SUMMARY
Recent discoveries of somatic mutations permit the recognition of subtypes of aldosterone-producing adenomas with distinct clinical presentations and pathological features. Here we describe three women with hyperaldosteronism, two who presented in pregnancy and one who presented after menopause. Their aldosterone-producing adenomas harbored activating mutations of CTNNB1, encoding β-catenin in the Wnt cell-differentiation pathway, and expressed LHCGR and GNRHR, encoding gonadal receptors, at levels that were more than 100 times as high as the levels in other aldosterone-producing adenomas. The mutations stimulate Wnt activation and cause adrenocortical cells to de-differentiate toward their common adrenal–gonadal precursor cell type.
Systematic screening detects primary aldosteronism in 5 to 10% of all patients with hypertension and in approximately 20% of patients with treatment-resistant hypertension.1,2 A unilateral aldosterone-producing adenoma is the most common potentially curable cause of hypertension in such cases.2 Early detection of a unilateral aldosterone-producing adenoma is important both to maximize the likelihood of a complete cure of hypertension by means of adenoma removal and to prevent the onset of resistant hypertension and the risk of long-term cardiovascular complications.3
The Wnt pathway, through β-catenin signaling, is critical for normal adrenocortical development and maintenance, in particular the zona glomerulosa of the cortex.4 Both the adrenal cortex and the gonads originate from a common progenitor-cell population in the urogenital ridge.5 Wnt signaling has been shown to maintain the undifferentiated state of adrenocortical progenitor cells even after migration to the adrenal subcapsule.6 This finding highlights the key role of the Wnt pathway in determining steroidogenic cell fate.
Pathogenic somatic mutations of CTNNB1 have been found in 27% of adrenocortical adenomas and in 31% of adrenocortical carcinomas.7 Some of these mutations arise in the part of exon 3 that encodes the consensus glycogen synthase kinase 3β–casein kinase 1 (GSK-3β–CK1) phosphorylation site and therefore result in loss of phosphorylation of β-catenin. This prevents the ubiquitination of β-catenin and leads to aberrant Wnt-pathway activation.8 Until now, β-catenin mutations in adrenocortical adenomas have been mainly associated with nonfunctioning tumors.7 Could some of these tumors be nonfunctioning only because they have not been exposed to the stimulus for hormone secretion?
Here we describe three patients with hyperaldosteronism, two presenting during pregnancy and one after menopause, who were discovered to have mutations in exon 3 of CTNNB1 in their adenomas. All three mutations were predicted to stabilize nonphosphorylated β-catenin and to activate the Wnt signaling pathway. All three aldosterone-producing adenomas expressed very high levels of the gonadal receptors luteinizing hormone–chorionic gonadotropin receptor (LH-CGR) and gonadotropin-releasing hormone receptor (GNRHR). It therefore seems likely that high circulating levels of human chorionic gonadotropin, luteinizing hormone, or gonadotropin-releasing hormone (as observed in pregnancy or at menopause) led to the identification of the aldosterone-producing adenomas in the three patients.
