Abstract
Background. The 4 serotypes of dengue virus, DENV-1–4, are the leading cause of arboviral disease globally. The ideal dengue vaccine would provide protection against all serotypes after a single dose.
Methods. Two randomized, placebo-controlled trials were performed with 168 flavivirus-naive adults to demonstrate the safety and immunogenicity of a live attenuated tetravalent dengue vaccine (TV003), compared with those of a second tetravalent vaccine with an enhanced DENV-2 component (TV005), and to evaluate the benefit of a booster dose at 6 months. Safety data, viremia, and neutralizing antibody titers were evaluated.
Results. A single dose of TV005 elicited a tetravalent response in 90% of vaccinees by 3 months after vaccination and a trivalent response in 98%. Compared with TV003, the higher-dose DENV-2 component increased the observed frequency of immunogenicity to DENV-2 in the TV005 trial. Both the first and second doses were well tolerated. Neither vaccine viremia, rash, nor a significant antibody boost were observed following a second dose.
Conclusions. A single subcutaneous dose of TV005 dengue vaccine is safe and induces a tetravalent antibody response at an unprecedented frequency among vaccinees. A second dose has limited benefit and appears to be unnecessary. Studies to confirm these findings and assess vaccine efficacy will now move to populations in regions where DENV transmission is endemic.
Clinical Trials Registration. NCT01072786 and NCT01436422.
Keywords: dengue vaccine, live attenuated tetravalent vaccine, clinical trial
(See the editorial commentary by McArthur and Edelman on pages 681–3.)
Dengue is emerging as the most important mosquito-borne viral disease [1]. More than 40% of the world's population is at risk, with 500 million infections annually. The largest burden of disease is currently in Southeast Asia and Central/South America. In the Americas alone, diagnosed dengue virus (DENV) infections increased 5-fold over the past decade to 2.3 million cases in 2013 [2–4]. The global threat of dengue has been influenced by the spread of the Aedes mosquito vector, urban crowding, and global climate change [5]. DENV is now endemic in 100 countries, and transmission has been reported in the United States [6].
There are 4 distinct circulating DENV serotypes (DENV-1–4); all can cause symptomatic disease. Infection with one serotype leads to long-lasting homotypic immunity to the infecting serotype and short-lived heterotypic immunity to the others [7, 8]. Clinical disease ranges from a self-limited febrile illness characterized by intense myalgias, headache, and rash to a severe life-threatening syndrome that includes plasma leakage, hemorrhagic complications, and circulatory collapse. Approximately 500 000 persons annually develop severe dengue, with 20 000 deaths [9]. Severe disease can be seen following primary infection with any of the 4 DENV serotypes. Epidemiologic studies have determined that preexisting immunity is a risk factor for severe disease following a second infection with a heterotypic serotype [10]. For this reason, dengue vaccines are designed to protect against all serotypes [11].
The live attenuated tetravalent dengue vaccine (LATV) was designed by the Laboratory of Infectious Diseases at the National Institutes of Health (Bethesda, Maryland). As previously reported, all monovalent vaccine components have a DENV genetic background and share a core attenuating, 30-nucleotide deletion in the 3′ untranslated region of the viral genome, yielding replication-deficient attenuated viruses [12]. One component (rDEN2/4Δ30) is chimeric, with the structural proteins of DENV-2 replacing those of DENV-4 [13]. Multiple monovalent components were tested for infectivity, safety, and immunogenicity in monkeys and humans before selection of strains for the tetravalent vaccine [14]. Initial clinical evaluation of several tetravalent admixtures has shown all to be safe and to elicit balanced immune response in healthy volunteers, although the DENV-2 component was less immunogenic than other serotypes [11]. In advance of testing in transmission-endemic settings, we sought to optimize the LATV admixture. Herein, we evaluate the safety and immunologic benefit from an increased dose of the DENV-2 component, as well as a second dose of vaccine at 6 months, and explored the kinetics of the neutralizing antibody response following vaccination.
MATERIALS AND METHODS
Ethics Statement
The studies were performed under an investigational new drug application reviewed by the Food and Drug Administration and approved by the institutional review boards at the University of Vermont and Johns Hopkins University. Informed consent was obtained in accordance federal and international regulations (21CFR50, ICHE6). External independent monitoring was performed, and the National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board reviewed all safety data every 6 months.
