Abstract
Background.
Few studies have examined patterns of health care utilization and costs during the period around incident dementia.
Methods.
Participants were drawn from the Washington Heights-Inwood Columbia Aging Project, a multiethnic, population-based, prospective study of cognitive aging of Medicare beneficiaries in a geographically defined area of northern Manhattan. Medicare utilization and expenditure were examined in individuals with clinically diagnosed dementia from 2 years before until 2 years after the initial diagnosis. A sample of non-demented individuals who were matched on socio-demographic and clinical characteristics at study enrollment was used as controls. Multivariable regression analysis estimated effects on Medicare utilization and expenditures associated with incident dementia.
Results.
During the 2 years before incident dementia, rates of inpatient admissions and outpatient visits were similar between dementia patients and non-demented controls, but use of home health and skilled nursing care and durable medical equipment were already higher in dementia patients. Results showed a small but significant excess increase associated with incident dementia in inpatient admissions but not in other areas of care. In the 2 years before incident dementia, total Medicare expenditures were already higher in dementia patients than in non-demented controls. But we found no excess increases in Medicare expenditures associated with incident dementia.
Conclusions.
Demand for medical care already is increasing and costs are higher at the time of incident dementia. There was a small but significant excess risk of inpatient admission associated with incident dementia.
Key Words: Incident dementia, Medicare, Health care use, Health care expenditures, Longitudinal follow-up
The disproportionately high cost of caring for patients with dementia has been extensively documented. As the prevalence of dementia increases from 4.7 million in the United States in 2010 to an expected 13.8 million by 2050, the associated costs of care are projected to rise from $203 billion to $1.2 trillion (1). To establish a comprehensive understanding of health care use and expenditures associated with dementia, an outline of health care utilization and costs during the incident period is critical. Deficits in cognition, and to a lesser extent, deficits in function, and neuropsychiatric symptoms that occur prior to a diagnosis of dementia have been documented in a number of studies (2–5). However, patterns of health care utilization during this period when individuals are progressing to dementia have received little attention (4,6–10).
With few exceptions, most studies have relied on diagnostic codes or medication use in claims data to identify individuals with dementia (8–15). This method of case identification often underestimates the presence of dementia and may exclude mild cases of dementia (14,16–20). This method may be particularly problematic in identifying incident dementia. When a dementia diagnosis first appears in the claims data, the incident window may already have been missed by some time. Partly because of the difficulties in identifying dementia cases using administrative claims, analyses of health care use around the time of dementia diagnosis have been inconsistent (6–9,11).
In this study, we aim to provide a better understanding of changes in health care utilization and costs during the time individuals progress to dementia. We utilize an epidemiologic study of cognitive aging in a cohort of Medicare beneficiaries for whom diagnoses of dementia and incident dates were clinically determined, with data on health care utilization separately obtained from Medicare claims. To explore changes in health care use and expenditures associated with incident dementia, we compared health care use and expenditures of those with dementia before and after incident date to a matched comparison sample of non-demented subjects. We hypothesized that regardless of dementia onset, Medicare use and expenditures would increase over time, but independent of other characteristics, there would be excess Medicare use and expenditures associated with incident dementia.
Methods
Participants
Participants were from the Washington Heights-Inwood Columbia Aging Project, a multiethnic, population-based, prospective study of cognitive aging of Medicare beneficiaries aged 65 and older residing in a geographically defined area of northern Manhattan. Lists of all Medicare or Medicaid recipients in the study area were obtained from the Health Care Financing Administration (now Centers for Medicare and Medicaid Services). Potential subjects were then drawn by stratified random sampling into one of six strata based on age (65–74, 75+) and ethnicity (Hispanics, non-Hispanic blacks, non-Hispanic whites based on self-report using 1990U.S. census format). All persons were sent a letter from Health Care Financing Administration explaining that they had been selected to participate in a study of aging by investigators at Columbia University. A total of 3,756 participants were followed in two (1992, 1999) cohorts using similar methods. Detailed descriptions of study methodology have been reported previously (21).
