Abstract
Abdominal pain is one of the most common reasons for outpatient and emergency department visits. We present one such case of early closure in a 32-year-old male with recurrent abdominal pain who was diagnosed with irritable bowel syndrome (IBS). Family history was suspicious for hereditary angioedema (HAE). The HAE workup came back positive, and the patient was started on prophylactic therapy, which led to an improvement in symptoms and quality of life. The purpose of this case is to create awareness among physicians to test for HAE in patients diagnosed with IBS who, based on their history or physical examination, have clinical suspicion for HAE.
Keywords: C4, hereditary angioedema, IBS, abdominal pain, C1 esterase inhibitor
A 32-year-old Caucasian male with a past medical history of irritable bowel syndrome (IBS) came to the emergency department with severe abdominal pain, intractable nausea, and vomiting, which began a few hours before presentation. He denied fever, skin rash, sick contact, or recent travel. Home medications included oxycodone/acetaminophen as needed for pain but no prior exposure to Angiotensin-converting enzyme (ACE) inhibitors or Angiotensin receptor blockers (ARB). There was no history of smoking or recreational drug use. He reported having recurrent attacks of abdominal pain, nausea, and diarrhea that had previously been attributed to IBS. Interestingly, he stated that his father had similar episodes of abdominal pain, but in his father's case, these episodes were associated with swelling of his hands and feet. However, he was not sure of his father's final diagnosis.
Physical examination showed an afebrile, middle-aged man in severe pain. His heart rate was 120 beats per minute, and BP was 100/65 mmHg. Abdominal exam revealed no distention; normal bowel sounds but diffuse abdominal tenderness with some guarding. The rest of the physical exam was negative, and there was no facial swelling or skin rash noted.
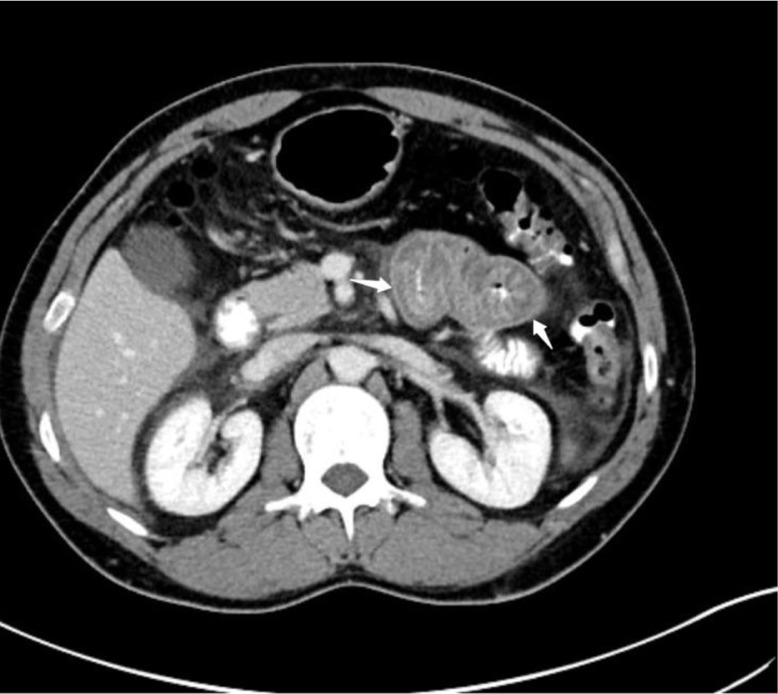
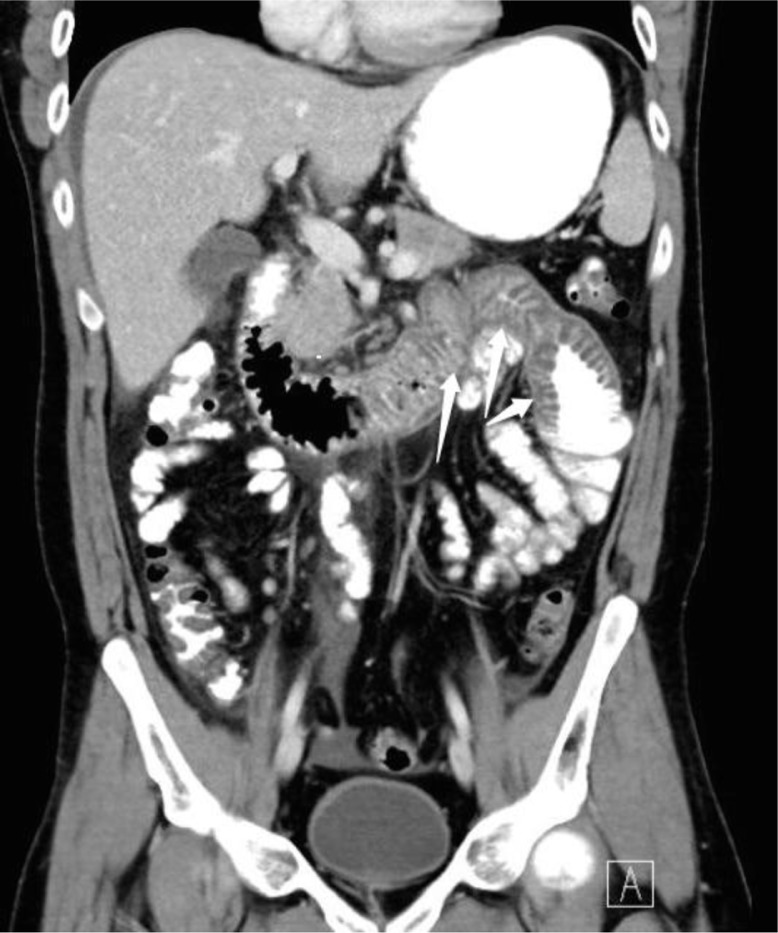
