Abstract
Monocyte and macrophage inflammation in parenchymal tissues during acute and chronic HIV and SIV infection plays a role in early anti-viral immune responses and later in restorative responses. Macrophage polarization is observed in such responses in the central nervous system (CNS) and the heart and cardiac vessels that suggest early responses are M1 type antiviral responses, and later responses favor M2 restorative responses. Macrophage polarization is unique to different tissues and is likely dictated as much by the local microenvironment as well as other inflammatory cells involved in the viral responses. Such polarization is found in HIV infected humans, and the SIV infected animal model of AIDS, and occurs even with effective anti-retroviral therapy. Therapies that directly target macrophage polarization in HIV infection have recently been implemented, as have therapies to directly block traffic and accumulation of macrophages in tissues.
Keywords: Macrophage polarization, Dorsal root ganglia, Central nervous system
Introduction
The local microenvironment influences distinct phenotypes of macrophages. In 2000, a M1-M2 paradigm was adopted to distinguish between classic and alternative activated macrophages, where macrophages predominately expressing the iNOS or arginase pathways were termed M1 or M2 macrophages, respectively [1]. M1 macrophages are thought to have efficient antigen presentation and pathogen killing and M1 macrophages secrete high amounts of pro-inflammatory cytokines and promote a TH1 cell response. M2 macrophages (expressing markers such as the scavenger receptor CD163) have high phagocytic activity, produce large amounts of anti-inflammatory cytokines, such as IL-10, and contribute to inflammation resolution. Current thinking suggests that the M1-M2 paradigm likely represents an extreme of macrophage phenotypes and is over simplistic [2]. Evidence suggests that macrophage phenotypes are not static but dynamic and tissue microenvironment-dependent and diseases play a role in the polarization of macrophages [3–5]. It has been shown that M1 and M2 macrophages can switch their phenotype based on the tissue microenvironment [6]. A recent perspective has proposed a common framework for macrophage-activation nomenclature that would replace the M1/M2 model using phenotype, function and context and is particularly useful with in vitro isolation and stimulation of macrophages [7]. For simplicity in this review, we will refer to the M1-M2 classification system.
In this review, we discuss macrophage polarization with human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infection and during acute and chronic infection of the central nervous system (CNS), peripheral nervous system (PNS) and the heart. We discuss the roles of M1-like MAC387+ and M2-like CD163+CD68+CD206+ macrophages in HIV-associated neurological disorders (HAND), peripheral neuropathy (PN) and cardiovascular disease (CVD). The discussion includes the biology of monocyte/macrophage polarization with HIV and SIV infection in tissues, traffic and accumulation of monocyte/macrophages as it relates to pathology. Further, we discuss macrophage-associated biomarkers of CNS, PNS and cardiac disease and the need for effective adjunctive therapies specifically targeting monocytes and macrophages in HIV infection.
Monocyte Traffic and Accumulation in CNS
In HIV and SIV infection, there are increased numbers of activated circulating monocytes [8–12] and turnover and accumulation of monocyte/macrophages in tissues including brain, dorsal root ganglia (DRG) and heart (discussed in the sections below) [13–16]. In SIV-infected monkeys, using bromodeoxyuridine (BrdU), an analogue of thymidine that is incorporated in DNA during replication it was shown that increased monocyte emigration from bone marrow (BM) is a better marker of AIDS progression than CD4+ T cell counts and plasma viral load [17]. Extending those observations, we found that the magnitude of BrdU+ monocytes (the absolute number and/or percent of BrdU+ cells in blood) of SIV-infected monkeys predicted how rapidly they would progress to AIDS and the severity of brain histopathology (macrophage accumulation (both M1 and M2-like macrophages) and productive viral infection)) [16]. Rappaport and colleagues made similar observations finding an expansion of CD16+CD163+ monocytes in blood that may replace or establish residence in the CNS of HIV-infected individuals [18]. Increased monocyte turnover and accumulation of macrophages in the CNS occurs in other organs including the DRG, heart, lung, liver and gut, of SIV-infected monkeys and HIV-infected individuals, even those on durable antiretroviral therapy (ART).