At the time of study entry, each participant underwent an in-person interview of general health and functional ability, followed by a standardized assessment including medical history, physical and neurological examination, and a neuropsychological battery. After baseline assessment, participants were followed at approximately 18-month intervals with similar assessments. Evaluations were conducted in either English or Spanish, based on the primary language or preference of the participant. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia Presbyterian Medical Center and Columbia University Health Sciences, the New York State Psychiatric Institute, and the Centers for Medicare and Medicaid Services Privacy Board. Written informed consent was obtained from all participants.
Identification of Incident Dementia
At baseline and each follow-up, diagnostic conferences were held by a group of neurologists, psychiatrists, and neuropsychologists using results from the neuropsychological battery as well as evidence of impairment in social or occupational function (22,23). A diagnosis of dementia was determined based on the Diagnostic and Statistical Manual of Mental disorders, Revised Fourth Edition criteria. The type of dementia was subsequently determined. Diagnosis of probable or possible Alzheimer’s disease was made based on criteria outlined by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association. Date when dementia was first diagnosed was recorded. To account for the time between the first date of dementia diagnosis and the last assessment date when the participant was considered cognitively normal, we used the midpoint between these two dates as the incident date for the current study. Participants who were never diagnosed with dementia during the study were considered non-demented cases. Because of the epidemiologic nature of the study, neither participants nor their primary care providers were notified of a research diagnosis of dementia.
Derivation of the Study Sample
Of the 3,756 participants who were followed, 2,476 were matched to the Medicare Beneficiary Summary file using social security number and Medicare beneficiary ID, and were identified to be enrolled in Medicare Fee-for-Service for 6 months or more during each year. The study sample included 2,383 individuals followed from their first Washington Heights-Inwood Columbia Aging Project visit or the beginning of Medicare data availability (January 1, 1999) and ended at death or end of study follow-up (December 31, 2010). Of these participants, 242 were incident cases and 1,805 were non-demented cases. Participants who were diagnosed with dementia at study enrollment were excluded.
Dementia severity at the time of first diagnosis was measured by the Clinical Dementia Rating (CDR) (24). 195 (80.6%) had CDR = 1, 32 (13.2%) had CDR = 2, and 15 (6.2%) had CDR ≥ 3. The current analysis included 227 participants with CDR = 1 or 2 at the time of first diagnosis.
Matching
Because characteristics that may have confounding effects on Medicare utilization and expenditures may differ substantially between incident and non-demented groups, we matched the participants without dementia to the participants with incident dementia at study enrollment using 1:1 matching with greedy matching algorithms. We used 1:1 matching to reduce biases that may arise from many-to-one matching (25,26). Variables used for matching include enrollment year, age, gender, race/ethnicity, education, Medicaid eligibility, comorbidities (measured by Charlson comorbidity index) (27), years of follow-up, and whether the participant died during the study. Participants were not matched on function (measured by Blessed Dementia Rating scale) (28) or cognition (measured by a global cognitive z-score) (29). To keep observation period as close as possible between incident and non-demented groups, we assigned the incident date of the participant with dementia as the index date for the matched non-demented participant.
Medicare Utilization and Expenditures Data
Medicare utilization and expenditures data were obtained from Medicare Standard Analytic Files. The following Medicare services were examined: inpatient care, outpatient care (including all care provided in ambulatory and hospital outpatient settings), durable medical equipment (DME), and because of low use of skilled nursing care, it was combined with home health care. There was no hospice use during the study period. Expenditures reflect actual payments from Medicare for all covered services. Medicare utilization and expenditure by quarter were computed for 2 years before and 2 years after incident date. For services that span multiple quarters, expenditures were apportioned by the number of days that care was received in each quarter. All expenditures were adjusted to 2012$ using the medical care component of the consumer price index (30).