CASE REPORTS
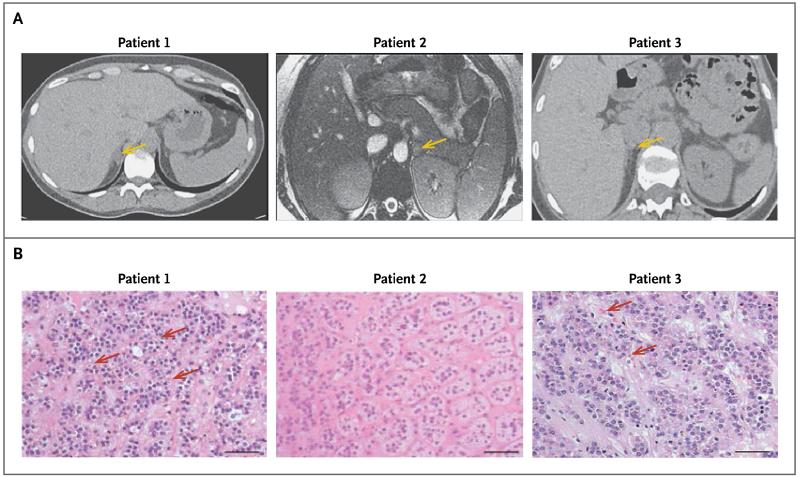
Patient 1, a 34-year-old woman (gravida 4, para 0030), presented in 2005 when she was 17 weeks pregnant; she reported edema and was found to have hypertension and hypokalemia (Table 1). Her blood pressure was 150/90 mm Hg, as compared with 120/70 mm Hg at the first prenatal visit, at 7 weeks.9 Her medical history included an ectopic pregnancy and resulting salpingectomy, two miscarriages, and removal of bilateral ovarian cysts. Her blood pressure was well controlled with amiloride, at a dose of 15 mg daily, until 37 weeks, and spontaneous labor 12 days later resulted in normal vaginal delivery of a healthy girl (birth weight, 3317 g).9 Hypokalemia was fully corrected after pregnancy, and the postpartum plasma aldosterone level fell by more than 50%. Computed tomography (CT) identified an adenoma in the right adrenal gland (Fig. 1A), and laparoscopic adrenalectomy was performed. On examination of the removed adrenal gland, the adenoma appeared as a well-circumscribed, yellow, lobulated nodule, 14 mm in diameter. Microscopic examination of the adenoma revealed eosinophilic spironolactone bodies, which are characteristic of zona glomerulosa–like adenomas (Fig. 1B). The tissue sample stained strongly for CYP11B2, which encodes aldosterone synthase, but it stained weakly for CYP11B1, which is responsible for cortisol production (see Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).10,11 Two months after surgery, the patient’s blood pressure was 105/68 mm Hg without medication, and all biochemical abnormalities had resolved (Table 1).
Table 1. Biochemical Measurements and Phenotypic Characteristics of the Tumors*.
| Patient No. | Age at Time of Surgery |
Measurements before Adrenalectomy | Measurements after Adrenalectomy | Phenotypic Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Aldosterone | Plasma Renin |
Serum Potassium |
Aldosterone | Plasma Renin |
Serum Potassium |
Aldosterone:Cortisol Ratio | Adenoma Size |
Adenoma Classification |
CTNNB1 Mutation |
|||
| Left adrenal vein |
Right adrenal vein |
|||||||||||
| yr | pmol/liter | mU/liter | mmol/liter | pmol/liter | mU/liter | mmol/liter | mm | |||||
| 1 | 34 | 2885 (1176) | <2.0 (<2.0) | 2.0 (4.1) | 172 | 2.0 | 5.0 | 4.1 | 47.2 | 14 | ZG-like | Ser33Cys |
|
| ||||||||||||
| 2† | 26 | 2590 (1268) | <2.0 (<2.0) | 2.0 (3.6) | NA | NA | NA | 5.1 | 0.9 | 17 | ZG-like | Ser45Phe |
|
| ||||||||||||
| 3 | 52 | 672 | <0.2 | 3.1 | 158 | 9.0 | 4.1 | 0.5 | 6.3 | 9 | ZG-like | Gly34Arg |
To convert the values for aldosterone to nanograms per deciliter, divide by 27.74. Numbers in parentheses show postpregnancy values in Patients 1 and 2. NA denotes not available, and ZG zona glomerulosa.
The husband of Patient 2 reported that the patient’s blood pressure was normal after adrenalectomy, but she left the United Kingdom before follow-up biochemical studies could be undertaken.
Figure 1. Adrenal Imaging and Histologic Features in Three Patients with Aldosterone-Producing Adenomas.
The images in Panel A show the adenomas in the three patients (arrows). Histologic examination of the adenomas after adrenalectomy (Panel B, hematoxylin and eosin) shows spironolactone bodies (red arrows), which are characteristic of zona glomerulosa–like aldosterone-producing adenomas. No spironolactone bodies were observed in the adenoma in Patient 2, but it nevertheless resembled a zona glomerulosa–like aldosterone-producing adenoma. Scale bars denote 50 μm.