Macrophage Polarization in CNS during HIV and SIV Infection
Populations of CNS macrophages can be distinguished by their anatomical location in the CNS and immune-phenotypic markers [10–12,15,16,18–21]. With regard to HIV and SIV infection, we and others have examined the resident parenchymal microglia, perivascular macrophages, and inflammatory macrophages (that are only present in the CNS with inflammation) [15]. Perivascular macrophages that are present in the CNS vasculature are CD14, CD16, and CD163 positive with high levels of CD45 and CCR2. The fact that perivascular macrophages are CD163+ and CCR2+ is consistent with an M2-polarized alternatively activated macrophage phenotype [12,15,19,21–23]. The resident parenchymal microglia have low to non-detectable CD14 and CD16 and variable to non-detectable CD163. These two cells types are the major constituents of HIV and SIV encephalitic lesions [12,19,21,24,25] where productive viral replication is often found. Perivascular CD163+ M2-like macrophages are also found in small lesions or perivascular cuffs along CNS vessels and some are HIV or SIV infected [12,21,26]. Another monocyte/macrophage cell type that is present in the CNS, but only with inflammation, is the MAC387 positive macrophage [15,27]. The antibody known as MAC387 is described as recognizing myeloid-related protein 14 (MRP14) and, to a lesser extent, the MRP8/MRP14 heterocomplex [28,29]. MRP14 is expressed on recently infiltrating monocytes/macrophages during early acute inflammation [30]. The MAC387+ cells are CD163 and CCR2 negative and thus similar to classically activated M1 macrophages, which are thought to amplify immune and inflammatory responses and are associated with higher suppression of HIV-1 replication [31]. Using BrdU injection in SIV-infected macaques it was demonstrated that 90% of recently trafficked BrdU+ macrophages in the brain are (M1-like) MAC387+ macrophages (that came from the bone marrow) and few were resident CD68+ or CD163+ (M2) cells [15]. Interestingly, these M1-like MAC387+ cells in the brain do not appear to be viral infected.
Comparing the number of MAC387+ macrophages in the CNS of chronically (1–2 years) SIV-infected monkeys with and without SIVE, rapid progressing SIV-infected animals (<6 months) with SIVE, and SIV-infected CD8 lymphocyte depleted animals with rapid (3–4 months) development to AIDS and SIVE and found that MAC387+ cells were present in early developing lesions [32] (Figure 1). In more established, chronic and severe SIVE lesions, CD68+CD163+ M2 macrophages accumulated and outnumber the MAC387+ M1-like macrophages, possibly in an attempt to dampen the anti-viral/pro inflammatory response (Figure 1) [15,16,33–35]. From this scenario, one can speculate that M1-like MAC387+ cells are recruited to sites of active infection and may be continuously recruited to the sites of infection. Such recruitment may contribute to the amplification of the inflammatory response leading to the recruitment of additional leukocytes and infected macrophages, including CD163+ M2 macrophages. Injection of dextran dyes directly into the ventricles in the brain to label perivascular cells, we found that the majority (approximately 80%) of M2 CD163+ macrophages found in SIVE lesions terminally were present early (prior to day 20 post infection) in the brain. Instead of trafficking from the periphery these M2 CD163+ macrophages were likely recruited to lesions by the M1-like MAC387+ macrophage from the CNS vasculature [36]. The majority of the M2 CD163+ macrophages in SIVE and HIVE lesions were productively infected. Early CNS invasion of MAC387+ M1-like macrophages was seen in the meninges and choroid plexus and late inflammation was characterized by M2 CD163+ macrophage accumulation in the perivascular space and in SIVE lesions [36] (Figure 1).
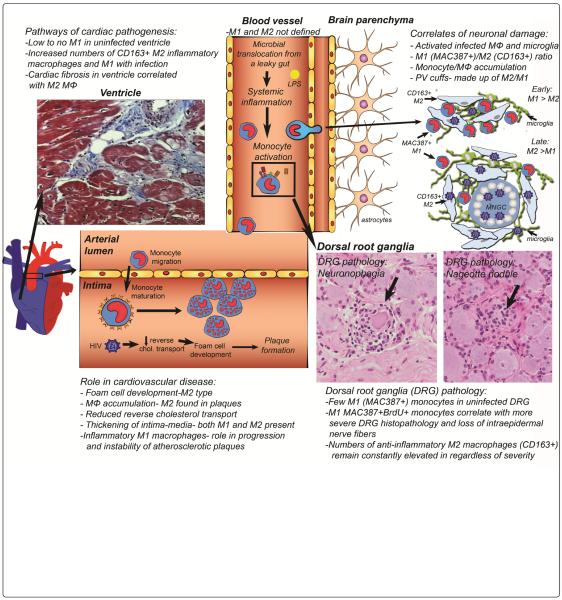
Figure 1.