Laboratory results, including serum amylase, lipase, Complete blood count (CBC), and urinalysis were unremarkable. A computed tomographic (CT) scan of his abdomen and pelvis with oral and intravenous contrast was performed. The CT scan showed a marked thickening of the fourth portion of the duodenum and the proximal jejunum, with no evidence of acute diverticulitis, bowel perforation, or bowel obstruction (Figs. 1 and 2). Given the recurrent nature of his symptoms, as well as the positive family history and CT scan findings, a C4 level and C1 esterase inhibitor (C1-INH) protein level and function study were done. The result was consistent with type 1 hereditary angioedema (HAE). The patient was subsequently started on danazol for prophylaxis, which led to an improvement in symptoms and a prospective decrease in the frequency of attacks.
Fig. 1.
Computed tomography showing marked wall thickening of small bowel.
Fig. 2.
Coronal view showing marked wall thickening of fourth duodenal portion and proximal jejunum.
Discussion
IBS represents one of the most common causes of abdominal pain, and it affects around 11% of the population around the world (1, 2). HAE is a rare cause of abdominal pain, and sometimes can be misdiagnosed as IBS. HAE is a rare autosomal dominant disorder with an estimated prevalence of 1 in 50,000 worldwide with no racial or sex differences, although women tend to have more severe disease (3, 4). In 1888, William Osler comprehensively described the clinical manifestation of HAE (5), and in 1963, Donaldson and Evans subsequently discovered that HAE was caused by a mutation of the C1-INH gene (6).
This C1-INH gene, SERPING1, is located on chromosome 11q11-q13.1 (4, 7). HAE accounts for about 75% of cases, with the remaining 20–25% of cases being sporadic (4, 7). C1-INH, a protein synthesized mainly by hepatocytes, belongs to the serine protease inhibitor family. Its deficiency or dysfunction leads to elevated levels of bradykinin, believed to account for most of the disease manifestations (4).