Patient 2, a 26-year-old primigravida, was admitted to the emergency department when she was 9 weeks pregnant, in 2013, with profound hypokalemia and hypomagnesemia (Table 1). Her husband had returned from work to find her unable to stand up. She reported fatigue, muscle cramps, polyuria, and polydipsia. Her blood pressure was 140/86 mm Hg, and she had a suppressed plasma renin level (<2 mU per liter) in the presence of marked hyperaldosteronism (aldosterone level, 2590 pmol per liter [93.4 ng per deciliter]). Her pregnancy was terminated at 10 weeks for personal reasons, and her blood pressure after termination was 110/66 mm Hg. The initial suspicion of Gitelman’s syndrome was ruled out post partum, when another test of her renin level again showed suppression (<2 mU per liter). The plasma aldosterone level remained elevated, at 1268 pmol per liter (45.7 ng per deciliter), despite near-normalization of plasma potassium levels as a result of potassium supplementation. Magnetic resonance imaging revealed a 17-mm nodule in her left adrenal gland (Fig. 1A); the gland was removed, and the findings on histologic and immunohistochemical examination of the adenoma (Fig. 1B, and Fig. S1 in the Supplementary Appendix) were similar to those in Patient 1.
Patient 3, a 52-year-old woman, was referred in 2008, because of postmenopausal early-morning headaches and hypertension that was progressively difficult to treat. She had undergone hysterectomy for fibroids and right salpingo-oophorectomy at the age of 40 years. By the time of the referral, her blood pressure was 190/100 mm Hg, despite treatment with five antihypertensive drugs, as compared with a pressure of 115/70 mm Hg at a medical examination 2 years before menopause (with menopause imputed from symptoms of flushing). Laboratory investigations revealed hypokalemia, an elevated plasma aldosterone level, and a suppressed plasma renin level (Table 1). CT showed a 9-mm adenoma in the right adrenal gland (Fig. 1A); the gland was removed laparoscopically, and the adenoma within it was found to have features characteristic of a zona glomerulosa–like adenoma, including compact cells, spironolactone bodies (Fig. 1B), and a high ratio of CYP11B2 to CYP11B1 on immunohistochemical analysis (Fig. S1 in the Supplementary Appendix). One month after surgery, her blood pressure was 118/79 mm Hg and aldosterone and renin levels were normal.
METHODS
GENETIC STUDIES
Using tissues obtained with approval from an ethics committee and with written informed consent from the three patients, we performed exome sequencing of DNA from the aldosterone-producing adenoma in Patient 1 with paired germ-line DNA from the adjacent adrenal gland. We also analyzed the adenoma from this patient by means of microarray analysis (see the Methods section in the Supplementary Appendix).11,12 The results have been deposited at the Gene Expression Omnibus (GEO accession number, GSE64957). Bioinformatic analysis of sequence data identified a somatic mutation in exon 3 of CTNNB1 (C→G, p.Ser33Cys) in the adenoma. Owing to a similar, zona glomerulosa–like histologic appearance of the adenomas in Patients 2 and 3 and our recollection (when evaluating Patient 2) that Patient 1 had presented while pregnant, we sought to determine whether the adenomas in Patients 2 and 3 shared genomic and transcriptomic features with the adenoma in Patient 1. Sanger sequencing of exon 3 of genomic DNA (gDNA) and of the whole complementary DNA sequence of CTNNB1 showed that the adenomas in Patients 2 and 3 also carried mutations in CTNNB1 (C→T, p.Ser45Phe, and G→A, p.Gly34Arg, respectively). On sequencing exon 3 of the gDNA in nine control aldosterone-producing adenomas from five men and from four women who were not pregnant at the time of presentation, we did not detect mutations in CTNNB1 (Table S1 in the Supplementary Appendix). These control adenomas were selected because of their previous inclusion in a semi-quantitative histologic analysis of cell type.10,12
FUNCTIONAL ANALYSES OF CTNNB1 MUTATIONS
After generation of the β-catenin mutants (Ser33Cys, Gly34Arg, and Ser45Phe) by means of site-directed mutagenesis, we measured Wnt–β-catenin activity using TCF/LEF luciferase reporter assays. Stabilization of mutants was shown by means of Western blotting, with the use of a monoclonal antibody that detects the transcriptionally active form of β-catenin (see the Methods section in the Supplementary Appendix).