M1 and M2 macrophages in HIV/SIV pathogenesis of brain, dorsal root ganglia, aorta and ventricle tissues. M1 and M2 monocytes are not well defined in blood vessels, but in tissues are defined as inflammatory M1 and anti-inflammatory M2 macrophages. In brain, the correlates of neuronal damage include: activated and infected macrophages and parenchymal microglia, M1 (MAC387)/M2 (CD163) ratio, monocyte/macrophage (MΦ) accumulation, and perivascular (PV) cuffs. In DRGs, few M1 monocytes are in uninfected DRGs. M1 macrophages correlate with severe DRG histopathology and loss of intraepidermal nerve fibers. M2 macrophages are elevated in DRGs, but do not correlate with severity of pathology. In heart, there are low to no M1 macrophages in uninfected tissue. The numbers of M1 and M2 macrophages increase with HIV infection and M2 macrophages correlates with cardiac fibrosis. Pathways of cardiac pathogenesis in aorta include the development of M2 foam cells, M1 and M2 macrophage accumulation in the intima, reduced reversed cholesterol transport and thickening of the intima-media. MNGC: Multi-Nucleated Giant Cell.
To determine whether on going monocyte/macrophage traffic is required for SIV-associated CNS damage and to maintain CNS lesions and productively infected cells, we blocked monocyte and T lymphocyte traffic using natalizumab (Biogen Idec) an anti-VLA-4 antibody in SIV-infected rhesus macaques [37]. Natalizumab is a humanized monoclonal antibody targeting the alpha subunit of the α4β1 adhesion molecule [38] and is approved for the treatment of relapsing-remitting multiple sclerosis (MS) [39]. Natalizumab prevents accumulation of leukocytes (monocyte/macrophages, T cells and B cells) in the brains of patients with MS [39] and in the gut of patients with Crohn's disease [40]. Natalizumab treatment of SIV-infected rhesus macaques resulted in stabilization of on-going neuronal injury (measured by NAA/Cr by 1H MRS), and decreased numbers of M1 and M2 monocytes/macrophages and productive infection (SIV p28+, RNA+) in the brain and gut [37]. BrdU pulse studies revealed that no BrdU+ cells trafficked to the brains of natalizumab treated animals, indicating that α4 blockade prevented BrdU+ monocyte/macrophages from entering. There were few scattered MAC387+ M1 inflammatory monocytes in the brains of treated animals suggest that despite SIV infection and CD8 lymphocyte depletion, very little inflammation occurred in the CNS following natalizumab treatment. Similar numbers of M2 CD68+ and M1-like MAC387+ monocyte/macrophages were seen in the lymph nodes of untreated and treated SIV-infected animals suggesting that natalizumab did not significantly affect traffic of these cells to lymph nodes [37] as has been previously reported by others.
Monocyte Traffic and Activation in DRG during SIV Peripheral Neuropathy
HIV-induced distal sensory polyneuropathy (DSP) is the most common neurological complication in HIV infection [43]. Previous research suggests that monocyte/macrophages traffic to the dorsal root ganglia (DRG) and inflict damage during HIV and SIV infection [42,44–46]. These studies used CD68+ or Iba-1 to characterize resident macrophages in the DRG [42,46,47]. We recently expanded on these phenotypes and used CD68 as a marker for resident macrophages and CD163 to label M2 perivascular macrophages. In addition, we used MAC387+ M1 cells and BrdU pulse to measure cells trafficking from bone marrow to the DRGs. In uninfected DRG, we characterized macrophage phenotypes surrounding the DRG neurons. Few macrophages (0.8 to 3%) were MAC387+ M1 cells inflammatory cells and less than 1% of cells were BrdU+ macrophages that are recent emigrants from BM (Figure 1). In contrast, CD68+ resident cells made up 12–15%, and CD163+ M2 macrophage made up 3–12% of the cells. CD3+ T lymphocytes comprised less than 10% of the cell population. In SIV-infected animals, resident CD68+ and CD163+ M2 macrophages were significantly increased. The traffic of monocyte/macrophages was increased with SIV infection and MAC387+ M1 and BrdU+ macrophages accumulated in DRGs (Figure 1). In this study we found that ~80% of all newly recruited BrdU+ monocytes in the DRGs were MAC387+, similar to that seen in the brain of SIV infected monkeys. Interestingly the number of M1-like MAC387+BrdU+ monocytes correlated with more severe DRG histopathology and loss of intraepidermal nerve fibers [14] (Figure 1). These findings regarding the association of cell traffic to DRG pathology is consistent with previously published data demonstrating a correlation between monocyte traffic and severity of SIV encephalitic brain lesions in the same model system [16].