Analysis
We first compared socio-demographic and clinical characteristics of the two groups at incidence. Quarterly Medicare utilization and expenditures during the 2 years before and 2 years after incidence were then compared for participants with dementia and matched controls. The main outcome variables are indicators for use of each type of Medicare services and Medicare expenditure. We used random effects logistic regression models to estimate Medicare utilization. We examined the appropriateness of distributional family and link functions and chose generalized linear models with gamma family and log link to estimate Medicare expenditures. Sensitivity analyses performed using ordinary least squares yielded substantively similar results (not shown). The main independent variables are an indicator for group (dementia vs controls), which estimates differences between demented and control groups in outcome before incidence, and an indicator for post-incidence (vs pre-incidence), which estimates changes in outcome in the control group. An interaction term for group and post-incidence estimates the excess effect of incident dementia. Because interaction terms in logistic regression models do not have straightforward interpretations (eg, the estimated odds ratios on the interaction term in the logistic regression are ratio of odds ratios) (31,32), we interpreted the interaction effects in the logistic models through predicted probabilities. Models controlled for age at incidence, gender, race/ethnicity, education, Medicaid eligibility, number of comorbid conditions, and an indicator for death within 2 years of incidence. An indicator for year also was included to control for secular changes that may produce an appearance of change with age. Because participants were followed over time, cluster robust standard errors were reported. All analyses were performed using Stata 13.0.
Results
Sample Characteristics at Incidence
Socio-demographic and clinical characteristics of dementia and control participants are in Table 1. On average, participants with dementia were enrolled into the study at age 77.3±6.8, followed for 7.0±4.3 years before they were first diagnosed with dementia at age 84.4±6.2, and then followed for another 4.0±4.5 years afterwards; 71.3% of participants with dementia were women, 63.1% were Hispanic, with 7.6±4.8 years of education. A little over half (54.5%) was eligible for Medicaid, and 17.2% of participants died during the study. Socio-demographic characteristics of the matched non-demented participants were similar. Participants with incident dementia had an average of 1.9±2.0 comorbid conditions. Hypertension (76.3%), arthritis (68.1%), diabetes (31.3%), peripheral vascular diseases (28.8%), and stroke (23.7%) were common. While the total number of comorbid conditions was similar between dementia and control samples, fewer control participants had a stroke (23.7% vs 8.9%, p < .01). At incident date, activities of daily living functioning was similar between the two groups, but cognitive scores were lower in the incident group (−0.17±0.62 vs −0.52±0.59, p < .01).
Table 1.
Descriptive Characteristics of Participants with Incident and Non-dementia
| Variables | Incident Dementia | Non-demented |
|---|---|---|
| Age at study enrollment, mean (SD) | 77.3 (6.8) | 76.8 (6.8) |
| Age at incidence, mean (SD) | 84.4 (6.2) | 83.3 (7.5) |
| Years from enrollment to incidence, mean (SD) | 7.0 (4.3) | 6.4 (5.5) |
| Years of follow-up from incidence, mean (SD) | 4.0 (4.5) | 5.3 (4.3) |
| Female (%) | 71.3 | 76.7 |
| Race/ethnicity (%) | ||
| White | 14.8 | 16.3 |
| Black | 21.3 | 21.7 |
| Hispanic | 63.1 | 58.9 |
| Years of education, mean (SD) | 7.6 (4.8) | 7.7 (4.5) |
| Medicaid (%) | 54.5 | 48.8 |
| Died during the study (%) | 17.2 | 15.3 |
| Number of months enrolled in Medicare FFS during the year, mean (SD) | 11.8 (0.9) | 11.7 (1.1) |
| Number of comorbid conditions, mean (SD) | 1.9 (4.5) | 2.0 (1.8) |
| BDRS score, mean (SD) | 0.8 (1.3) | 0.6 (1.3) |
| Composite cognitive score (z- scores), mean (SD) | −0.5 (0.6) | −0.2 (0.6) |
| Medicare utilization (%) | ||
| Inpatient | 16.8 | 10.9 |
| Outpatient | 96.3 | 98.4 |
| Skilled nursing/home health care | 21.3 | 13.2 |
| DME | 38.1 | 20.9 |
| Total Medicare expenditures, mean $ (SD) | 3,604 (6,139) | 3,437 (5,004) |
Notes: All clinical characteristics are measured at incident date. BDRS = Blessed Dementia Rating scale; FFS = Fee-for-Service.