EX VIVO STUDIES OF ALDOSTERONE-PRODUCING ADENOMAS
A microarray assay was performed on 42 RNA samples (14 trios of samples from adenomas, adjacent zona fasciculata, and adjacent zona glomerulosa, including samples from Patient 1) (see the Methods section in the Supplementary Appendix).12 Gene expression of the adenoma in Patient 3 had been assayed by microarray previously.10
We measured LHCGR, GNRHR, and gonadal transcription factor GATA4 messenger RNA (mRNA) levels in the adenomas and adjacent adrenal tissue from all three patients, using a quantitative polymerase-chain-reaction (qPCR) assay with specific primers. Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded adrenal sections (4 μm in thickness) for β-catenin (BD Biosciences) and LHCGR (Sigma). To confirm a direct effect of CTNNB1 mutation on the expression of receptors for luteinizing hormone and human chorionic gonadotropin, immunofluorescence staining was performed on zona glomerulosa–like adenoma cells after transfection with wild-type or mutated CTNNB1 (see the Methods section in the Supplementary Appendix).
RESULTS
IDENTIFICATION OF MUTATIONS IN EXON 3 OF CTNNB1
Whole-exome sequencing of the aldosterone-producing adenoma from Patient 1 identified a heterozygous somatic mutation (C→G, p.Ser33Cys) in exon 3 of CTNNB1. Mutation in CTNNB1 was absent in the nine control adenomas from men and from women who had not presented during pregnancy. Sanger sequencing identified heterozygous mutations in the same exon of CTNNB1 in the aldosterone-producing adenomas in the other two patients (C→T, p.Ser45Phe, in Patient 2, and G→A, p.Gly34Arg, in Patient 3) (Fig. S2 in the Supplementary Appendix).
MUTANT β-CATENINS AND THE WNT PATHWAY
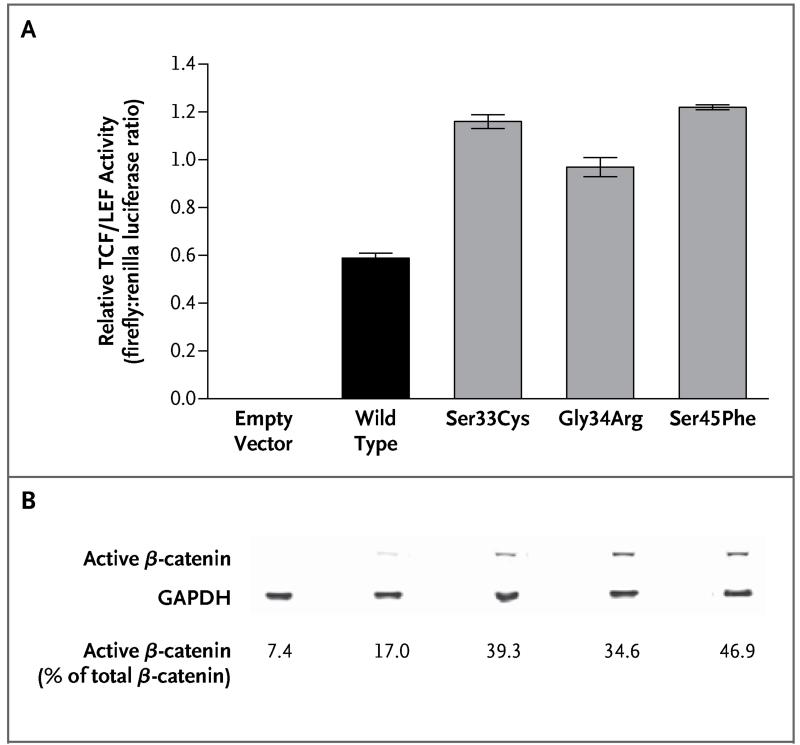
In HEK293T cells transfected with mutated CTNNB1, there was increased transcriptional activity of a TCF/LEF-responsive luciferase construct, as compared with the vector control and with wild-type CTNNB1 (Fig. 2A). Western blotting revealed increased expression of active β-catenin (nonphosphorylated at residues Ser33, Ser37, and Thr41) by the three mutant cell lines (Fig. 2B). In addition, a greater proportion of total β-catenin was in its active form (Fig. S3 in the Supplementary Appendix).
Figure 2. Relative TCF/LEF Activity and Immunoblots after Transfection with Control and CTNNB1 Constructs.