In addition to traffic and accumulation of macrophages in the brain, a recent study showed macrophage invasion into visceral tissues (kidney, spleen, and lymph node) in patients with HIV encephalitis (HIVE) [41]. They demonstrated an altered monocyte/macrophage homeostasis in HIV infection, which may contribute to comorbid conditions during AIDS [41]. The notion that macrophage accumulation in parenchymal tissues in HIV infection is a global phenomenon is further supported by data presented below in the DRGs and hearts of SIV-infected macaques [13,14,42].
In SIV-infected CD8-depleted macaques, the ratio of M2 (CD163+) to M1 (MAC387+) macrophages in DRGs decreased with more severe DRG pathology (Burdo, unpublished). It appears the numbers of anti-inflammatory M2 macrophages (CD163+) remained elevated across all pathology subgroups (mild, moderate and severe), but numbers of the pro-inflammatory M1 macrophages (MAC387+) increased, resulting in a decreased M2/M1 ratio in this rapid model. Because SIV-infected CD8-depleted macaques progress rapidly (3–4 months) to AIDS it is possible that with chronic SIV, where disease progression is slower, there may be a shift from M1 to M2 macrophages in the DRG. This hypothesis is consistent with our observation that rapid progressors (<6 months to AIDS) have an accumulation of M1-like MAC387+ macrophages in SIVE brain lesions in contrast to conventional progressors (>6 months to AIDS), which had more M2-like macrophages than M1-like macrophages [15]. This is likely due to MAC387+ cells being recruited to active sites of infection and inflammation that occurs with acute disease. In contrast, M2-like cell activation is more likely to be elevated in chronic disease where anti-inflammatory properties would be beneficial.
To determine whether ongoing monocyte/macrophage traffic is required for SIV-associated DRG damage, we used using natalizumab to block traffic (as discussed above). Natalizumab treated animals had less severe DRG pathology, decreased numbers of recently recruited M1 MAC387+BrdU+, and fewer productively SIV-infected macrophages compared to non CD8-depleted SIV infected animals (Burdo, unpublished). Overall, these results suggest a role of monocyte traffic in DRG histopathology and demonstrate a potential benefit of drugs that directly target monocyte traffic to inhibit HIV peripheral neuropathy.
Macrophage Infiltration and Pathology in the Heart during SIV/HIV Infection
HIV infection with stable ART is characterized by a state of chronic inflammation that is recognized as a risk factor for cardiovascular disease (CVD) in both HIV-infected and uninfected individuals, where monocyte/macrophages are involved in the development of fibrosis [48,49] atherosclerosis [50,51], and ongoing inflammation. Immune activation in the HIV-infected population plays a role in CVD development. Levels of sCD14 correlate with coronary artery calcification [52], and plasma sCD163 correlates with non-calcified, vulnerable coronary plaques [53] and correlate with the number of inflammatory macrophages in the ascending aorta of HIV infected individuals, as shown by FDG-PET imaging [54]. More recent studies have shown that acute/early macrophage inflammation in vessel walls precedes calcification [55], supporting the role of inflammation in the development of cardiovascular disease.
The simian immunodeficiency virus (SIV) infection of rhesus macaques that closely mimics the progression of HIV in humans has been used to study CVD [13,56–58]. Acutely SIV-infected rhesus macaques, infected with different strains of SIV (<6 weeks infection) have no cardiac pathology [59]. However, in chronic infection two thirds of animals whom succumb to AIDS developed myocarditis, vessel occlusion and inflammation [59]. In these animals, the inflammatory infiltrate, most often studied in the in the left ventricle, consists of scattered CD3+ T-lymphocytes and CD68+ macrophages [59]. An early study in monkeys found increased numbers of M2 CD163+ macrophages in uninflammed hearts of SIV-infected rhesus macaques compared to SIV-infected animals with myocarditis, and suggested that the M2 CD163+ macrophages may play an anti-inflammatory or protective role [60]. More recent studies have found increased numbers of CD163 inflammatory macrophages with SIV infection and AIDS [13,61], but increased numbers of CD163+ macrophages did not correlate with functional decline of the heart [61] (Figure 1).
