Abstract
Background
Leukemia seriously threatens human life and health. MicroRNAs can regulate cell growth, proliferation, and death. This article investigated the role of miR-29 on regulating leukemia cell growth, proliferation, and apoptosis.
Material/Methods
miR-29 and scramble miRNA were transfected to K562 cells. MTT assay, colony formation assay, caspase-3 activity detection, and flow cytometry were applied to test miR-29 effect on cell growth, proliferation, and apoptosis. Western blot was used to detect Forkhead box protein M1 (FoxM1) protein expression. After we transfected miR-29, K562 cells were transfected with FoxM1 siRNA to test cell apoptosis.
Results
K562 cell growth and proliferation were inhibited after transfection with miR-29. Apoptosis phenome and caspase-3 activation were observed. FoxM1 level decreased. SiRNA FoxM1 enhanced miR-29-induced K562 cell apoptosis. FoxM1 overexpression suppressed miR-26-induced K562 cell apoptosis.
Conclusions
MiR-29 restrained K562 cell growth and proliferation. MiR-29 induced K562 cell apoptosis through down-regulating FoxM1.
MeSH Keywords: Amlodipine, Buthionine Sulfoximine, K562 Cells
Background
Leukemia is caused by several factors, including genetic, virus, chemical, and radiation factors. Leukemia has become the sixth leading cause of death among all cancers [1]. Currently, the main treatments for leukemia are radiotherapy, chemotherapy, stem cell transplantation, molecular target therapy, and immune therapy [2]. Although various therapies are available, molecular-targeted therapy is applicable to almost all types of leukemia. Leukemia-targeted therapy focuses on protein or other molecules closely related to occurrence and development. At present, there are few targets for leukemia therapy. Therefore, in this study we explored potential targets for leukemia-targeted therapy from the cellular level.
MicroRNAs is an important member of the small non-coding RNA family, which plays a key role in cell growth, proliferation, death, autophagy, and multiple signaling pathways through regulating target genes transcription, expression, and activity [3]. At present, miRNAs with clearly defined functions includes miR-29, miR-150, miR-150, miR-181a, miR-23a, miR-148/152, miR-221/222, miR-483-3p, miR-30e, miR-397, and miR-126 [4]. Our study speculated that miR-29 may play a role through regulating transcription factors, while the other miRNAs’ regulating effect is unclear [3–5]. The miR-29 family comprises 3 isoforms arranged in 2 clusters: miR-29b-1/miR-29a in chromosome 7q32 and mir-29b-2/miR-29c in chromosome 1q23. Interestingly, chromosome 7q32 is a frequent region of deletion in myelodysplasia and therapy-related acute myeloid leukemia (AML). In fact, miR-29 family members have been shown to be downregulated in CLL, lung cancer, invasive breast cancer, AML, and cholangiocarcinoma [3–5]. miR-29 can inhibit breast cancer cell growth. We investigated the regulating effect of miR-29 on leukemia cell growth, proliferation, and apoptosis [6].
Forkhead box protein M (FoxM) is a conservative transcription factor widely expressed, from single-cell yeasts to mammals [7]. It belongs to the helix-turn-helix protein family and can be divided into 17 subtribes with more than 100 protein members according to their DNA binding site homology size. Among them, FoxM1 is a transcription factor related to cell proliferation, growth, and death [8]. At present, the role of FoxM1 and miRNAs in regulating K562 cells still needs further investigation.
Thus, the aim of this study was to explore the effect of miR-29 on K562 cell growth, proliferation, and apoptosis.
Material and Methods
Reagents and cell line
3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) was purchased from Beyotime Biotechnology Co. FoxM1 plasmid was from the plasmid library in our laboratory. FoxM1 primary antibody and horseradish peroxidase-labeled secondary antibody were from Sigma. GAPDH primary antibody was bought from Cell Signaling. Cell culture medium RPMI-1640 and the fetal bovine serum were purchased from Santa Cruz. K562 cells were provided by the American type culture collection (ATCC). RNA extraction and reverse transcription RT-PCR kit was purchased from Beijing Dingguo Changsheng Biotechnology Co., LTD. FITC-Annexin V and caspase 3 activity detection kits were from Beyotime Biotechnology Research Institute. MiR-29 and scramble miRNA were designed and produced by Sangon.
Cell culture
K562 cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum in a humid atmosphere containing 5% CO2 at 37°C [9].