Panel A shows relative TCF/LEF activity, measured as the ratio of firefly luciferase to renilla luciferase, with the use of a luciferase-tagged TCF/LEF-responsive promoter in HEK293T cells. Results are from five experiments performed 48 hours after transfection with empty vector and with wild-type and mutated CTNNB1 constructs. I bars indicate standard errors, and P<0.001 for all comparisons of the mutated constructs with wild-type CTNNB1. Panel B shows immunoblots for active endogenous β-catenin in HEK293T cells 72 hours after transfection with empty vector and with wild-type and mutated CTNNB1 constructs. Values are percentages of total endogenous β-catenin that was active (i.e., nonphosphorylated at residues Ser33, Ser37, and Thr41). Immunoblots obtained 24 hours after transfection showed similar results (Fig. S3 in the Supplementary Appendix). GAPDH denotes glyceraldehyde-3-phosphate dehydrogenase.
UP-REGULATION OF GONADAL RECEPTORS IN MUTANT ADENOMAS
Microarray analysis showed LHCGR to be the most overexpressed gene (×748), followed by GNRHR (×120), in the aldosterone-producing adenoma from Patient 1, as compared with 13 other adenomas (7 zona fasciculata–like and 6 zona glomerulosa–like adenomas with no CTNNB1 mutation) (Fig. 3A). These increases in LHCGR and GNRHR were confirmed by means of a qPCR assay in all three CTNNB1-mutant adenomas (Fig. 3B).
Figure 3. Ex Vivo Studies of Aldosterone-Producing Adenomas.
Panel A shows the results of a microarray analysis of LHCGR and GNRHR expression in the aldosterone-producing adenoma (APA) in Patient 1, as compared with six zona glomerulosa (ZG)–like control adenomas and seven zona fasciculata (ZF)–like control adenomas. T bars indicate standard errors. Panel B shows the results of a quantitative polymerase-chain-reaction (qPCR) assay, which confirms that expression of both genes is much higher in the APAs of the three patients than in the six ZG-like and seven ZF-like control APAs. The log2 factor change refers to the difference between the tumor and the adjacent nontumorous adrenal tissue in the expression of LHCGR and GNRHR. Panel C shows immunohistochemical staining for β-catenin and luteinizing hormone–chorionic gonadotropin receptor (LHCGR) on adenoma cells after adrenalectomy in the three patients, as compared with adjacent adrenal tissue. (For each patient, adenoma cells are shown on the left, and adjacent adrenal tissue is shown on the right.) Scale bars denote 50 μm. Panel D shows immunofluorescence staining for LHCGR proteins in ZG-like adenoma cells transfected with mutant (Ser33Cys) CTNNB1. Red indicates LHCGR, green GFP (green fluorescent protein)–tagged mutant β-catenin, and blue nuclear DNA stained with 4′,6-diamidino-2-phenylindole (DAPI).
Immunohistochemical analysis showed greater β-catenin expression and more abundant membrane staining of LHCGR in the mutant adenomas than in the adjacent adrenal tissue (Fig. 3C). Transfection of primary zona glomerulosa–like adenoma cells with mutant CTNNB1 led to expression of LHCGR, as shown by immunofluorescence staining, whereas untransfected cells on the same slide did not stain for LHCGR (Fig. 3D) (for findings in controls, see Fig. S4 and S5 in the Supplementary Appendix).
DISCUSSION
There have been previous reports of CTNNB1 mutations in adrenal tumors7 and of aberrant G-protein-coupled receptors (including LHCGR and GNRHR) in aldosterone-producing adenomas,13-16 as well as a case report of a zona glomerulosa–like adenoma in a patient presenting during pregnancy.17 Our findings link these observations and provide a molecular basis for the further observation that pregnancy can unmask the zona glomerulosa type of aldosterone-producing adenoma. We have previously reported that such adenomas have a distinct set of somatic mutations, as compared with the classic, more zona fasciculata–like aldosterone-producing adenomas, which usually harbor a mutation in KCNJ5.10,11
Despite the known association between CTNNB1 activation and aldosterone-producing adenomas, to our knowledge, only one of the three mutations we describe here, Ser45Phe, has previously been identified in adrenal tissue; the same residue is mutated in immortalized H295R cells, derived from an adrenocortical carcinoma. All three mutations are predicted to affect a GSK-3β phosphorylation consensus motif and could thus impair β-catenin degradation and up-regulate canonical Wnt activity (Fig. 2A) as a result of elevated levels of active β-catenin (Fig. 2B, and Fig. S3 in the Supplementary Appendix).