The disease is divided into three different types, depending on the level and function of C1-INH:
Type 1 accounts for approximately 85% of patients and is characterized by decreased production of C1-INH, caused by deletions or insertions of single or multiple nucleotides into the C1-INH gene.
Type 2 accounts for about 15% of patients, with normal or elevated levels of dysfunctional C1-INH due to point mutations in SERPING1 (4, 7).
Type 3 has normal C1-INH level and function. It is further divided into HAE with normal C1-INH and FXII mutation and HAE of unknown origin (U-HAE) (4, 8, 9).
HAE typically presents in the first or second decades of life (4, 10). The average time between the onset of symptoms and diagnosis is 8–10 years (3, 8).
The skin is the most commonly involved organ, followed by the gastrointestinal and respiratory systems (10). The cutaneous presentation is characterized by non-pitting edema of the face, extremities, and genitalia. Gastrointestinal symptoms are the second most common complaints.
In one retrospective study of 221 patients with HAE by Bork et al., 93.3% of patients had recurrent abdominal symptoms (10). Gastrointestinal symptoms included abdominal pain, nausea, vomiting, constipation, or diarrhea. The primary pathophysiology is edema of the stomach and bowel walls. In some cases, the fluid loss (third spacing) can lead to hypovolemic shock (7). Physical exam can be positive for abdominal tenderness and ascites. Abdominal sonogram often shows mucosal thickening and free peritoneal fluid (11, 12). Abdominal symptoms may be the only presenting symptoms of HAE, and these symptoms may precede the skin manifestation by many years (8).
CT scans of the abdomen show small bowel or colonic wall thickening with increased contrast enhancement, prominent mesenteric vessels, and mild to moderate ascites, which resolve after an acute attack (11, 13).
Endoscopy is relatively contraindicated when acute HAE is a possible differential because of the risk of inducing life-threatening laryngeal edema. However, endoscopic findings, if performed, have included diffuse erythema and mucosal edema, with bulging masses of gastric mucosa resembling a submucosal tumor (14).
Diagnosis of HAE is often challenging if skin manifestations are absent. A positive family history can help, as was the case in our patient. If HAE is suspected, the C4 complement level can serve as a screening test due to its high sensitivity and high negative predictive value (9, 15, 16). The C4 level is typically less than 30% of the mean normal level in untreated HAE (15, 16). If the C4 level is low, C1-INH level and function should be checked (16). The three tests should be repeated in 1–3 months to minimize diagnostic error, given the low prevalence of HAE (9, 15, 16).
The diagnosis of the third type of HAE with normal C1-INH function is either genetic (in the case of FXII mutation) or clinical (for unknown origin). The international working group has published the criteria to diagnose HAE of unknown origin. These criteria are:
Presence of clinical symptoms
One or more family member with similar symptoms
The exclusion of familial and hereditary chronic urticaria with urticaria-associated angioedema
Normal C1-INH activity and protein in plasma, and no HAE-associated mutation in FXII gene (9).
The C1q level can be used to distinguish between HAE and acquired angioedema. C1q should be normal in HAE (17).
Treatment of patients with HAE is aimed at decreasing morbidity and mortality. The main cause of mortality is airway obstruction due to acute laryngeal edema. There are currently three approved medications for the treatment of acute attacks: plasma-derived C1-INH, the bradykinin B2 receptor antagonist icatibant, and kallikrein inhibitor ecallantide. All have been shown to be safe and efficacious for the treatment of acute HAE attacks (8, 18, 19).