Medicare Utilization
Utilization rates during the 2 years before and 2 years after incident date for demented and non-demented control participants are shown in Figure 1, and multivariable estimates of risks of using each type of Medicare service are in Table 2. Results showed that during the pre-incident period, adjusted odds of having an inpatient admission were similar between demented and non-demented groups. In non-demented controls, the odds of having an inpatient admission during post-incident period was higher compared to pre-incident period but the estimate was marginally significant (odds ratio [OR] = 1.324, 95% confidence interval [CI] = [0.980, 1.788], p < .10). Predicted probability of inpatient admissions increased from pre-incident to post-incident period in both demented (10.8% to 14.2%, p < .01) and control participants (8.8% to 10.0%, p < .05). The increase in the predicted probability of inpatient admissions in participant with dementia was higher than the increases in non-demented controls (2.1%, 95% CI = [0.025, 4.23], p < .05), suggesting a small but significant increase in inpatient admissions associated with incident dementia.
Figure 1.
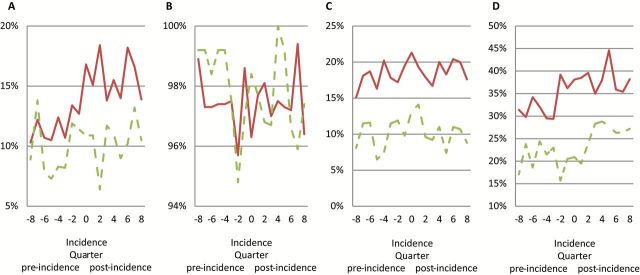
Quarterly utilization rate by Medicare service types for (A) inpatient care, (B) outpatient care, (C) home health and skilled nursing, (HHA + SNF) and (D) durable medical equipment (DME) 2 years pre- and 2 years post-dementia incidence by dementia status. Solid lines are for incident dementia cases, dashed lines are for matched non-demented cases.
Table 2.
Random Effects Logistic Regression Results on Medicare Utilization
| Variables | Inpatient | Outpatient | HHA + SNF | DME |
|---|---|---|---|---|
| OR (SE) | OR (SE) | OR (SE) | OR (SE) | |
| [95% CI] | [95% CI] | [95% CI] | [95% CI] | |
| Dementia (reference = non-demented) |
1.255 (0.241) | 0.394 (0.207)* | 2.818 (0.573)*** | 2.966 (0.826)*** |
| [0.861, 1.830] | [0.140, 1.105] | [1.893, 4.197] | [1.719, 5.119] | |
| Post-incidence (reference = pre-incidence) |
1.324 (0.203)* | 0.268 (0.111)*** | 1.161 (0.187) | 2.558 (0.354)*** |
| [0.980, 1.788] | [0.119, 0.603] | [0.847, 1.591] | [1.950, 3.355] | |
| Post-incidence × dementia | 1.982 (0.378)*** | 0.313 (0.166)** | 3.093 (0.634)*** | 5.663 (1.580)*** |
| [1.364, 2.881] | [0.111, 0.884] | [2.070, 4.621] | [3.277, 9.785] |
Notes: Models controlled for age at incidence, gender, race/ethnicity, education, Medicaid eligibility, number of comorbid conditions, and an indicator for death within 2 years of incidence diagnosis of dementia.
*** p < .01, ** p < .05, *p < .1.
During the pre-incident period, the odds of using outpatient care was lower in participants with dementia compared to non-demented controls but the estimate was marginally significant (OR = 0.394, 95% CI = [0.140, 1.105], p < .10). Compared to pre-incident period, the odds of using outpatient care decreased in non-demented controls in the post-incident period (OR = 0.268, 95% CI = [0.119, 0.603], p < .01). Comparison of predicted probabilities of outpatient visits showed no difference between demented and control participants in pre- and post-incident periods, suggesting that there was no excess increase in the probability of outpatient visits associated with incident dementia.
During the pre-incident period, the odds of using home health and skilled nursing care was already higher in dementia patients (OR = 2.818, 95% CI = [1.893, 4.197]), p < .01). In non-demented controls, the odds of using home health and skilled nursing care did not change post-incident compared to pre-incident period. Compared to pre-incident period, predicted probability of using home health and skilled nursing care remained stable during the post-incident period in both dementia (15.5% vs 14.0%) and control participants (8.4% vs 7.6%).