We used a SIV-infected, CD8-depletion model, in which macaques undergo rapid progression to AIDS (3–4 months), to study macrophage activation and traffic to the heart in AIDS [13,14,16,42,62]. In this study of twenty-three rhesus macaques, seventeen animals were SIV-infected and twelve were also CD8-lymphocyte depleted. We found few scattered macrophages in the heart tissues of uninfected controls [13] (Figure 1). These data were similar to reports of normal human heart where low numbers of resident CD68+ macrophages were found in different heart regions usually in proximity to blood vessels [63]. In the CD8-lymphocyte depleted, SIV-infected monkeys, we found significant increases in the number M2 CD163+, resident CD68+, and M1-like MAC387+ macrophages compared to non-CD8-lymphocyte, SIV-infected animals [13] (Figure 1). The incidence of cardiac fibrosis, measured by collagen deposition, was also increased in CD8-depleted animals compared to SIV-infected non-CD8 lymphocyte depleted animals, suggesting that macrophages play a role in cardiac fibrosis [13]. Moreover, with increased numbers of M2 CD163+, resident CD68+, and M1-like MAC387+ macrophages, we found increased percentage of cardiac fibrosis, and a statistically significant positive correlation with the number of inflammatory macrophages and cardiac fibrosis (Figure 1). In the same study, using BrdU to examine traffic of newly released monocyte/macrophages from the bone marrow (BM) we found that the majority of BrdU+ macrophages trafficked to the heart late in infection. These BrdU+ cells were predominantly M1-like MAC387+ recently recruited macrophages (79.5 ± 5.0%) [13]. We have previously demonstrated and discussed that these cells are early players in what is likely anti-viral responses [15]. During the early phase of cardiac inflammation, it is likely that the M1 macrophages are recruited to and infiltrate the heart expressing pro-inflammatory cytokines and mediate cardiac fibrosis [64]. The microenvironment of the heart may cause differentiation of M1 and/or M2 macrophages in the development of fibrosis [65]. In the context of our study described above M1 MAC387+ macrophages traffic to the heart with the development of AIDS as potential anti-viral agents that initiate the inflammatory process, while CD163+ M2 macrophages are recruited from the monocyte pool that differentiate and may begin the process of fibrosis.
A follow up study examining the effects of directly blocking monocyte/macrophage traffic to the heart using natalizumab (as described for studies in the CNS and DRG) was done to determine if decreased recruitment of monocyte/macrophages resulted in a decrease in cardiac fibrosis. We found few M1 or M2 macrophages in cardiac tissues of animals sacrificed early, adding evidence in this infection model that cardiac inflammation occurs later during infection with progression to AIDS (Walker, in press JAHA 2015). Natalizumab treatment resulted in a significant decrease in the numbers M2 CD163+ and resident CD68+ macrophages present in the heart, along with a trend of decreased numbers of M1-like MAC387+ macrophages. Additionally, blocking macrophage traffic to the heart reduced the frequency and severity of cardiac fibrosis (Walker, in press JAHA 2015). This suggests that blocking traffic of macrophages directly can decrease the number of M1 macrophages that begin the inflammatory process, as well as decrease M2 macrophages that play a role in cardiac fibrosis [65].
A range of macrophage phenotypes exist within atherosclerotic plaques including CD68+, CD163+, HAM56+, MAC387+ cells, all of which likely play a role in plaque stability and rupture [66]. Inflammatory M1 macrophages are thought to play a role in the progression and instability of atherosclerotic plaques [67], while M2 macrophages are thought to be anti-inflammatory and athero-protective [68] (Figure 1). However M2 macrophages take up lipids more readily and become foam cells more easily [69] than M1 macrophages. Additionally, M2 macrophages secrete matrix metalloproteinases (MMPs) that are found in human atherosclerotic plaques [70,71] leading to the breakdown of collagen and compromise of the fibrous cap region, which provides structure and strength, ultimately leading to plaque rupture [72]. Overall, whether M2 macrophages are athero-protective is not well-defined. In sections of normal aorta, HAM56+ macrophages were rare and scattered in the intima of the aortic wall. As atherosclerotic plaques progress, the number of HAM56+ macrophages increased and were scattered throughout the lesion [73]. A recent study examining twenty human atherosclerotic plaques from carotid arteries found both M1 and M2 macrophages present throughout plaques within the core and cap regions (Figure 1). Numbers of CD64+ and CD86+ macrophages (M1) negatively correlated with cap thickness of atherosclerotic plaques, while numbers of CD163+ macrophages (M2) did not [74]. Other studies have shown that both M1 and M2 polarized macrophages are present in atherosclerotic plaques and there is increased accumulation with disease severity [75]. Spatial differences between macrophage subsets with M1 macrophages were predominant in shoulder regions of atherosclerotic plaques, which are prone to rupture, providing evidence these M1 macrophages play a role in plaque destabilization [75]. Additional reports underscore the role of macrophages in plaque destabilization specifically that ruptured cap regions have an increased in macrophage density [76]. Additionally, using Optical Coherence Tomography (OCT) it was shown that stable plaques have fewer macrophages compared to unstable, ruptured plaques within the same individual [77]. Pleotropic effects of statins further support this notion; it has been shown that treatment resulting in the reduction of macrophages in plaques leads to stabilization [78,79]. Recently, statin use has been effective in decreasing the volume of high-risk plaques and the number of high-risk features (positive remodeling and low attenuation) in HIV-infected patients [80].
