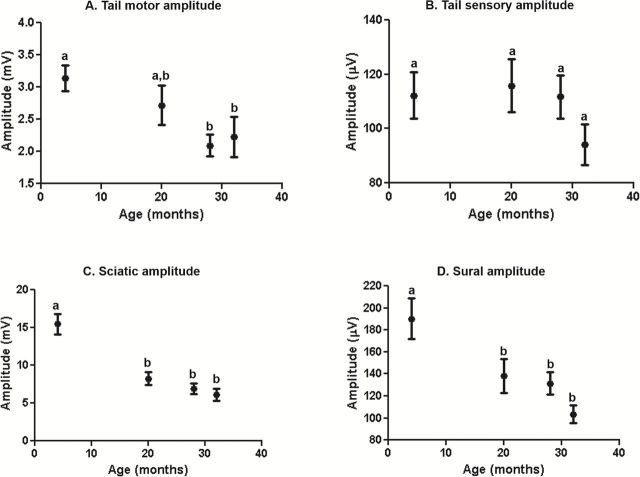
We also measured CMAP and SNAP amplitude in female mice (Figure 4A). CMAP is the summation of individual motor unit action potentials, and is indicative of axonal integrity. Tail motor CMAP was similar for 4- and 20-month-old female mice and was significantly lower in the 28- and 32-month-old female mice compared to the 4-month-old mice. SNAP amplitude in the tail remained constant over the life span of the female mice (Figure 4B). We measured CMAPs during the ankle-foot measurement of the sciatic NCV and found a significant decrease in CMAP in the 20-, 28-, and 32-month-old groups relative to the 4-month-old group (Figure 4C). Interestingly, the SNAP amplitude of the sural nerve was significantly reduced by 20 months of age in the female mice (Figure 4D), suggesting loss of amplitude precedes the reduction in NCV.
Figure 4.
Effect of age on CMAP and SNAP amplitude in female mice. The tail motor amplitude (A), tail sensory amplitude (B), sciatic CMAP amplitude (C), and sural nerve amplitude (D) is shown for female C57Bl/6 mice. Data were obtained with 18–24 mice per age and expressed as the mean with the standard error. Tukey’s post-test was used to determine significant differences between age groups after two-way ANOVA determined a significant effect of age. Values that are statistically different are denoted with different letters.
Effect of Age and Dietary Restriction on NCV
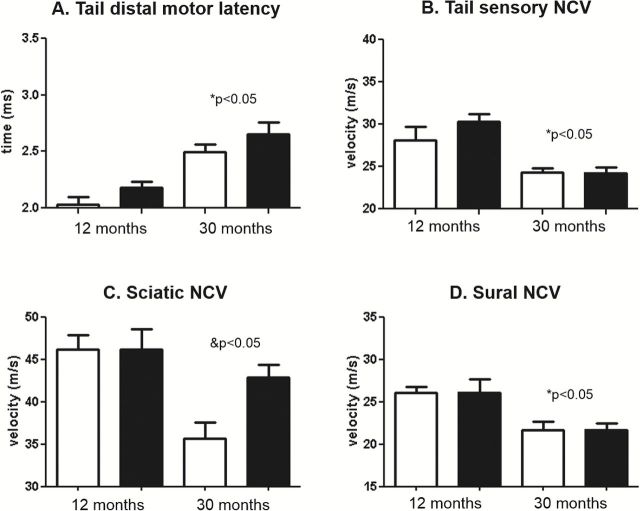
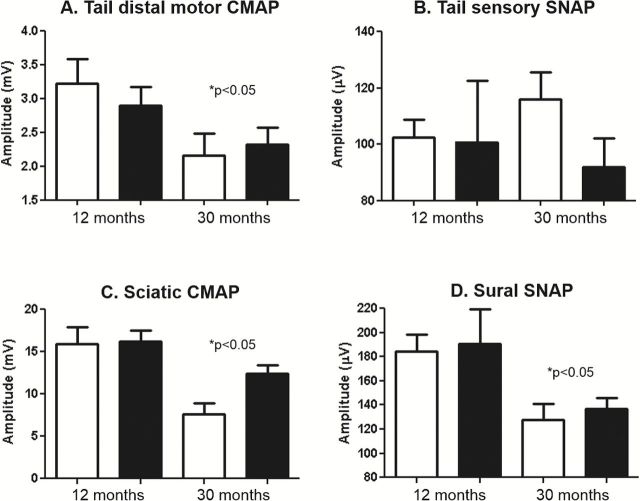
Dietary restriction (DR) is the most studied manipulation to increases life span in rodents, and DR has been shown to delay or prevent most biochemical and physiological processes that change with age, including alterations in the sciatic nerve (20). Therefore, it was of interest to determine if DR would prevent the functional decline in the peripheral nervous system during aging. We measured NCV for the motor and sensory nerves of tail and hind limb at 12- and 30-months of age in female mice fed ad libitum or a DR diet. As shown in Figure 5, we found similar age-related changes in NCV in tail and hind limb in the female mice that were housed at our institution as found in Figure 3 for mice from the NIA aging colony. Significant effects of age were found for all NCV measurements. We were surprised to find that DR had no effect on tail motor latency, tail sensory, or sural NCV either at 12 or 30 months of age, ie DR did not prevent the age-related decline in NCV in these nerves. However, a significant age*diet interaction was found for sciatic NCV, and post-test analysis revealed that DR partially prevented the age-related decline in sciatic NCV (Figure 5C). As shown in Figure 6, we also measured CMAP and SNAP amplitude in the 12- and 30-month-old female mice fed ad libitum or a DR diet. Significant effect of age was found in all measurements except tail SNAP amplitude. No effect of diet was found for CMAP or SNAP amplitude, although we did notice a trend for sciatic CMAP amplitude (p = 0.85).