During the pre-incident period, the odds of using DME was already higher in dementia patients (OR = 2.966, 95% CI = [1.719, 5.119]), p < .01). In non-demented controls, the odds of using DME in the post-incident period was higher (OR = 2.558, 95% CI = [1.950, 3.355]), p < .01) compared to pre-incident period. Compared to pre-incident period, predicted post-incidence probability of using DME increased in both dementia patients (42.9% vs 34.8%, p < .01) and non-demented controls (32.1% vs 22.3%, p < .01). The magnitude of increases in utilization was similar between the two groups, suggesting no excess increase in the probability of DME use associated with incident dementia.
Medicare Expenditures
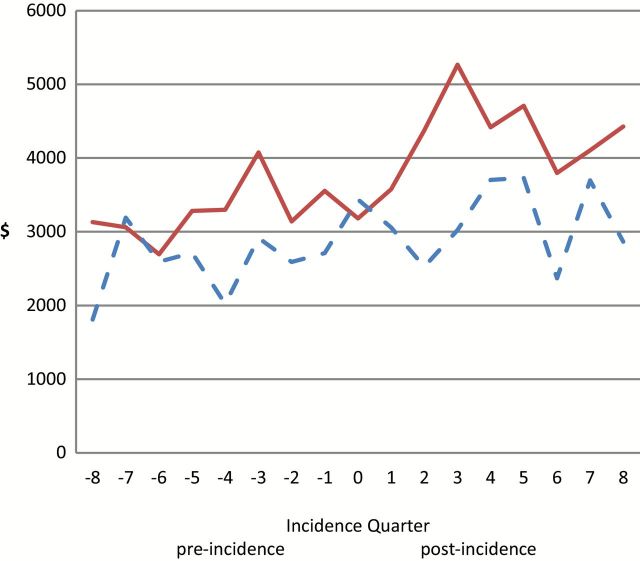
Quarterly Medicare expenditures during the 2 years before and after incident date are presented in Figure 2. Multivariable analysis showed that after controlling for other covariates, during the pre-incident period, Medicare expenditures was 65% higher in participants with dementia than matched non-demented controls (p < .01) (Table 3). After incidence, Medicare expenditures increased 43% in non-demented controls and almost doubled in dementia patients but the magnitude of the effect was marginally significant (p < .10). In terms of actual cost, adjusted Medicare expenditures was $1,003 higher in the dementia patients than non-demented controls (p < .01) during the pre-incident period. After incidence, Medicare expenditures increased $1,572 in non-demented controls and $1,895 in dementia patients, but estimates of excess increases associated with dementia was statistically insignificant (p = .318).
Figure 2.
Quarterly Medicare expenditures 2 years pre- and 2 years post-dementia incidence by dementia status. Solid lines are for incident dementia cases, dashed lines are for matched non-demented cases.
Table 3.
Generalized Linear Regression Models on Medicare Expenditures
| Variables | Total Medicare Expenditures | |
|---|---|---|
| Coefficient (SE) | Cost Ratio [95% CI] | |
| Dementia (reference = non- demented) | 0.503 (0.152) | 1.654 [1.228–2.228]*** |
| Post-incidence (reference = pre-incidence) | 0.358 (0.140) | 1.43 [1.086–1.883]* |
| Post-incidence × dementia | 0.683 (0.146) | 1.979 [1.485–2.637]*** |
Notes: Models controlled for age at incidence, gender, race/ethnicity, education, Medicaid eligibility, number of comorbid conditions, and an indicator for death within 2 years of incidence diagnosis of dementia.
*** p < .01, *p < .1.
Discussion
This study compared Medicare utilization and expenditures in a cohort of Medicare beneficiaries with clinically diagnosed dementia 2 years before and 2 years after their initial diagnosis to a matched control sample of non-demented individuals with similar characteristics. During the 2 years before the initial dementia diagnosis, rates of inpatient admissions and outpatient visits were similar between dementia patients and non-demented controls, but use of home health and skilled nursing care and DME were already higher in dementia patients. Two years after incident dementia, inpatient admissions and DME use both increased significantly but use of outpatient and home health and skilled nursing care remained stable in dementia patients. During the same period of time, except for increased DME use, and marginally significant increases in inpatient admissions, utilization of outpatient and home health and skilled nursing care in non-demented controls remained stable. Results showed a small but significant excess increase in inpatient admissions in participants with dementia compared with non-demented controls associated with incident dementia. In the 2 years before incidence, total Medicare expenditures were already higher in dementia patients than non-demented controls. But we found no excess increases in Medicare expenditures associated with incident dementia.