Data from our lab investigating macrophage accumulation in the aorta of HIV-infected individuals shows there is a significant increase in the numbers of both M1 and M2 macrophages compared to uninfected individuals (Walker, unpublished). Numbers of CD68+ and M1-like MAC387+ macrophages are significantly increased in the intima of the aorta and positively correlate with an increase in intima-media thickness, as well as numbers of CD163+ and CD206+ macrophages (Walker, unpublished). It is possible that MAC387+ macrophages enter the vessel wall during acute inflammation and recruit other macrophages during the inflammatory process resulting in an increase in intima thickness of the aorta. While the M1 and M2 categories might represent extreme ends of the spectrum, it appears that macrophages in general, and inflammation, play a role in the development of HIV-associated CVD.
Targeting Macrophages in HIV Infection
Evidence that chronic immune activation in HIV-infected patients on ART is associated with comorbities, including neurologic and cardiac complications, underscores the need for development of adjunctive therapies targeting macrophages. Such therapies could target blocking monocyte traffic, inhibiting macrophage function at the tissue site or switching macrophage phenotype. Here, we have described our proof-of-concept studies using anti-VLA-4 antibody to block traffic of monocyte/macrophages. Use of natalizumab in SIV-infected rhesus macaques we blocked traffic of M1-like MAC387+ macrophage and BrdU+ monocytes migrating from the bone marrow and stopped M2 CD163+ activation. Natalizumab is effective in blocking cell traffic but it is not selective to M1 or M2 macrophages.
Because macrophage are a heterogeneous population with M1 inflammatory and M2 anti-inflammatory properties, altering macrophage phenotype may function to diminish the tissue specific inflammatory milieu. Studies targeting macrophage polarization with the goal of switching from the classically activated, pro-inflammatory M1 subtype to alternatively activated, anti-inflammatory M2 phenotype are currently being examined in numerous diseases. Fasudil, a Rho-kinase inhibitor, has been used successfully to alleviate symptoms of experimental autoimmune encephalomyelitis (EAE) that is a model for Multiple Sclerosis (MS) [81]. Fasudil treatment inhibited expression of M1 markers and increased expression of M2 markers on macrophages. Polarization of macrophages toward an M2 phenotype also decreased expression of pro-inflammatory cytokines IL-1β. TNF-α, and MCP-1 may suggest that the benefits of fasudil in EAE mice might be due to switching the macrophage phenotype from M1 to M2 [81]. Also in mice, small interfering RNAs (siRNA) have been delivered orally to target TNF-α produced by macrophages [82]. TNF-α and IL-1β production was inhibited in mice receiving the siRNA and challenged with Lipopolysaccharide (LPS), suggesting that an oral delivery of siRNA could be beneficial in treating inflammatory disease in humans.
Statins, which are traditionally used to treat dyslipidemia by targeting cholesterol, have also been shown to exert anti-inflammatory effects and are associated with clinical benefit. Statin use has recently been found to be beneficial in the treatment and prevention of CVD in HIV-infected patients [83,84]. Statins may help minimize chronic monocyte activation and subsequent arterial inflammation, as well as the development of inflammatory plaques, and in fact have been shown to shrink active plaques [83]. Previous data has shown that statins effect the composition of atherosclerotic plaques in monkeys by decreasing the total number of macrophages present [85]. In rats, in a model of glomerulonephritis, atorvastatin increased M2 macrophages and production of IL-10 [86]. Thus, statins may be useful in increasing M2 anti-inflammatory macrophage and aid in lesion resolution.
In summary, M1 and M2 macrophages clearly play a role in early, late and chronic HIV and SIV function and tissue pathology associated with infection. Directly targeting macrophages by either blocking their traffic to sites of inflammation, or skewing M1 to M2 phenotypes holds clinical promise in HIV infection. Caveats exist of course and in the case of HIV these would include accounting for the global effects of such treatments on off target tissues. Likely, the best future approaches will be ones that chemically push macrophages to non-disease appropriate phenotypes specific for the target tissues that are specific for the tissue microenvironment. Regardless, the biology of these interesting cells provides the exciting opportunity for future therapeutic work.