Figure 5.
Effect of age and dietary restriction on nerve conduction velocity (NCV) in female mice. The tail distal motor latency (A), tail sensory nerve conduction velocity (B), sciatic nerve conduction velocity (C), and sural nerve conduction velocity (D) is shown for female C57Bl/6 mice at 12 and 30 months of age fed ad libitum (white bars) or a DR diet (black bars). Data were obtained with 6–16 mice per group and expressed as means with standard error. An age–diet interaction was found to be significant for sciatic NCV (&p < .05) and post-tests revealed a significant decline in aged ad-libitum fed, but not DR, mice relative to diet-matched young. A significant effect of age (*p < .05) was found for all other measurements.
Figure 6.
Effect of age and dietary restriction on compound muscle action potential (CMAP) and sensory neuron action potential (SNAP) amplitude in female. The tail motor CMAP amplitude (A), tail sensory amplitude (B), sciatic CMAP amplitude (C), and sural nerve amplitude (D) is shown for female C57Bl/6 mice at 12 and 30 months of age fed ad libitum (white bars) or a DR diet (black bars). Data were obtained with 6–16 mice per group and expressed as means with standard error. A significant effect of age (*p < .05) was found for all measurements.
Discussion
NCV is an excellent measure of nerve function because it is relatively uninvasive, reproducible, can be used longitudinally, and is commonly used in humans to assess nerve function and diagnose presence of neuromuscular disease, myopathies, sensory disorders or trauma (7). Therefore, NCV is a measurement of functional status of nerves that can be used as part of a battery of assays to assess the healthspan of mice and these measures are directly applicable to humans. A recent study of 1,200 men and women aged 21–96 years showed that motor NCV in the peroneal nerve decline with age (10). Although nerve function is commonly measured in humans by NCV, this measure has been used to a limited extent in rodents, primarily on the effect of diabetes and peripheral nerve disease on NCV (11,12). Previous studies have not found an age-related decline in NCV in mice when looking at middle-aged mice (21), but our study confirms a recent study that found reduced NCV in late life (22). Thus, it is important to determine functional measurements later in life. Mice have become the primary mammalian model for aging research because of their relatively short life span and the ability to use genetic, pharmacological, and nutritional interventions; therefore, we evaluated the usefulness of NCV in assessing nerve function as one measure of healthspan. We studied both male and female mice over a wide range of ages and tested multiple nerves to determine if changes were sex-dependent, the ages that showed the greatest change, and whether polyneuropathy occurred during aging. We specifically chose to study NCV over the entire life span of the mouse, especially later in life, because of its relevance to human quality of life.