Trial Design and Study Setting
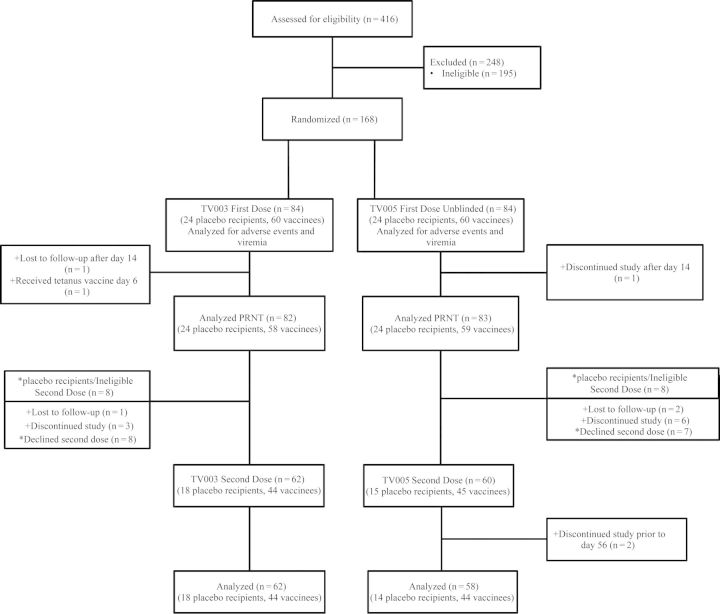
Two phase 1 randomized, double-blind, placebo-controlled trials were conducted in Baltimore, Maryland, and Burlington, Vermont. Study subjects were enrolled between August 2010 and March 2013 under study protocols CIR268 and CIR279 (clinical trial registration NCT01072786 and NCT01436422). Both studies evaluated the safety and immunogenicity of a single dose of different tetravalent admixtures of the LATV. To determine the effect of a second vaccination on immunogenicity (frequency of seroconversion, tetravalent response, and mean neutralizing antibody titer), a second dose of the same vaccine was administered 6 months following the first dose. The 2 studies (CIR268 and CIR279) differed slightly in postvaccination follow-up; the immunologic end point was study day 42 for CIR268 and study day 90 for CIR279 (Supplementary Table 1). For study CIR268, only volunteers previously vaccinated received a second dose of vaccine or placebo (4:1 ratio) following unblinding of the original study and reenrollment into a blinded substudy of the second dose. In contrast, for volunteers in CIR279, the study remained blinded, and volunteers received a second dose of either vaccine or placebo (Figure 1). Study outcomes included vaccine safety, vaccine viremia (characterized by mean peak titer, day of onset, and duration), and antibody response (characterized by geometric mean titer [GMT] of neutralizing antibodies and the frequency and distribution of seroconversion). The serologic response was characterized as a 50% plaque-reduction neutralization titer (PRNT50), measured at multiple time points following vaccination.
Figure 1.
Enrollment and follow up of volunteers evaluating the National Institutes of Health live attenuated tetravalent dengue vaccine with 2 tetravalent admixtures and a second dose at 6 months. Volunteers from study 268 are denoted by asterisks, and volunteers from study 279 are denoted by plus signs. Abbreviation: PRNT, plaque-reduction neutralization titer.
For each study, volunteers were block randomized in groups of 7 such that 5 would receive vaccine and 2 would receive placebo at the first vaccination. The study pharmacist randomized subjects using a random-number generator. Study teams from both the clinic and laboratory remained blinded to treatment assignment. Unblinding was performed after all subjects in a randomization block reached postvaccination study day 42 for study CIR268 and day 270 for CIR279.
Study Population
Healthy adult volunteers aged 18–50 years were recruited and enrolled from both sites. Eligibility criteria included seronegativity for flaviviruses (DENV-1–4, and West Nile, St. Louis encephalitis, and yellow fever viruses); seronegativity for hepatitis B, hepatitis C, and human immunodeficiency viruses; and normal findings of blood hematology, serum chemistry, and physical examinations.
Vaccines
Supplementary Table 2 lists the 4 vaccine components and doses used in each tetravalent admixture. Previous clinical trials of the component vaccines describe their safety, immunogenicity, and attributes of viral replication [11, 13–22]. Prior to use, the monovalent vaccine viruses were stored at −80°C ± 15°C and then thawed, diluted, and combined as an admixture immediately prior to vaccination. Vaccines were diluted to yield a potency of 3 log10 plaque-forming units (PFU) for each serotype (TV003), with the exception of the DENV-2 component in the TV005 admixture, which had a potency of4 log10 PFU. Viral titers were confirmed following the preparation of all admixtures. Placebo inoculations consisted of diluent only (qualified Leibovitz L-15 medium).
Clinical Procedures and Evaluation
On the day of first vaccination (day 0), subjects were randomized to receive vaccine or placebo, which was administered by subcutaneous injection of a 0.5-mL dose. Subjects were followed as outpatients and recorded their oral temperature 3 times daily for 16 days. For subjects enrolled in CIR268, clinical assessments and physical examinations were performed every other day through study day 16 and on study days 21, 28, 42, and 180 as described elsewhere [11]. For subjects enrolled in CIR279, clinical assessments and physical examinations were performed on study days 3, 8, 10, 12, 14, 16, 21, 28, 56, 90, and 180. For both studies, the schedule of study visits after the second dose was the same as the schedule following the first visit, with the exception of the study day 150 visit, which was not performed following the second vaccination.