These results corroborate those of others that showed higher health care use in patients with prevalent dementia compared with non-demented control groups (6,7,10,12,14). They also build on existing research that showed deficits in cognition and function prior to a diagnosis of dementia (2–5). Results are consistent with a recent study that showed increased hospitalizations and out-of-pocket cost in individuals who reported that their physicians had identified them as having memory problems compared with those who did not report memory problems (4). Our study differs from others in that we followed participants from before the onset of dementia and used a comparison sample of non-demented individuals with otherwise similar characteristics as controls to examine excess health care utilization before and after dementia diagnosis. It is worth noting that while participants with dementia and controls were matched at study enrollment, after several years of follow-up, when participants with dementia were first diagnosed, socio-demographic characteristics of the two groups remained similar except for worse cognition in the participants with dementia.
In studies that used claims-based diagnoses to identify dementia patients, increased utilization and costs in the period just prior to diagnosis of AD may be related to a variety of factors including early manifestations of cognitive impairment, diagnostic work-up, or treatment of other conditions. Increased utilization could also be related to the delayed recognition of dementia in studies based on claims data. Labeling a person as having dementia also may affect decisions of physicians, patients, and their families, even affecting patients’ perceptions of their ability to recover from acute unrelated diseases (33). It is unclear how participants’ awareness of their memory problem may affect health care utilization (10). Because of the epidemiologic nature of our study, participants and their primary care providers were not notified of a study diagnosis of dementia. Our estimates based on clinical assessment of incident dementia are therefore less likely to reflect the effects of having a dementia diagnosis and more likely to reflect the true underlying effect of dementia on health care utilization and expenditures.
There are several limitations to this study. First, because we are looking at the time around incident diagnosis, our estimates do not reflect the extraordinary high health care use and costs associated with dementia in later stages. Second, our results represent the experience of a racial/ethnically diverse, vulnerable population in a large urban area and may not be representative of experiences in the general population. Third, our estimates reflect only utilization and payment for services covered by Medicare, so services provided by other payers (eg, Medicaid, Veterans Administration), out-of-pocket costs, and costs of informal care and of lost productivity are unaccounted for.
Our study also has a number of strengths. Because we begin with a known population of older adults in a defined community, whose dementia status has been determined clinically and separately from administrative claims, we are able to avoid biases such as under-recognition or under-coding of dementia, or other measurement limitations that are inherent to studies based on administrative claims only, as well as biases that may arise from using self-reported health care utilization (4,14,16,17). The frequent follow-up of the current study sample ensures increased accuracy of identification of incident dementia and provides increased confidence of our results. While our sample may not be representative of the general population, studies on racial/ethnically diverse, vulnerable population are scarce. These data highlight the impact of dementia on health care utilization around the time of disease onset and its effects on different types of services. These data underline the importance of dementia and may have implications in the provision of health care planning. As incidence of dementia continue to increase, it is important to identify factors that contribute to these trends and develop effective strategies to manage the disease.