Age and height are significant contributors to variation in NCV, and while sex does not affect NCV in healthy, young humans (9), the rate of decline with age is significantly slower in females than in males (10). We showed that NCV was similar between 4 and 20 months of age, but decreased after 20 months of age in both tail and hind limb nerves of male and female mice. NCV continued to decrease late in life in some nerves. We also found reduced amplitude with age in both tail and hind limb motor CMAP, suggesting either a loss of nerve fibers or failure of muscle fiber recruitment. Interestingly, hind limb CMAP amplitude had declined by 20 months, while sciatic NCV was not reduced until 28 months in females, suggesting axonal atrophy precedes the decline in NCV in females. We also found that female mice have slower sensory NCV at all ages and experience a slower age-related decline in motor NCV compared to males. This observation is consistent with a recent study demonstrating that women are protected from the age-related decline in NCV (10). The physiological and molecular processes that confer this protection are prime targets for future studies, and these mechanisms may be useful in designing novel treatments for peripheral nerve diseases.
The loss of peripheral nerve function during aging can affect the function of its target organs. For example, dysfunction of motor neurons is associated with muscle atrophy. Sarcopenia is the age-related loss of muscle mass and is observed in many animal models, including mice. In humans, approximately 1% of lean mass is lost each year after 50 years of age (23), and muscle strength declines at approximately three times that rate (24). Diabetic patients with peripheral neuropathy are more likely to have muscle atrophy (25), suggesting peripheral nerves are key contributors to muscle strength. While NCV has been shown to contribute to muscle strength in humans independent of the effect of age (26), the relationship between decreased NCV and loss of skeletal muscle during aging is not clear. Another study found that CMAP amplitude, but not NCV, is associated with muscle mass in both men and women, suggesting motor neuron number may be important for predicting sarcopenia in humans (27). Similarly, declines in sensory nerve NCV might contribute to declines in the auditory, visual, and vestibular systems as well as nociception and thermosensation in older adults. Sensory NCV declines with age in humans (28), and in addition to the well-known decline in auditory and visual function with age, there are reports of reduced function of other sensory systems in older adults (29–33). Similar to muscle function, the decline in sensory function during aging may be due to peripheral nerve dysfunction (34,35). Given the importance of sensory function in health and quality of life for humans, the relationship between age-related peripheral nerve dysfunction and sensory decline is a critical field for further research.
DR extends life span in mice as well as most animal models studied and delays numerous age-related pathologies and improves many physiological functions that decline with age. It has been argued that DR increases life span by delaying aging. DR prevents cellular and molecular changes in peripheral nerves and prevents muscle atrophy and neuromuscular junction abnormalities during aging. For example, lifelong DR prevents age-related structural changes and oxidative stress in the sciatic nerve (4,20). DR also prevents alterations at the neuromuscular junction and preserves muscle mass during aging (16,36). Thus, we hypothesized DR would prevent age-related changes in NCV. While DR partially prevented the age-related decline in NCV, it had no effect on the age-related decline in NCV and CMAP amplitude in tail motor and sciatic nerves and SNAP amplitude in tail sensory and sural nerves. Although DR has been shown to improve a large number of physiological functions that are altered with age, there are reports showing that some functions that change with age are not preserved by DR. For example, DR mice are equally susceptible to influenza as ad-libitum fed mice and do not respond properly to wound healing (37,38). Thus, it is important to assess the effect of manipulations that increase life span on a number of physiological functions rather than assuming that a manipulation that increases life span will improve all aspects of healthspan.