Adverse Events (AEs)
AEs and laboratory results were captured and recorded as previously described and were graded for intensity and relationship to vaccination [11, 23]. Abnormal clinical laboratory data were also graded as mild, moderate, or severe, using standardized toxicity tables. Dengue vaccine–like rash was defined as in previous studies. Vaccine-related infection was defined as recovery of vaccine virus from the blood and/or seroconversion to DENV as measured by the PRNT50 assay, described below.
Virus Quantitation and Serologic Response
Serum samples collected at follow-up visits every other day through day 16 were tested for viable virus by amplification and direct titration on Vero cell monolayers as described elsewhere [24]. Virus titration was performed for each serotype as described, using serotype-specific monoclonal antibodies. The neutralizing antibody response was measured by the PRNT assay, using the lowest serum dilution that gave a 50% reduction in viral foci (PRNT50) in accordance with other LATV evaluations [24]. PRNT50 assays used an initial serum dilution of 1:5. Seroconversion after first dose was defined as a PRNT50 of ≥1:10 by study day 42 for CIR268 and by study day 90 for CIR279 (a 4-fold rise in titer of 1:2.5 assigned to samples with undetectable titers <1:5). A boost in neutralizing antibody from a second dose of vaccine was defined as a ≥4-fold rise in serum neutralizing antibody titer by study day 270, compared with study day 180.
Data Analysis
A per-protocol analysis was performed. χ2 analyses with likelihood ratios were used to determine statistically significant differences in the frequency of adverse events or demographic characteristics, as well as significant differences in the percentage of viremic, seroconverted, and tetravalent responders, between the TV003 and TV005 groups. Differences in mean peak titers, mean day of onset, and mean duration of viremia between the TV003 and TV005 groups were determined by Mann–Whitney U analyses. Paired t tests were used to determine differences between peak serologic responses between first and second doses of tetravalent admixtures. Statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 21.0; Armonk, New York) and JMP (version 9.0.2; SAS Institute, Cary, North Carolina) software.
RESULTS
As shown in Figure 1, 416 volunteers were assessed for eligibility, and 168 were randomized to receive vaccine or placebo. A total of 84 volunteers received the first dose of TV003, and 82 volunteers (58 vaccinees and 24 placebo recipients) met per-protocol criteria for serologic analysis. Sixty-two volunteers (44 vaccinees and 18 placebo recipients) received the second dose of TV003. TV005, the admixture with an increased dose of DENV-2, was given as a first dose to 84 volunteers, and 83 subjects (59 vaccinees and 24 placebo recipients) were analyzed. Of these, 60 subjects received a second dose, and 58 subjects (44 vaccinees and 14 placebo recipients) were analyzed. There were no significant differences in demographic data. For TV003 and TV005, males composed 52.4% and 59.5%, respectively, of the study groups; the mean ages were 29.3 and 30.0 years, respectively; 39.3% and 45.2%, respectively, were African American, 57.1% and 48.8%, respectively, were white, 1.2% and 3.6%, respectively, were Hispanic, and 3.4% and 5.9%, respectively, were of other races/ethnicities. Similarly, demographic differences were not seen between vaccine and placebo recipients (data not shown).
Safety and Reactogenicity
Both admixtures of vaccine were well tolerated in all volunteers after both the first and second dose. Table 1 lists the AEs through study day 28 following each vaccination. As seen in previous studies, mild rash was common following the first vaccination, occurring in approximately 60% of volunteers, and was more common in non–African American subjects (P ≤ .001) owing to the increased visibility of faint rash in non–African American subjects [11]. In contrast, rash was seen only in a single volunteer following the second vaccination. Mild, transient neutropenia and elevated alanine aminotransferase levels were seen in 2%–8% and 2%–7% of TV003 and TV005 volunteers, respectively, and were infrequent or absent following the second dose. No significant differences in AEs between TV003 and TV005 participants were observed. Five serious AEs unrelated to vaccination were reported among vaccinees: hospitalizations following assault, Lyme disease, cholecystitis, and depression. One unrelated serious AE was reported in a placebo recipient (fractured jaw). No other differences in AEs were seen by race/ethnicity.
Table 1.