Conclusion
The diagnosis of HAE in our patient brings the diagnosis of IBS into question. IBS is a diagnosis of exclusion, and it should be considered after excluding other causes. Clinicians should keep HAE in mind in patients suspected of having IBS or in those who present with recurrent unexplained abdominal symptoms, as early diagnosis can lead to prompt treatment and relief of symptoms.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50. doi: 10.1046/j.1365-2036.2003.01456.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12641512. [DOI] [PubMed] [Google Scholar]
- 2.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3921083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longhurst H, Cicardi M. Hereditary angio-oedema. Lancet. 2012;379:474–81. doi: 10.1016/S0140-6736(11)60935-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22305226. [DOI] [PubMed] [Google Scholar]
- 4.Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161:2417–29. doi: 10.1001/archinte.161.20.2417. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11700154. [DOI] [PubMed] [Google Scholar]
- 5.Osler W. Hereditary angioneurotic oedema. Am J Med Sci. 1888;95:362–7. [Google Scholar]
- 6.Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C’ 1-esterase. Am J Med. 1963;35:37–44. doi: 10.1016/0002-9343(63)90162-1. Available from: http://www.amjmed.com/article/0002-9343(63)90162-1/abstract. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Johnston DT. Hereditary and acquired complement component 1 esterase inhibitor deficiency: a review for the hematologist. Acta Haematol. 2012;127:208–20. doi: 10.1159/000336590. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22456031. [DOI] [PubMed] [Google Scholar]
- 8.Ali MA, Borum ML. Hereditary angioedema: what the gastroenterologist needs to know. Clin Exp Gastroenterol. 2014;7:435–45. doi: 10.2147/CEG.S50465. doi: http://dx.doi.org/10.2147/CEG.S50465. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4242071/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602–16. doi: 10.1111/all.12380. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24673465. [DOI] [PubMed] [Google Scholar]
- 10.Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119:267–74. doi: 10.1016/j.amjmed.2005.09.064. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16490473. [DOI] [PubMed] [Google Scholar]
- 11.LoCascio EJ, Mahler SA, Arnold TC. Intestinal angioedema misdiagnosed as recurrent episodes of gastroenteritis. West J Emerg Med. 2010;11(4):391–4. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2967696/ [PMC free article] [PubMed] [Google Scholar]
- 12.Sofia S, Casali A, Bolondi L. Sonographic findings in abdominal hereditary angioedema. J Clin Ultrasound. 1999;27:537–40. doi: 10.1002/(sici)1097-0096(199911/12)27:9<537::aid-jcu9>3.0.co;2-l. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10525217. [DOI] [PubMed] [Google Scholar]
- 13.De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. JR Am J Roentgenol. 2001;176:649–52. doi: 10.2214/ajr.176.3.1760649. Available from: http://www.ajronline.org/doi/abs/10.2214/ajr.176.3.1760649. [DOI] [PubMed] [Google Scholar]
- 14.Hara T, Shiotani A, Matsunaka H, Yamanishi T, Oka H, Ishiguchi T, et al. Hereditary angioedema with gastrointestinal involvement: endoscopic appearance. Endoscopy. 1999;31:322–4. doi: 10.1055/s-1999-14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10376461. [DOI] [PubMed] [Google Scholar]
- 15.Gompels MM, Lock RJ, Morgan JE, Osborne J, Brown A, Virgo PF. A multicentre evaluation of the diagnostic efficiency of serological investigations for C1 inhibitor deficiency. J Clin Pathol. 2002;55(2):145–7. doi: 10.1136/jcp.55.2.145. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769585/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139(3):379–94. doi: 10.1111/j.1365-2249.2005.02726.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15730382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markovic SN, Inwards DJ, Frigas EA, Phyliky RP. Acquired C1 esterase inhibitor deficiency. Ann Intern Med. 2000;132:144–50. doi: 10.7326/0003-4819-132-2-200001180-00009. Available from: http://annals.org/article.aspx?articleid=713235. [DOI] [PubMed] [Google Scholar]
- 18.Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67:147–57. doi: 10.1111/j.1398-9995.2011.02751.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22126399. [DOI] [PubMed] [Google Scholar]
- 19.Zuraw BL, Bernstein JA, Lang DM, Craig T, Dreyfus D, Hsieh F, et al. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491–3. doi: 10.1016/j.jaci.2013.03.034. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23726531. [DOI] [PubMed] [Google Scholar]