In addition to showing a decline in NCV during aging, our data give us some insight into potential mechanisms involved in the age-related decline in peripheral nerves during aging. NCV is the speed at which an action potential is propagated along an axon, and NCV is dependent on a number of features, including temperature, axon diameter and myelination. Our data are consistent with a loss of myelination in peripheral nerves during aging. Structural changes in peripheral with age are well documented in humans (26), cats (39), and rats (40) and include loss of axons and demyelination (41,42). Demyelination of peripheral nerves can cause nerve dysfunction by impairing conduction of electrical signals along the nerve. Myelinated fibers in particular are susceptible to age-related alterations (43), and indications of remyelination and regeneration are often seen in peripheral nerves during aging (5,44). Consistent with reductions in NCV during aging, the number of regenerating myelinated fibers in the sciatic nerve is reduced at 24 months of age in C57Bl/6 mice (45), and myelin thickness in the sciatic nerve is reduced in large fibers at 28 months of age in C57Bl/6 mice (46). We observed an age-related decline in sciatic NCV at 28, but not 20, months of age relative to 4-month-old mice. Thus, the period between 20 and 30 months of age is a critical time frame for age-related changes in peripheral nerves. Furthermore, C57Bl/6 mice were reported to have higher levels of peripheral neuropathy at 24 months of age compared to other strains (47), suggesting that C57Bl/6 mice are an appropriate model for the peripheral nerve changes seen in humans.
Axonal vacuoles, collagen accumulation, macrophage infiltration and onion bulbs are some of the structural changes commonly observed in peripheral nerves during aging (41,44). Furthermore, nerve fiber loss may occur during aging (5,41). We found reduced CMAP and SNAP amplitude in aged mice, suggesting axonal atrophy occurs during aging, a finding consistent with data from humans (48). Interventions that prevent demyelination, repair myelin or prevent motor neuron death could prevent age-related peripheral nerve dysfunction and contribute to healthspan during aging. Demyelination and axonal loss need to be comprehensively studied at the molecular level to determine the mechanism behind the declining NCV during aging.
Supplementary Data
Supplementary material can be found at http://biomedgerontology.oxfordjournals.org/
Funding
This study was funded as part of a grant from NIA to the San Antonio Nathan Shock Aging Center to study healthspan in mice (1P30-AG13319) and VA Merit Grants to H.V.R. and A.R.
Supplementary Material
Acknowledgments
We thank Alex Bokov for his assistance in interpreting statistical analyses.