Adverse Events Following First and Second Dose of the Live Attenuated Tetravalent Dengue Vaccine Admixtures TV003 and TV005, Compared With Placebo
| Adverse Event | Dose 1 |
Dose 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| TV003 (n = 60) | TV005 (n = 60) | Placebo (n = 48) | LR | TV003 (n = 44) | TV005 (n = 45) | Placebo (n = 32) | LR | |
| Injection site | ||||||||
| Erythema | 5.0 | 5.0 | 4.2 | 0.973 | 11.4 | 11.1 | 0.0 | 0.040 |
| Pain | 0.0 | 3.3 | 4.2 | 0.162 | 2.3 | 4.4 | 3.1 | 0.847 |
| Tenderness | 5.0 | 1.7 | 4.2 | 0.563 | 0.0 | 2.2 | 0.0 | 0.369 |
| Induration | 3.3 | 1.7 | 0.0 | 0.303 | 2.3 | 6.7 | 0.0 | 0.169 |
| Systemic | ||||||||
| Fever | 0.0 | 3.3 | 0.0 | 0.125 | 2.3 | 0.0 | 3.1 | 0.381 |
| Headache | 36.7 | 50.0 | 35.4 | 0.215 | 25.0 | 20.0 | 25.0 | 0.817 |
| Rash | 61.7 | 61.7 | 0.0 | <0.001 | 0.0 | 2.2 | 0.0 | 0.369 |
| Neutropeniaa | 8.3 | 6.7 | 4.2 | 0.671 | 0.0 | 2.2 | 3.1 | 0.389 |
| Elevated ALT levelb | 5.0 | 3.3 | 2.1 | 0.709 | 6.8 | 2.2 | 3.1 | 0.533 |
| Myalgia | 8.3 | 10.0 | 6.3 | 0.778 | 4.6 | 8.9 | 9.4 | 0.635 |
| Arthralgia | 3.3 | 0.0 | 2.1 | 0.242 | 2.3 | 2.2 | 3.1 | 0.965 |
| Retro-orbital pain | 6.7 | 6.7 | 8.3 | 0.933 | 2.3 | 0.0 | 3.1 | 0.381 |
| Fatigue | 16.7 | 28.3 | 12.5 | 0.096 | 11.4 | 17.8 | 18.8 | 0.596 |
| Nausea | 11.7 | 16.7 | 16.7 | 0.675 | 13.6 | 0.0 | 9.4 | 0.010 |
| Prolonged PT | 6.7 | 5.0 | 8.3 | 0.784 | 2.3 | 4.4 | 10.0 | 0.345 |
| Prolonged PTT | 6.7 | 0.0 | 6.3 | 0.042 | 4.6 | 8.9 | 3.3 | 0.547 |
Data are % of subjects, unless otherwise indicated. Adverse events experienced by recipients of TV003 and TV005 from both studies (CIR268 and CIR279) are presented together. In addition, adverse events experienced by placebo recipients from both the TV003 and TV005 cohorts of both trials are presented together.
Abbreviations: ALT, alanine aminotransferase; LR, likelihood ratio; PT, protime; PTT, prothrombin time.
a Defined as an absolute neutrophil count of ≤ 1500 cells/mm3 in the CIR268 study and ≤1000 cells/mm3 in the CIR279 study.
b Elevations ranged from 1.3 to 2.0 times the upper limit of normal.
Detection of Viremia
Following the first dose of vaccine, low levels of viremia were detected in 76% of all vaccinees (91 of 120 in both the TV003 and TV005 groups) during the initial 16 days after vaccination (Table 2). Viremia was generally detected between days 8 and 16 and was short lived (duration, 1–3 days) in most volunteers. Mean peak titers were low (ie, <1.0 log10 PFU/mL) for all serotypes (range, 0.50–0.64 log10 PFU/mL). Following vaccination with either TV003 or TV005, the DENV-2 component, rDEN2/4Δ30, was detected less frequently than the other serotypes. However, the incidence of rDEN2/4Δ30 viremia was significantly higher in the TV005 group (P = .034; P = .136 after adjustment for multiple comparisons).
Table 2.
Low-Level Viremia Is Observed for All Vaccine Components Following 1 Dose of Live Attenuated Tetravalent Dengue Vaccine Admixture TV003 or TV005
| Admixture, Component | Subjects With Viremia, % | Peak Titer, Log10 PFU/mL, Mean ± SE | Maximum Titer, log10 PFU/mL | Time of Onset, d, Mean (Range) | Duration, d, Mean (Range) |
|---|---|---|---|---|---|
| TV003 (n = 60) | |||||
| rDEN1Δ30 | 25.0 | 0.61 ± 0.07 | 1.4 | 10.9 (8–15) | 2.1 (1–5) |
| rDEN2/4Δ30 | 6.7a | 0.50 ± 0.00 | 0.5 | 7.5 (6–10) | 1.0 (all 1) |
| rDEN3Δ30/31 | 38.3 | 0.56 ± 0.03 | 1.2 | 9.4 (5–14) | 2.4 (1–7) |
| rDEN4Δ30 | 30.0 | 0.52 ± 0.02 | 0.7 | 9.3 (6–16) | 1.9 (1–6) |
| Overall | 75.0 | … | … | … | … |
| TV005 (n = 60) | |||||
| rDEN1Δ30 | 28.3 | 0.62 ± 0.08 | 1.7 | 12.3 (8–16) | 2.2 (1–7) |
| rDEN2/4Δ30 | 21.7a | 0.50 ± 0.00 | 0.5 | 9.1 (2–14) | 1.8 (1–8) |
| rDEN3Δ30/31 | 41.7 | 0.60 ± 0.03 | 1.0 | 9.2 (6–14) | 2.8 (1–9) |
| rDEN4Δ30 | 30.0 | 0.64 ± 0.07 | 1.5 | 10.1 (6–16) | 2.2 (1–10) |
| Overall | 77.0 | … | … | … | … |
Results for each admixture are combined for CIR268 and CIR279 studies. Serum sampling schedule for viremia analysis was identical for each study. The days of viremia testing are shown in Supplementary Table 1.