MTT assay
K562 cell growth and viability were detected by MTT assay according to the literature [10]. Cells were seeded in 12-well plates at a density of 8×105 cells/well and incubated for 72 h at 37°C. After addition of 5 mg/ml MTT to each well, plates were incubated for 6 h at 37°C. Another 100 μL DMSO was added and absorbance of each well at 492 nm was read using a spectrophotometer [11].
Colony formation assay
K562 cell colony formation ability was measured according to the literature [12]. Cells were plated in soft agar at different cell numbers and cultured for 12 days. The colonies were stained with Giemsa for 30 min after fixation with 10% formaldehyde for 30 min.
Transfection
K562 cells were seeded into 96-well plates. We transfected 150 nM FoxM1 siRNA (sequence: 5′-GGTTGTTACGATTACTTCC-3′, 5′-TGATAAGAGTGGCCTTCTC-3′) or miR-29 to cells by using Lipo2000. K562 cells were cultured in 37°C and 5% CO2 for 24 h after transfection. The cells were further used for apoptosis assay and Western blot [13].
Flow cytometry: Cells were incubated at Annexin-V-FITC combining buffer at 1×105 cells. Flow cytometry was used to detect apoptosis of the transfected K562cells at 488 nm by determining the relative amount of Annexin V-FITC-positive cells [14].
Western blot
The cells were harvested and homogenized with lysis buffer. Total protein was separated by denaturing 10% SDS – polyacrylamide gel electrophoresis and FoxM1 protein level was detected [15].
Caspase-3 activity assay
K562 cells caspase-3 activity was determined according to the manual [16]. The cells were harvested and resuspended in RIPA. The cells were incubated for 60 min at 37°C after adding 2 mM Ac-DEVD-pNA. Absorbance of each well at 492 nm was read using a spectrophotometer [17].
Statistical analysis
All statistical analyses were performed using SPSS13.0 software. Numerical data are presented as means and standard deviation. Differences between multiple groups were analyzed by one-way ANOVA. P < 0.05 was considered as a significant difference.
Results
MiR-29 transfection inhibited K562 cells growth
As shown in Figure 1, 1 μg miR-29 transfection restrained K562 cells growth significantly (P=0.0012), while cells growth presented no obvious difference when transfected with miRNA, so we omitted the non-transfection group in the following experiments.
Figure 1.
MiR-29 transfection inhibited K562 cells growth.
MiR-29 transfection suppressed K562 cells proliferation
Colony formation assay showed that 1 μg miR-29 transfection obviously suppressed K562 colony formation ability compared with the miRNA transfection group (P=0.029) (Figure 2).
Figure 2.
MiR-29 transfection suppressed K562 cells proliferation.
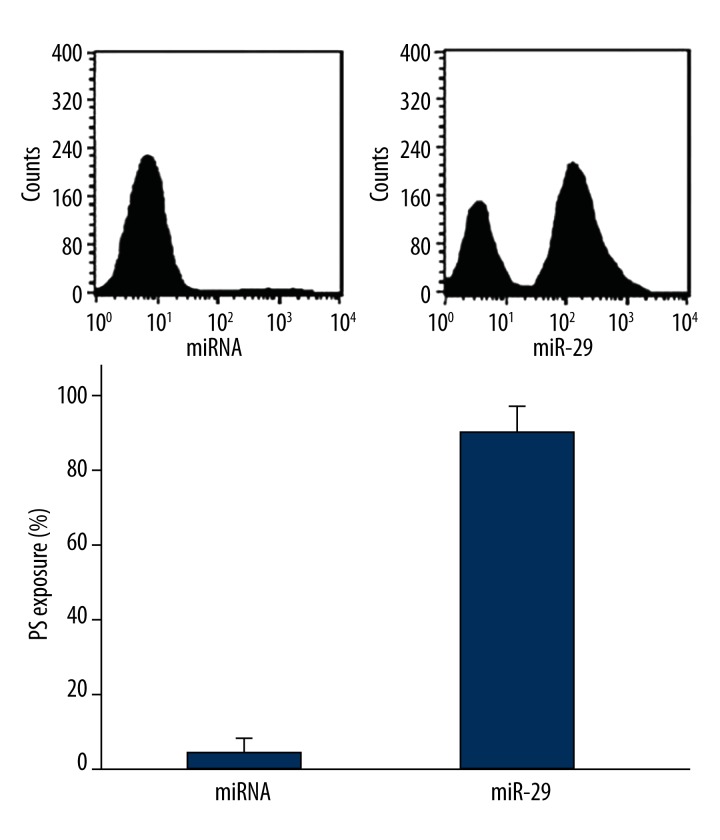
MiR-29 transfection induced K562 cell apoptosis
Flow cytometry revealed that phosphatidylserine eversion level increased significantly after transfection with 1 μg miR-29 (P=0.0024) (Figure 3).