References
- 1. Hughes RA. Peripheral neuropathy. BMJ. 2002;324:466–469. 10.1136/bmj.324.7335.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hadley SH, Bahia PK, Taylor-Clark TE. Sensory nerve terminal mitochondrial dysfunction induces hyperexcitability in airway nociceptors via protein kinase C. Mol Pharmacol. 2014;85:839–848. 10.1124/mol.113.091272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yezierski RP, King CD, Morgan D, Carter CS, Vierck CJ. Effects of age on thermal sensitivity in the rat. J Gerontol A Biol Sci Med Sci. 2010;65:353–362. 10.1093/gerona/glq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 2010;13:65–74. 10.1089/rej.2009.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. 10.1111/j.1529-8027.2000.00026.x [DOI] [PubMed] [Google Scholar]
- 6. Hamilton R, Bhattacharya A, Walsh M, et al. Elevated protein carbonylation, and misfolding in sciatic nerve from db/db and Sod1(-/-) mice: plausible link between oxidative stress and demyelination. PLoS One. 2013;8:e65725. 10.1371/journal.pone.0065725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson E. Clinical value of motor nerve conduction velocity determination. J Am Med Assoc. 1960;172:2030–2035. 10.1001/jama.1960.03020180040007 [DOI] [PubMed] [Google Scholar]
- 8. Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. 10.1002/mus.880030207 [DOI] [PubMed] [Google Scholar]
- 9. Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24:1134–1141. 10.1002/mus.1124 [DOI] [PubMed] [Google Scholar]
- 10. Di Iorio A, Cherubini A, Volpato S, et al. Markers of inflammation, vitamin E and peripheral nervous system function: the InCHIANTI study. Neurobiol Aging. 2006;27:1280–1288. 10.1016/j.neurobiolaging.2005.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85+ years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2001;56. 10.1093/gerona/56.1.M25 [DOI] [PubMed] [Google Scholar]
- 12. Strotmeyer E, de Rekeneire N, Schwartz A, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008;31:1767–1772. 10.2337/dc08-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strotmeyer E, Cauley J, Schwartz A, et al. ; Health ABC Study. Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2006;21:1803–1810. 10.1359/jbmr.060725 [DOI] [PubMed] [Google Scholar]
- 14. Djouhri L, Dawbarn D, Robertson A, Newton R, Lawson SN. Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci. 2001;21:8722–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Djouhri L, Lawson SN. Increased conduction velocity of nociceptive primary afferent neurons during unilateral hindlimb inflammation in the anaesthetised guinea-pig. Neuroscience. 2001;102:669–679. 10.1016/S0306-4522(00)00503-0 [DOI] [PubMed] [Google Scholar]
- 16. Jang Y, Lustgarten M, Liu Y, Van Remmen H. Calorie Restriction (CR) Protects against Oxidative Stress-induced Muscle Atrophy by Preserving Mitochondrial Function and Muscle Integrity Even in the Absence of Antioxidant Enzyme CuZnSOD. Free Radical Biol Med . 2010;49:S81. 10.1016/j.freeradbiomed.2010.10.203 [Google Scholar]
- 17. Oh S, Hayes J, Sims-Robinson C, Sullivan K, Feldman E. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett. 2010;483:127–131. 10.1016/j.neulet.2010.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz A, Walther C, Morrison H, Bauer R. In vivo electrophysiological measurements on mouse sciatic nerves. J Vis Exp. 2014;e51181. 10.3791/51181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:B379–B389. 10.1093/gerona/57.11.b379 [DOI] [PubMed] [Google Scholar]
- 20. Rangaraju S, Hankins D, Madorsky I, et al. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8:178–191. 10.1111/j.1474-9726.2009.00460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verdú E, Butí M, Navarro X. Functional changes of the peripheral nervous system with aging in the mouse. Neurobiol Aging. 1996;17:73–77. 10.1016/0197-4580(95)02010-1 [DOI] [PubMed] [Google Scholar]
- 22. Sims-Robinson C, Hur J, Hayes J, et al. The role of oxidative stress in nervous system aging. PLoS One. 2013;8:e68011. 10.1371/journal.pone.0068011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56. 10.1093/gerona/56.5.B198 [DOI] [PubMed] [Google Scholar]