Abbreviations: PFU, plaque-forming unit; SE, standard error.
a P = .034 (unadjusted) and P = .136 (adjusted for multiple comparisons).
The majority of all subjects with viremia (55 [60%]) had 1 serotype detected, 28 (31%) had 2 serotypes detected, and 7 (8%) had 3 serotypes detected. There was no significant difference in mean peak titer, mean day of onset of viremia, mean duration of viremia, or number of serotypes detected between the TV003 and TV005 groups. Viremia parameters did not differ by race or sex. Following the second dose of vaccine, viremia involving the DENV3 component, rDEN3Δ30/31, was detected in a single volunteer (0.5 log10 PFU/mL) on only day 11 after vaccination.
Serologic Responses
Kinetics of Antibody Titers
In CIR268, neutralizing antibody through study day 42 was the serologic end point, but some peak antibody titers occurred after day 42 [11]. In CIR268, 2 subjects (10%) and 4 subjects (20%) who received TV003 and TV005, respectively, developed a tetravalent antibody response by seroconverting to additional serotypes between study days 42 and 180 (Table 3). In CIR279, an additional 10% of subjects who received TV003 or TV005 developed a tetravalent antibody response between study days 56 and 90. These data demonstrate that antibody responses to the DENV serotypes may differ among individuals and that seroconversion or peak titers may be missed if the antibody response is measured too early or too infrequently.
Table 3.
Seroconversion to All 4 Dengue Virus Serotypes (Tetravalent Response) May Increase in Volunteers up to 180 Days After Vaccination With TV003 and TV005
| Study, Admixture | Subjects, No. | Subjects With T-PRNT, No. (%) |
Subjects With New T-PRNT After Day 42/56, No. (%) | ||
|---|---|---|---|---|---|
| Day 42/56 | Day 90 | Day 180 | |||
| CIR268 | |||||
| TV003 | 20 | 10 (50) | NA | 12 (60) | 2 (10) |
| TV005 | 20 | 12 (60) | NA | 16 (80) | 4 (20) |
| CIR279 | |||||
| TV003 | 38 | 24 (63) | 28 (74) | 28 (74) | 4 (11) |
| TV005 | 39 | 31 (80) | 35 (90) | 35 (90) | 4 (10) |
Specimens were collected on study day 42 for CIR268 and study day 56 for CIR279 (Supplementary Table 1).
Abbreviations: NA, not available; T-PRNT, tetravalent antibody response, as measured by plaque-reduction neutralization titer.
Impact of Increased DENV-2 Dose (TV005 Versus TV003)
All volunteers were seronegative for flaviviruses at the onset of the trial, as per inclusion criteria. Both the TV003 and TV005 admixtures induced consistently high frequencies of seroconversion to each serotype following a single dose (Table 4), but the DENV-2 component induced the lowest response in the TV003 recipients (76%), compared with the other serotypes. Nevertheless, DENV-2 seroconversion significantly improved following vaccination with TV005 (97%; P = .007; P = .028 after adjustment), which contains an increased dose of the DENV-2 component (4 log10 PFU), compared with the TV003 admixture. A single dose of the TV005 admixture resulted in seroconversion frequencies of 92%, 97%, 97%, and 97% for DENV-1–4, respectively.
Table 4.