Acknowledgement
This worked was supported by NIH/NINDS R01 NS082116 (awarded to THB) and NIH/NINDS R01 NS40327 (awarded to KCW).
References
- 1.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liddiard K, Taylor PR. Understanding local macrophage phenotypes in disease: shape-shifting macrophages. Nat Med. 2015;21:119–120. doi: 10.1038/nm.3798. [DOI] [PubMed] [Google Scholar]
- 4.Wolfs IM, Donners MM, de Winther MP. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost. 2011;106:763–771. doi: 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- 5.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 9.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 10.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 11.Kim WK, Sun Y, Do H, Autissier P, Halpern EF, et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010;87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JA, Sulciner ML, Nowicki KD, Miller AD, Burdo TH, et al. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res Hum Retroviruses. 2014;30:685–694. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakritz JR, Bodair A, Shah N, O'Donnell R, Polydefkis MJ, et al. Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy. Am J Pathol. 2015;185:1912–1913. doi: 10.1016/j.ajpath.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, et al. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 21.Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borda JT, Alvarez X, Mohan M, Hasegawa A, Bernardino A, et al. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172:725–737. doi: 10.2353/ajpath.2008.070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabriek BO, Van Haastert ES, Galea I, Polfliet MM, Döpp ED, et al. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- 24.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- 26.Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, et al. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- 27.Esiri MM, Morris CS. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non-neoplastic diseases. J Neurol Sci. 1991;101:59–72. doi: 10.1016/0022-510x(91)90018-3. [DOI] [PubMed] [Google Scholar]
- 28.Hessian PA, Fisher L. The heterodimeric complex of MRP-8 (S100A8) and MRP-14 (S100A9). Antibody recognition, epitope definition and the implications for structure. Eur J Biochem. 2001;268:353–363. doi: 10.1046/j.1432-1033.2001.01894.x. [DOI] [PubMed] [Google Scholar]
- 29.Goebeler M, Roth J, Teigelkamp S, Sorg C. The monoclonal antibody MAC387 detects an epitope on the calcium-binding protein MRP14. J Leukoc Biol. 1994;55:259–261. doi: 10.1002/jlb.55.2.259. [DOI] [PubMed] [Google Scholar]
- 30.Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 31.Cassol E, Cassetta L, Alfano M, Poli G. Macrophage polarization and HIV-1 infection. J Leukoc Biol. 2010;87:599–608. doi: 10.1189/jlb.1009673. [DOI] [PubMed] [Google Scholar]
- 32.Soulas C, Autissier P, Schuetz A, Valcour V, Chalermchai T, et al. A Comprehensive Phenotypic Analysis of Monocyte Subsets: Modulation of Activation Markers and Chemokine Receptors in ART-Naïve HIV-1-Infected Individuals. 18th Conference on Retroviruses and Opportunistic Infections (CROI), Hynes Convention Center; Boston, Massachusetts. 2011. [Google Scholar]
- 33.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. 2014;28:2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowlin BT, Burdo TH, Midkiff CC, Salemi M, Alvarez X, et al. SIV Encephalitis Lesions Are Composed of CD163(+) Macrophages Present in the Central Nervous System during Early SIV Infection and SIV-Positive Macrophages Recruited Terminally with AIDS. Am J Pathol. 2015;185:1649–1665. doi: 10.1016/j.ajpath.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, et al. Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533. doi: 10.1371/journal.ppat.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Y, Schürpf T, Springer TA. How natalizumab binds and antagonizes α4 integrins. J Biol Chem. 2013;288:32314–32325. doi: 10.1074/jbc.M113.501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Yednock TA. Novel approaches to treating inflammatory bowel disease: targeting alpha-4 integrin. Am J Gastroenterol. 2003;98:2372–2382. doi: 10.1111/j.1572-0241.2003.08703.x. [DOI] [PubMed] [Google Scholar]
- 41.Fischer T, Wyatt CM, D'Agati VD, Croul S, McCourt L, et al. Mononuclear phagocyte accumulation in visceral tissue in HIV encephalitis: evidence for increased monocyte/macrophage trafficking and altered differentiation. Curr HIV Res. 2014;12:201–212. doi: 10.2174/1570162x12666140713165141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdo TH, Orzechowski K, Knight HL, Miller AD, Williams K. Dorsal root ganglia damage in SIV-infected rhesus macaques: an animal model of HIV-induced sensory neuropathy. Am J Pathol. 2012;180:1362–1369. doi: 10.1016/j.ajpath.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 45.Hahn K, Robinson B, Anderson C, Li W, Pardo CA, et al. Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Exp Neurol. 