Figure 3.
MiR-29 transfection induced K562 cell apoptosis.
MiR-29 transfection induced caspase-3 activation in K562 cells
Caspase-3 activity degree increased markedly after transfected with 1 μg miR-29 (P=0.012) (Figure 4).
Figure 4.
MiR-29 transfection induced caspase-3 activation in K562 cells.
MiR-29 transfection decreased FoxM1 protein level
Western blot analysis showed that 1 μg miR-29 transfection decreased FoxM1 protein level (Figure 5).
Figure 5.
MiR-29 transfection decreased FoxM1 protein level.
FoxM1 knockdown enhanced miR-29 induced K562 cell apoptosis
Phosphatidylserine eversion suggested that after FoxM1 knockdown by siRNA, 0.1 μg miR-29-induced K562 cell apoptosis increased significantly (P=0.0053) (Figure 6).
Figure 6.
FoxM1 knockdown enhanced miR-29-induced K562 cell apoptosis.
FoxM1 overexpression inhibited miR-29 induced cell apoptosis
Flow cytometry indicated that after FoxM1 plasmid was transfected, 1 μg miR-29 induced K562 cell apoptosis decreased obviously (P=0.0029) (Figure 7).
Figure 7.
FoxM1 overexpression inhibited miR-29-induced cell apoptosis.
Discussion
MiRNAs play a role in tumorigenesis and development, which has significance for tumor diagnosis and treatment. In this study, we investigated the regulating effect of miR-29 on K562 cells and its possible mechanism. The results showed that miR-29 overexpression inhibits human leukemia K562 cells growth and proliferation, and promotes K562 cells apoptosis, which is consistent with previous results [5].
FoxM1 is a conservative transcription factor that can mediate cell growth, proliferation, and apoptosis through regulating gene expression [18]. At present, whether FoxM1, synergetically or antagonistically with miRNAs, can regulate K562 cells growth and apoptosis has not yet been determined [19,20]. The results showed that miR-29 overexpression down-regulated FoxM1 expression level. FoxM1 knockdown enhanced miR-29 transfection-induced K562 cells apoptosis. FoxM1 overexpression inhibited cell apoptosis caused by miR-29. MiR-29 activating Caspase-3 in K562 cells suggests that miR-29 induces K562 cell apoptosis.
In this study, there were 3 main results demonstrating that FoxM1 protein plays an important role in miR-29 induced K562 cells apoptosis: (1) Western blot analysis showed that miR-29 significantly decreased FoxM1 protein expression in K562 cells; (2) siRNA FoxM1 enhanced miR-29 transfection-induced K562 cell apoptosis; and (3) FoxM1 overexpression inhibited cell apoptosis caused by miR-29. These data prove that FoxM1 protein plays a key role in miR-29-induced K562 cell apoptosis. It also reveals that an intervention strategy targeting FoxM1 protein may be a new approach for leukemia treatment.
Many miRNAs are believed to act as tumor suppressors, although the evidence supporting those claims is merely correlative. Substantial experimental data are lacking, and miRNA knockout mice that develop or are predisposed to cancer have not been yet reported. It is noteworthy that most of the miRNAs with a clear tumor suppressor role (miR-15- a/16-1, miR-29s, and let-7) have more than 1 genomic location, and although they are transcribed from different precursors, the mature miRNA is identical. The different loci could be differentially regulated; for example, in HeLa cells the mature miR-29b is preferentially transcribed from the miR-29b-1/miR-29a locus in chromosome 7q32, whereas the other locus, miR-29b-2/miR-29c in chromosome 1q23, is silenced.
This study has 3 limitations: (1) the lack of clinical leukemia specimens at different development stages. Western blot detection of FoxM1 protein expression in leukemia and normal tissue level could reveal the relationship between FoxM1 level and leukemia progression; (2) the lack of clinical specimens using different chemotherapy drugs. Western blot detection of FoxM1 protein in leukemia and normal tissue can further determine their relationship; and (3), the lack of a leukemia rat animal model treated with miR-29, which can explore the effect of miR-29 in leukemia treatment from the animal level and provide information for clinical practice.
Conclusions
Our results revealed that miR-29 transfection can inhibit K562 cells growth and proliferation. MiR-29 induced K562 cells apoptosis through down-regulating FoxM1. FoxM1 might be a potential target for killing tumor cells.