Seroconversion Frequencies Following 1 or 2 Doses of the Live Attenuated Tetravalent Dengue Vaccine With a Lower (TV003) or Higher (TV005) Dose of the Dengue Virus (DENV) Serotype 2 (DENV-2) Component in Study CIR279
| Admixture | Dose | Subjects, No. | Subjects Who Seroconverted, by Serotype, No. (%)a |
|||
|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||
| TV003 | 1 | 38 | 35 (92) | 29 (76)b | 37 (97) | 38 (100) |
| TV005 | 1 | 39 | 36 (92) | 38 (97)b | 38 (97) | 38 (97) |
| TV003 | 2 | 34 | 33 (97) | 32 (94) | 34 (100) | 34 (100) |
| TV005 | 2 | 33 | 31 (94) | 33 (100) | 33 (100) | 33 (100) |
Data are presented only from the CIR279 trial because data from day 90 after vaccination were not available for CIR268.
a Once a subject seroconverted, defined as a 50% plaque-reduction neutralization titer of ≥10, they maintained this designation for all remaining analyses.
b P = .007 (unadjusted) and P = .028 (adjusted), by the Fisher exact test.
In addition to seroconversion, significantly higher mean peak antibody GMTs against DENV-2 were observed following the first dose of TV005, compared with TV003 (91 vs 39; P = .004 after adjustment; Table 5). DENV-3 and DENV-4 mean antibody titers were also increased following TV005 receipt, but the opposite was true for DENV-1 (35 vs 63). GMT changes in DENV-1, DENV-3, and DENV-4 antibody were not statistically different after adjustment for multiple comparisons. Additionally, as shown in Supplementary Figure 1, at all measured time points (ie, days 28, 42/56, and 90) after the first dose, the proportion of vaccinees who seroconverted to DENV-2 was significantly higher in the TV005 admixture (P < .0002, P = .021, and P < .0001, respectively, after adjustment for multiple comparisons). The GMT of DENV-2 was higher in the TV005 admixture at each time point, but the GMT was only significantly higher at day 90 (P = .006; data not shown).
Table 5.
Dengue Virus (DENV) Serotype–Specific Geometric Mean Neutralizing Antibody Titers (GMTs) Following 1 or 2 Doses of Live Attenuated Dengue Vaccine Admixtures TV003 or TV005 in Study CIR279
| Admixture, No. of Subjects | Dose | Day | Reciprocal Peak GMT (Median), by Serotypea |
|||
|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||
| TV003 | ||||||
| 38 | 1 | 28–90 | 63 (58) | 39 (50) | 83 (72) | 144 (152) |
| 34 | 1 | 180 | 13 (12) | 11 (9) | 37 (33) | 38 (41) |
| 34 | 2 | 208–270 | 18 (15) | 23 (31) | 68 (53) | 78 (76) |
| TV005 | ||||||
| 39 | 1 | 28–90 | 35 (32) | 91 (99) | 100 (99) | 205 (218) |
| 33 | 1 | 180 | 10 (9) | 35 (40) | 25 (28) | 40 (40) |
| 33 | 2 | 208–270 | 15 (15) | 55 (65) | 36 (34) | 75 (74) |
Data are presented only from the CIR279 trial because data from day 90 after vaccination were not available for CIR268.
a GMTs were determined on the basis of the 50% plaque-reduction neutralization titer.
Although both admixtures induced seroconversion to all 4 serotypes (a tetravalent response) by day 90 in the majority of volunteers, this response trended higher following TV005 receipt (90% for TV005 vs 74% for TV003; P = .083). An additional 18% and 8% of vaccines mounted a trivalent response, for an overall trivalent or greater response after a single dose in 92% and 98% of vaccinees for TV003 and TV005, respectively (Table 6). Overall, when TV003 and TV005 were combined, African American subjects had a lower tetravalent seroconversion rate (57%), compared with non–African Americans (86%; P = .001).
Table 6.
Most Vaccinees Achieve a Tetravalent Dengue Virus Antibody Response Following Administration of Live Attenuated Dengue Vaccine Admixtures TV003 or TV005 in Study CIR279
| Admixture,a No. of Subjects | Dose | Subjects With Antibody Response, %, by Valenceb |
||||
|---|---|---|---|---|---|---|
| Tetravalent | Trivalent | Bivalent | Monovalent | None | ||
| TV003 | ||||||
| 38 | 1 | 74 | 18 (92) | 8 (100) | 0 (100) | 0 |
| 34 | 2 | 91 | 9 (100) | 0 (100) | 0 (100) | 0 |
| TV005 | ||||||
| 39 | 1 | 90 | 8 (98) | 0 (98) | 2 (100) | 0 |
| 33 | 2 | 94 | 6 (100) | 0 (100) | 0 (100) | 0 |
Seroconversion was indicated by serotype-specific 50% plaque-reduction neutralization titer of ≥10 by day 90 after vaccination. Data are presented only from the CIR279 trial because data from day 90 after vaccination were not available for CIR268.
a There is no statistical difference in trivalent and tetravalent responses in dose 2 between TV003 and TV005.
b Data are cumulative and were measured on the basis of seroconversion to individual serotypes.