2008;210:30–40. doi: 10.1016/j.expneurol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laast VA, Shim B, Johanek LM, Dorsey JL, Hauer PE, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laast VA, Pardo CA, Tarwater PM, Queen SE, Reinhart TA, et al. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J Neuropathol Exp Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- 48.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdelbaky A, Corsini E, Figueroa AL, Subramanian S, Fontanez S, et al. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis. 2015;238:165–172. doi: 10.1016/j.atherosclerosis.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Letvin NL, King NW. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 57.Simon MA, Chalifoux LV, Ringler DJ. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992;8:327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- 58.Lackner AA. Pathology of simian immunodeficiency virus induced disease. Curr Top Microbiol Immunol. 1994;188:35–64. doi: 10.1007/978-3-642-78536-8_3. [DOI] [PubMed] [Google Scholar]
- 59.Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, et al. Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation. 2000;101:185–193. doi: 10.1161/01.cir.101.2.185. [DOI] [PubMed] [Google Scholar]
- 60.Yearley JH, Pearson C, Carville A, Shannon RP, Mansfield K. Phenotypic Variation in Myocardial Macrophage Populations Suggests a Role for Macrophage Activation in SIV-Associated Cardiac Disease. AIDS Research and Human Retroviruses. 2007;23:512–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 61.Kelly KM, Tarwater PM, Karper JM, Bedja D, Queen SE, et al. Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS. 2012;26:815–823. doi: 10.1097/QAD.0b013e3283518f01. [DOI] [PubMed] [Google Scholar]
- 62.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azzawi M, Hasleton PS, Kan SW, Hillier VF, Quigley A, et al. Distribution of myocardial macrophages in the normal human heart. J Anat. 1997;191:417–423. doi: 10.1046/j.1469-7580.1997.19130417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M, Zheng J, Miao Y, Wang Y, Cui W, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]
- 66.Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 67.Bobryshev YV1. Dendritic cells and their role in atherogenesis. Lab Invest. 2010;90:970–984. doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- 68.Bouhlel MA, Derudas B, Rigamonti E, R Dièvart, J Brozek, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Oh J, Riek AE, Weng S, Petty M, Kim D, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikkari ST, Geary RL, Hatsukami T, Ferguson M, Forough R, et al. Expression of collagen, interstitial collagenase, and tissue inhibitor of metalloproteinases-1 in restenosis after carotid endarterectomy. Am J Pathol. 1996;148:777–783. [PMC free article] [PubMed] [Google Scholar]
- 71.Rajavashisth TB, Xu XP, Jovinge S, Meisel S, Xu XO, et al. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99:3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 72.Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361–366. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gown AM, Tsukada T, Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986;125:191–207. [PMC free article] [PubMed] [Google Scholar]
- 74.Medbury HJ, James V, Ngo J, Hitos K, Wang Y, et al. Differing association of macrophage subsets with atherosclerotic plaque stability. Int Angiol. 2013;32:74–84. [PubMed] [Google Scholar]
- 75.Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- 77.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–979. doi: 10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 78.Williams JK, Sukhova GK, Herrington DM, Libby P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol. 1998;31:684–691. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- 79.Shiomi M, Ito T, Tsukada T, Yata T, Watanabe Y, et al. Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques. Effect of pravastatin sodium on atherosclerosis in mature WHHL rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1938–1944. doi: 10.1161/01.atv.15.11.1938. [DOI] [PubMed] [Google Scholar]
- 80.Lo J, You SM, Liebau J, Lee H, Grinspoon S. Effects of low-dose growth hormone withdrawal in patients with HIV. JAMA. 2010;304:272–274. doi: 10.1001/jama.2010.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu C, Li Y, Yu J, Feng L, Hou S, et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One. 2013;8:e54841. doi: 10.1371/journal.pone.0054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet. 2015;2:e52–63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grinspoon SK. Cardiovascular disease in HIV: traditional and nontraditional risk factors. Top Antivir Med. 2014;22:676–679. [PMC free article] [PubMed] [Google Scholar]
- 85.Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–1458. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- 86.Fujita E, Shimizu A, Masuda Y, Kuwahara N, Arai T, et al. Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. Am J Pathol. 2010;177:1143–1154. doi: 10.2353/ajpath.2010.090608. [DOI] [PMC free article] [PubMed] [Google Scholar]