Funding
This research was supported by grants from the National Institute on Aging (AG07370, AG037212). C.W.Z. was also supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. 10.1016/j.jalz.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Small BJ, Bäckman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex. 2007;43:826–834. [DOI] [PubMed] [Google Scholar]
- 3. Bäckman L. Memory and cognition in preclinical dementia: what we know and what we do not know. Can J Psychiatry. 2008;53:354–360. [DOI] [PubMed] [Google Scholar]
- 4. Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Analysis of cognitive, functional, health service use, and cost trajectories prior to and following memory loss. J Gerontol B Psychol Sci Soc Sci. 2013;68:562–567. [DOI] [PubMed] [Google Scholar]
- 5. Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Depressive, functional status, and neuropsychiatric symptom trajectories before an Alzheimer’s disease diagnosis. Aging Ment Health. 2014;18:110–116. 10.1080/13607863.2013.814100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albert SM, Glied S, Andrews H, Stern Y, Mayeux R. Primary care expenditures before the onset of Alzheimer’s disease. Neurology. 2002;59:573–578. [DOI] [PubMed] [Google Scholar]
- 7. Eisele M, van den Bussche H, Keller D, et al. Utilization patterns of ambulatory medical care before and after the diagnosis of dementia in Germany–results of a case-control study. Dement Geriatr Cogn Disord. 2010;29:475–483. 10.1159/000310350 [DOI] [PubMed] [Google Scholar]
- 8. Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. 10.1001/jama.2011.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geldmacher DS, Kirson NY, Birnbaum HG, et al. Pre-diagnosis excess acute care costs in Alzheimer’s patients among a US Medicaid population. Appl Health Econ Health Policy. 2013;11:407–413. 10.1007/s40258-013-0038-9 [DOI] [PubMed] [Google Scholar]
- 10. Suehs BT, Davis CD, Alvir J, et al. The clinical and economic burden of newly diagnosed Alzheimer’s disease in a medicare advantage population. Am J Alzheimers Dis Other Demen. 2013;28:384–392. 10.1177/1533317513488911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leibson C, Owens T, O’Brien P, et al. Use of physician and acute care services by persons with and without Alzheimer’s disease: a population-based comparison. J Am Geriatr Soc. 1999;47:864–869. [DOI] [PubMed] [Google Scholar]
- 12. Sloan FA, Taylor DH., Jr Effect of Alzheimer disease on the cost of treating other diseases. Alzheimer Dis Assoc Disord. 2002;16:137–143. [DOI] [PubMed] [Google Scholar]
- 13. Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. [DOI] [PubMed] [Google Scholar]
- 14. Lin PJ, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimers Dement. 2010;6:334–341. 10.1016/j.jalz.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 15. Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8:108. 10.1186/1472-6963-8-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer’s disease solely from Medicare claims records. J Am Geriatr Soc. 1999;47:215–219. [DOI] [PubMed] [Google Scholar]
- 17. Fillit H, Geldmacher DS, Welter RT, Maslow K, Fraser M. Optimizing coding and reimbursement to improve management of Alzheimer’s disease and related dementias. J Am Geriatr Soc. 2002;50:1871–1878. [DOI] [PubMed] [Google Scholar]
- 18. Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55:929–937. [DOI] [PubMed] [Google Scholar]
- 19. Ostbye T, Taylor DH, Jr, Clipp EC, Scoyoc LV, Plassman BL. Identification of dementia: agreement among national survey data, medicare claims, and death certificates. Health Serv Res. 2008;43(1 Pt 1):313–326. 10.1111/j.1475-6773.2007.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor DH, Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. 10.3233/JAD-2009-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. [DOI] [PubMed] [Google Scholar]
- 22. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23. Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. [DOI] [PubMed] [Google Scholar]
- 24. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 25. Parsons LS.Using SAS® Software to perform a case-control match on propensity score in an observational study. Paper 225-25. Paper presented at: Proceedings of the Twenty-Fifth Annual SAS Users Group International Conference. Proceedings of the Twenty-Fifth Annual SAS. Users Group International Conference 2000; SAS Institute Inc, Cary, NC. [Google Scholar]
- 26. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–1240. 10.1016/j.jclinepi.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 28. Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. [DOI] [PubMed] [Google Scholar]
- 29. Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Pt 2):1842–1849. 10.1212/01.wnl.0000304038.37421.cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bureau of Labor Statistics. Consumer price index 2012. http://www.bls.gov/cpi/home.htm. Accessed May 15, 2015.. 10.1093/geronb/gbs078
- 31. Ai C, Norton EC. Interaction terms in logit and probit models. Economics Letters. 2003;80(1):123–129. [Google Scholar]
- 32. Buis ML. Stata tip 87: Interpretation of interactions in nonlinear models. The Stata Journal. 2010;10(2):305–308. [Google Scholar]
- 33. Mold JW, Hamm RM, Jafri B. The effect of labeling on perceived ability to recover from acute illnesses and injuries. J Fam Pract. 2000;49:437–440. [PubMed] [Google Scholar]