Effect of a Second Vaccine Dose
The impact on immunogenicity was assessed in volunteers receiving a second dose of LATV 6 months following the first dose (Figure 1). Differences were compared between dose 1 and dose 2 for both admixtures; second-dose differences between TV003 and TV005 were also examined. Six of 8 subjects who remained seronegative to DENV-2 following the first dose of TV003 seroconverted following the second dose, increasing the seroconversion rate from 76% to 94% (Table 4). Only 1 of 3 subjects seronegative to DENV-1 following the first dose of TV003 seroconverted following the second dose. For TV005, >92% of subjects seroconverted following the first dose, and we did not observe a significant increase in seroconversion following the second dose for any serotype. When mean peak neutralizing antibody titers were examined (Table 5), the use of a second dose was not associated with boosts in mean titers for any serotype with either TV003 or TV005 in a pair-wise analysis (which including all subjects receiving both doses).
DISCUSSION
In advance of efficacy trials with this promising LATV in transmission-endemic regions, we performed 2 randomized, controlled phase 1 trials of different admixtures in healthy flavivirus-naive adults to optimize the formulation and dose schedule. This work demonstrates that a single subcutaneous dose of vaccine is well tolerated and prompts a robust and balanced neutralizing antibody response to all DENV serotypes and that a second dose is likely unnecessary. Earlier formulations of the LATV containing a 3 log10 PFU dose of rDEN2/4Δ30 (TV003) induced a relatively less robust DENV-2 neutralizing antibody response than the responses to DENV-1, DENV-3, and DENV-4 [11]. This lower response was likely the result of 2 unique properties of rDEN2/4Δ30: it is a chimeric virus strain and has a slightly higher median infectious dose in humans (10 PFU) than the other serotypes in the tetravalent vaccine (median human infectious dose, < 10 PFU) [13]. Our data show that vaccination with a new tetravalent formulation (TV005) containing a 10-fold increase in dose of the DENV-2 serotype (4 log10 PFU) significantly improves seroconversion frequencies and overall antibody titers to the DENV-2 component of the vaccine while maintaining the immunogenicity of the other serotypes. A single dose of TV005 induced seroconversion to all 4 DENV serotypes in an unprecedented 90% of vaccinated subjects. Importantly, this frequency of seroconversion is attainable in flavivirus-naive subjects (as in this study) and supports the use of the vaccine in target populations that are unprimed by previous DENV exposure.
The immunogenicity of TV005 is improved, compared with that of TV003, owing to the increased dose of the DENV-2 component in this admixture. Additionally, the 90-day window of serum sampling allowed for a more comprehensive measure of immunogenicity, characterized by an 11% increase in seroconversion, compared with the 45/56-day window (Table 3). The increased dose of the DENV-2 component contributed to increased infectivity, evidenced by increased incidence of rDEN2/4Δ30 viremia in TV005 recipients, compared with TV003 recipients (Table 2). Nevertheless, as with our previous observations following LATV and monovalent vaccine administration, the peak level of viremia remains low (≤1.7 log10 PFU/mL) and is well below levels associated with symptomatic illness or transmission to mosquitoes [25]. The percentage of volunteers (21.7%) with DENV-2 viremia following TV005 is also commensurate with frequency of viremia seen for the other serotypes. Nevertheless, increases in reactogenicity were not noted following use of the new formulation.
This work also evaluated the safety and immunogenicity implications of a second dose of either the TV003 or TV005 admixture. Importantly, clinical safety issues were not evident after the second dose. For TV005, this second dose revealed no improvement in immunogenicity when given 6 months after the first. Similar data were found for TV003, with the exception of the expected increase in the frequency of seroconversion to DENV-2 (from 76% to 94%). More specifically, the second dose was not associated with a boost in mean peak neutralizing antibody titer for any serotype with either TV003 or TV005. A second dose also did not increase the overall percentage of volunteers mounting a tetravalent or trivalent response. Importantly, the second dose was associated with an absence of vaccine viremia and rash in all but 1 of 89 volunteers (1.1%). The data confirm that a single dose of TV003 or TV005 imparts sterilizing immunity against a second dose of vaccine given at 6 months. Our data differ from those for the Sanofi Pasteur CYD or Takeda DENVax tetravalent candidate vaccines, in which multiple doses are required for seroconversion to all 4 serotypes and in which viremia is detected following the second or third doses [26, 27]. In advance of phase 3 efficacy trials and to demonstrate the ability of LATV to protect against natural DENV infection, LATV vaccinees will be challenged 6 months after vaccination with a mildly attenuated heterotypic DENV-2 challenge strain. This work will be particularly important since a clear neutralizing titer cutoff or correlate of protection has not yet been clearly defined for DENV infection.