Arising from a common adrenal–gonadal primordium, mesenchymal cells develop into two distinct populations — adrenal precursor cells and bipotential gonadal precursor cells — which ultimately develop into the adrenal cortex and the ovaries or testis, respectively,5 under the regulation of steroidogenic factor 1 (SF1). SF1-dependent transcription of genes controlling adrenal–gonadal cell fate is regulated by Wnt signaling cascades, in which β-catenin has a pivotal role.18 The influence of β-catenin on LHCGR was anticipated on the basis of experiments on granulosa cells of the preovulatory follicle, where β-catenin activates the LHCGR promoter, together with the transcription factors SF1 and TCF3. Transduction of granulosa cells with a constitutively active β-catenin mutant leads to an increase in LHCGR mRNA expression by a factor of 3.19
Adrenal-cell renewal is thought to occur through the amplification, centripetal migration, and differentiation of initially undifferentiated subcapsular mesenchymal progenitor cells.20 We conclude that the three mutations affecting β-catenin in aldosterone-producing adenomas, which we describe here, activate aldosterone production by unleashing the ability of these adrenal progenitor cells to switch on gonadal genes (Fig. 3A, 3B, and 3C). The production of aldosterone is latent until the aberrant gonadal receptors are stimulated by pregnancy hormones. Of the two receptors, LHCGR appears to be the more relevant, since it is more highly overexpressed than GNRHR. But gonadotropin-releasing hormone, the agonist for GNRHR, is expressed in the adrenal gland12 and may be secreted by the placenta into the maternal circulation during the first half of pregnancy.21
Our explanation for the latency of aldosterone production is supported by the appearance of luteinizing hormone receptor protein in primary, zona glomerulosa–like, aldosterone-producing adenoma cells transfected with mutant CTNNB1 (Fig. 3D) and by the increase in GATA4 both in the three CTNNB1-mutated adenomas and in the cells overexpressing wild-type or mutated CTNNB1 (Fig. S6 and S7, respectively, in the Supplementary Appendix). As a marker of gonadal differentiation, the transcription factor GATA4 should be absent in adrenal cells, so its elevated levels point to dedifferentiation toward their common adrenal–gonadal precursor cell type. CTNNB1 mutation may need to occur in specific adrenocortical cell types, such as the subcapsular progenitor cells, for the full effects to be seen. Our transfections had a moderate effect in zona glomerulosa–like aldosterone-producing adenoma cells but had no effect in the adrenocortical carcinoma–derived H295R cells; these cells also had little endogenous LHCGR expression, despite their intrinsic CTNNB1 mutation. Indeed, adrenocortical carcinomas commonly have CTNNB1 mutations, yet previous transcriptome analysis of 33 carcinomas showed above-background LHCGR expression in only 4.22
Patient 3 did not present during pregnancy; rather, she had hypertensive headaches shortly after menopause. This can be explained by the fact that luteinizing hormone is increased by a factor of approximately 3 after menopause because of the lack of ovarian estrogen feedback.23 Whereas the sharp increase in human chorionic gonadotropin levels during early pregnancy appears to cause a more explosive presentation, postmenopausal latency (and the accompanying rise in luteinizing hormone) is likely to be of longer duration. The apparent infrequency of primary aldosteronism at menopause most likely reflects the relative rarity of these mutations. The presentation of Patient 3 is reminiscent of a previous case of aberrant LHCGR adrenal expression in a woman with transient Cushing’s syndrome during each of her four pregnancies, which reappeared after menopause.24
Clinically, primary aldosteronism during pregnancy is perilous for both mother and fetus, as shown by a recent case of overlooked primary aldosteronism resulting in delivery of an infant who was small for gestational age.25 Provision of a molecular explanation will help to increase awareness of aldosterone-producing adenomas as the probable cause of hypertension, hypokalemia, or both early in pregnancy.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (Cardiovascular) (NF-SI-0512-10052, to Prof. Brown), the Agency for Science, Technology and Research (A*STAR) Singapore (to Ms. Teo), Wellcome Trust (085686/Z/08/A, to Ms. Teo), the British Heart Foundation (FS/14/75/31134, to Ms. Garg; and FS/11/35/28871, to Ms. Haris Shaikh), Cambridge Overseas Trust (to Dr. Zhou), NIHR Cambridge Biomedical Research Centre (Metabolic) (to Dr. Gurnell), and Medical Research Council (U105192713, to Dr. Bienz); the Tunku Abdul Rahman Centenary Fund, Cambridge (to Dr. Azizan); and the Austin Doyle Award (Servier Australia) (to Dr. Azizan).