Footnotes
Source of support: Departmental sources
References
- 1.Napier RJ, Norris BA, Swimm A, et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015;11:e1004770. doi: 10.1371/journal.ppat.1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chendamarai E, Ganesan S, Alex AA, et al. Comparison of newly diagnosed and relapsed patients with acute promyelocytic leukemia treated with arsenic trioxide: insight into mechanisms of resistance. PLoS One. 2015;10:e0121912. doi: 10.1371/journal.pone.0121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang FQ, Zhang HM, Chen SJ, et al. MiR-506 is down-regulated in clear cell renal cell carcinoma and inhibits cell growth and metastasis via targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Sun Y, He Y, et al. MicroRNA-338-3p inhibits cell proliferation in hepatocellular carcinoma by target forkhead box P4 (FOXP4) Int J Clin Exp Pathol. 2015;8:337–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Hernandez E, Duenas-Gonzalez MT, Arellano-Galindo J, et al. Survival of Mexican children with acute myeloid leukaemia who received early intensification chemotherapy and an autologous transplant. Biomed Res Int. 2015;2015:940278. doi: 10.1155/2015/940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duhachek-Muggy S, Zolkiewska A. ADAM12-L is a direct target of the miR-29 and miR-200 families in breast cancer. BMC Cancer. 2015;15:93. doi: 10.1186/s12885-015-1108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bicknell KA. Forkhead (FOX) transcription factors and the cell cycle: measurement of DNA binding by FoxO and FoxM transcription factors. Methods Mol Biol. 2005;296:247–62. doi: 10.1385/1-59259-857-9:247. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Chen W, Miao R, et al. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurhayati RW, Ojima Y, Nomura N, Taya M. Promoted megakaryocytic differentiation of K562 cells through oxidative stress caused by near ultraviolet irradiation. Cell Mol Biol Lett. 2014;19:590–600. doi: 10.2478/s11658-014-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimi R, Mahdavi M, Pejman S, et al. Inhibition of cell proliferation and induction of apoptosis in K562 human leukemia cells by the derivative (3-NpC) from dihydro-pyranochromenes family. Acta Biochim Pol. 2015;62:83–88. doi: 10.18388/abp.2014_825. [DOI] [PubMed] [Google Scholar]
- 11.Ghasemian M, Mahdavi M, Zare P, et al. Spiroquinazolinone-induced cytotoxicity and apoptosis in K562 human leukemia cells: alteration in expression levels of Bcl-2 and Bax. J Toxicol Sci. 2015;40:115–26. doi: 10.2131/jts.40.115. [DOI] [PubMed] [Google Scholar]
- 12.Eide CA, Adrian LT, Tyner JW, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer Res. 2011;71:3189–95. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu XS, Lin ZY, Du J, et al. BCR/ABL mRNA targeting small interfering RNA effects on proliferation and apoptosis in chronic myeloid leukemia. Asian Pac J Cancer Prev. 2014;15:4773–80. doi: 10.7314/apjcp.2014.15.12.4773. [DOI] [PubMed] [Google Scholar]
- 14.Smolarek D, Hattab C, Buczkowska A, et al. Studies of a murine monoclonal antibody directed against DARC: reappraisal of its specificity. PLoS One. 2015;10:e0116472. doi: 10.1371/journal.pone.0116472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Yang H, Song Z, Gu S. Copper excess in liver HepG2 cells interferes with apoptosis and lipid metabolic signaling at the protein level. Turk J Gastroenterol. 2014;25:S116–21. doi: 10.5152/tjg.2014.5064. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Zhao L, Liu J, et al. Cisplatin induces platelet apoptosis through the ERK signaling pathway. Thromb Res. 2012;130:81–91. doi: 10.1016/j.thromres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Liu J, Sun R, et al. Calpain activator dibucaine induces platelet apoptosis. Int J Mol Sci. 2011;12:2125–37. doi: 10.3390/ijms12042125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Chen X, Gu Y, et al. FOXM1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and mediates sensitivity to cisplatin in A549 cells via the JNK/mitochondrial pathway. Neoplasma. 2015;62:61–71. doi: 10.4149/neo_2015_008. [DOI] [PubMed] [Google Scholar]
- 19.Zhou M, Zeng J, Wang X, et al. MiR-370 sensitizes chronic myeloid leukemia K562 cells to homoharringtonine by targeting Forkhead box M1. J Transl Med. 2013;11:265. doi: 10.1186/1479-5876-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zeng J, Zhou M, et al. The tumor suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer. 2012;11:56. doi: 10.1186/1476-4598-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]