Data from the 2 trials reported here support the overall safety of the LATV and its development as the first single-dose dengue vaccine. Thus far, 168 volunteers have received the TV003 or TV005 tetravalent vaccines, 84 volunteers have received other LATV admixtures (TV001, TV002, or TV004), and 450 have received monovalent vaccines [11, 13, 15, 17, 20–23]. In aggregate, we have compiled detailed safety information on >700 recipients of monovalent or tetravalent vaccine. The vaccine is well tolerated, with only transient mild neutropenia and mild elevations in the alanine aminotransferase level in 2%–5% of volunteers. Clinical features of dengue fever or severe dengue, such as fever or hemorrhagic manifestations, have not been seen. As with past trials, a mild maculopapular rash is seen in approximately 60% of volunteers after first vaccination, particularly in those with light skin. In almost all cases, the rash is asymptomatic and is not self-reported by the volunteer. Interestingly, the appearance of vaccine-associated rash is positively correlated with tetravalent seroconversion (P < .0001).
Our observations will next undergo confirmation in a vaccine-challenge model, followed by testing in dengue-endemic settings that will include phase 3 efficacy trials. Further evaluations will assess (1) the durability of response to a single dose, (2) the significance of DENV-specific cellular immune responses, (3) the ability of rash to predict vaccine take, and (4) effect of preexisting flavivirus exposure on safety and immunogenicity. These evaluations will enhance our knowledge of dengue immunopathogenesis and, most importantly, will lead to a tetravalent vaccine that can play a critical public health role in the control of this expanding infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases Intramural Research Program, National Institutes of Health (contract HHSN272200900010C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Beatty ME, Stone A, Fitzsimons DW, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis 2010; 4:e890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012; 6:e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PAHO/WHO. Five-fold increase in dengue cases in the Americas over the past decade. 29 May 2014. http://www.paho.org/hq/index.php?option=com_content&view=article&id=9657&Itemid=1926. Accessed 5 November 2014.
- 4.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB. Dengue. Lancet 2007; 370:1644–52. [DOI] [PubMed] [Google Scholar]
- 6.Locally acquired dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 2010; 59:577–81. [PubMed] [Google Scholar]
- 7.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1:30–50. [DOI] [PubMed] [Google Scholar]
- 8.Innis BL. Antibody responses to dengue virus infection. In: Gubler DJ, Kuno G, eds. Dengue and dengue haemorrhagic fever. New York: CAB International, 1997:221–43. [Google Scholar]
- 9.Simmons CP, Farrar JJ, Nguyen Vv, Wills B. Dengue. N Engl J Med 2012; 366:1423–32. [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB. Antibodies determine virulence in dengue. Ann N Y Acad Sci 2009; 1171(suppl 1):E48–56. [DOI] [PubMed] [Google Scholar]
- 11.Durbin AP, Kirkpatrick BD, Pierce KK, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 2013; 207:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaney JE, Jr, Durbin AP, Murphy BR, Whitehead SS. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol 2006; 19:10–32. [DOI] [PubMed] [Google Scholar]
- 13.Durbin AP, McArthur JH, Marron JA, et al. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum Vaccin 2006; 2:255–60. [DOI] [PubMed] [Google Scholar]
- 14.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 2011; 29:7242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin AP, McArthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin 2006; 2:167–73. [DOI] [PubMed] [Google Scholar]
- 16.Durbin AP, Schmidt A, Elwood D, et al. Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis 2011; 203:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durbin AP, Whitehead SS, McArthur J, et al. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis 2005; 191:710–8. [DOI] [PubMed] [Google Scholar]
- 18.Lindow JC, Borochoff-Porte N, Durbin AP, et al. Primary vaccination with low dose live dengue 1 virus generates a proinflammatory, multifunctional T cell response in humans. PLoS Negl Trop Dis 2012; 6:e1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindow JC, Durbin AP, Whitehead SS, Pierce KK, Carmolli MP, Kirkpatrick BD. Vaccination of volunteers with low-dose, live-attenuated, dengue viruses leads to serotype-specific immunologic and virologic profiles. Vaccine 2013; 31:3347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright PF, Durbin AP, Whitehead SS, et al. Phase 1 trial of the dengue virus type 4 vaccine candidate rDEN4{Delta}30–4995 in healthy adult volunteers. Am J Trop Med Hyg 2009; 81:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McArthur JH, Durbin AP, Marron JA, et al. Phase I clinical evaluation of rDEN4Delta30–200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg 2008; 79:678–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Troyer JM, Hanley KA, Whitehead SS, et al. A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg 2001; 65:414–9. [DOI] [PubMed] [Google Scholar]
- 23.Durbin AP, Whitehead SS, Shaffer D, et al. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis 2011; 5:e1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg 2001; 65:405–13. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2–9. [DOI] [PubMed] [Google Scholar]
- 26.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis 2010; 201:370–7. [DOI] [PubMed] [Google Scholar]
- 27.Osorio JE, Velez ID, Thomson C, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.