- 24. Goodpaster B, Park S, Harris T, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 25. Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–2385. 10.2337/diacare.27.10.2382 [DOI] [PubMed] [Google Scholar]
- 26. Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano). 1998;10:471–478. 10.1007/ BF03340161 [DOI] [PubMed] [Google Scholar]
- 27. Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–1154. 10.1016/j.neurobiolaging.2005.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Werner RA, Franzblau A, D’Arcy HJ, Evanoff BA, Tong HC. Differential aging of median and ulnar sensory nerve parameters. Muscle Nerve. 2012;45:60–64. 10.1002/mus.22233 [DOI] [PubMed] [Google Scholar]
- 29. Peiffer A, Hugenschmidt C, Maldjian J, et al. Aging and the interaction of sensory cortical function and structure. Hum Brain Mapp. 2009;30:228–240. 10.1002/hbm.20497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poole J. Age Related Changes in Sensory System Dynamics Related to Balance. Phys Occup Ther Geriatr. 1992;10:55–66. 10.1080/j148v10n02_04 [Google Scholar]
- 31. Aviv JE, Martin JH, Jones ME, et al. Age-related changes in pharyngeal and supraglottic sensation. Ann Otol Rhinol Laryngol. 1994;103:749–752. [DOI] [PubMed] [Google Scholar]
- 32. Gibson S, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. [DOI] [PubMed] [Google Scholar]
- 33. Kaneda H, Maeshima K, Goto N, Kobayakawa T, Ayabe-Kanamura S, Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem Senses. 2000;25:331–337. 10.1093/chemse/25.3.331 [DOI] [PubMed] [Google Scholar]
- 34. Shaffer S, Harrison A. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87:193–207. 10.2522/ptj.20060083 [DOI] [PubMed] [Google Scholar]
- 35. Richardson J. The clinical identification of peripheral neuropathy among older persons. Arch Phys Med Rehabil. 2002;83:1553–1558. 10.1053/apmr.2002.35656 [DOI] [PubMed] [Google Scholar]
- 36. Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. 10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gardner E. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–694. 10.1093/gerona/60.6.688 [DOI] [PubMed] [Google Scholar]
- 38. Reed MJ, Penn PE, Li Y, et al. Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice. Mech Ageing Dev. 1996;89:21–43. 10.1016/0047-6374(96)01737-X [DOI] [PubMed] [Google Scholar]
- 39. Adinolfi AM, Yamuy J, Morales FR, Chase MH. Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging. 1991;12:175–179. 10.1016/0197-4580(91)90058-R [DOI] [PubMed] [Google Scholar]
- 40. Schmelzer J, Low P. Electrophysiological studies on the effect of age on caudal nerve of the rat. Exp Neurol. 1987;96:612–620. 10.1016/0014-4886(87)90223-8 [DOI] [PubMed] [Google Scholar]
- 41. Ceballos D, Cuadras J, Verdú E, Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat. 1999;195 (Pt 4):563–576. 10.1046/j.1469-7580.1999.19540563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108 (Pt 4):897–924. 10.1093/brain/108.4.897 [DOI] [PubMed] [Google Scholar]
- 43. Sato A, Sato Y, Suzuki H. Aging effects on conduction velocities of myelinated and unmyelinated fibers of peripheral nerves. Neurosci Lett. 1985;53:15–20. 10.1016/0304-3940(85)90090-4 [DOI] [PubMed] [Google Scholar]
- 44. Thomas PK, King RH, Sharma AK. Changes with age in the peripheral nerves of the rat. An ultrastructural study. Acta Neuropathol. 1980;52: 1–6. 10.1007/bf00687222 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka K., de F. Myelinated fiber regeneration after crush injury is retarded in sciatic nerves of aging mice. J Comp Neurol. 1991;308:180–187. 10.1002/cne.903080205 [DOI] [PubMed] [Google Scholar]
- 46. Verdier V, Csárdi G, de Preux-Charles AS, et al. Aging of myelinating glial cells predominantly affects lipid metabolism and immune response pathways. Glia. 2012;60:751–760. 10.1002/glia.22305 [DOI] [PubMed] [Google Scholar]
- 47. Tabata H, Ikegami H, Kariya K. A parallel comparison of age-related peripheral nerve changes in three different strains of mice. Exp Anim. 2000;49:295–299. [DOI] [PubMed] [Google Scholar]
- 48. Kurokawa K, Mimori Y, Tanaka E, Kohriyama T, Nakamura S. Age-related change in peripheral nerve conduction: compound muscle action potential duration and dispersion. Gerontology. 1999;45:168–173. 10.1159/000022081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.