We thank Dr. James Brenton and his group for the generous gift of OVCAR-3 cells; Ms. Diana Walters and the Human Research Tissue Bank of Addenbrooke’s Hospital for help with collection of fresh adrenal tissue; and Dr. Jonathan Roland, Peterborough and Stamford Foundation Hospitals NHS Foundation Trust (United Kingdom), and Dr. Nadia Schoenmakers, Institute of Medical Science, University of Cambridge, for referring Patient 1 and providing details of the patient’s obstetrical history.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–6. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 4.Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- 5.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–71. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Berthon A, Martinez A, Bertherat J, Val P. Wnt/β-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351:87–95. doi: 10.1016/j.mce.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Tissier F, Cavard C, Groussin L, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 8.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ali NA, El-Sandabesee D, Steel SA, Roland JM. Conn’s syndrome in pregnancy successfully treated with amiloride. J Obstet Gynaecol. 2007;27:730–1. doi: 10.1080/01443610701667098. [DOI] [PubMed] [Google Scholar]
- 10.Azizan EA, Lam BY, Newhouse SJ, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97(5):E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 11.Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–60. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Shaikh LH, Neogi SG, et al. DACH1, a zona glomerulosa selective gene in the human adrenal, activates transforming growth factor-β signaling and suppresses aldosterone secretion. Hypertension. 2015;65:1103–10. doi: 10.1161/HYP.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saner-Amigh K, Mayhew BA, Mantero F, et al. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91:1136–42. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- 14.Albiger NM, Sartorato P, Mariniello B, et al. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol. 2011;164:405–12. doi: 10.1530/EJE-10-0879. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Hattangady NG, Ye P, et al. Aberrant gonadotropin-releasing hormone receptor (GnRHR) expression and its regulation of CYP11B2 expression and aldosterone production in adrenal aldosterone-producing adenoma (APA) Mol Cell Endocrinol. 2014;384:102–8. doi: 10.1016/j.mce.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Ghorayeb N, Bourdeau I, Lacroix A. Multiple aberrant hormone receptors in Cushing’s syndrome. Eur J Endocrinol. 2015 May 13; doi: 10.1530/EJE-15-0200. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Shigematsu K, Nishida N, Sakai H, et al. Primary aldosteronism with aldosterone-producing adenoma consisting of pure zona glomerulosa-type cells in a pregnant woman. Endocr Pathol. 2009;20:66–72. doi: 10.1007/s12022-009-9060-8. [DOI] [PubMed] [Google Scholar]
- 18.Gummow BM, Winnay JN, Hammer GD, et al. Convergence of Wnt signaling and SF-1 on transcription of the rat inhibin α gene. J Biol Chem. 2003;278:26572–9. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- 19.Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol. 2013;27:1295–310. doi: 10.1210/me.2013-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim AC, Barlaskar FM, Heaton JH, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–63. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siler-Khodr TM, Khodr GS, Valenzuela G. Immunoreactive gonadotropin-releasing hormone level in maternal circulation throughout pregnancy. Am J Obstet Gynecol. 1984;150:376–9. doi: 10.1016/s0002-9378(84)80142-8. [DOI] [PubMed] [Google Scholar]
- 22.Barlaskar FM, Spalding AC, Heaton JH, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94:204–12. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JWW. Hormonal profiles after the menopause. Br Med J. 1976;2:784–7. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone–dependent Cushing’s syndrome. N Engl J Med. 1999;341:1577–81. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi K, Hoshide S, Nagashima S, Maekawa T, Sasano H, Kario K. An adverse pregnancy-associated outcome due to overlooked primary aldosteronism. Intern Med. 2014;53:2499–504. doi: 10.2169/internalmedicine.53.2762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.