Abstract
Background
HbA1c is the standard measure to monitor glucose control in diabetes and is a marker of future cardiovascular risk. Fructosamine and glycated albumin are markers of short-term glycemic control but their associations with cardiovascular outcomes are uncharacterized.
Methods and Results
We measured glycated albumin and fructosamine in 11,104 participants with and without diabetes in the community-based Atherosclerosis Risk in Communities (ARIC) Study in 1990–1992 (baseline). We evaluated associations of fructosamine and glycated albumin with risk of coronary heart disease, ischemic stroke, heart failure and mortality. We compared associations to those observed for HbA1c. During two decades of follow-up there were 1096 new cases of coronary heart disease, 605 of ischemic stroke, 1432 of heart failure, and 2860 deaths. Elevated baseline concentrations of fructosamine and glycated albumin were significantly associated with each of the outcomes even after adjustment for traditional cardiovascular risk factors, with especially strong associations in persons with diabetes. Associations were of similar magnitude to those observed for HbA1c and—as has been previously observed for HbA1c—the associations tended to be J-shaped, with an elevation of risk at the lowest levels of each biomarker.
Conclusions
The acceptance of new measures of hyperglycemia is partly dependent on establishing their association with long-term outcomes. We found that fructosamine and glycated albumin were associated with vascular outcomes and mortality and that these associations were similar to those observed for HbA1c.
Keywords: epidemiology, hyperglycemia, biomarkers, fructosamine, glycated albumin, cardiovascular disease, mortality
Hemoglobin A1c (HbA1c) reflects exposure to glucose concentrations over an approximate 2–3 month period and has long been the standard measure used clinically to monitor glycemic control in persons with diabetes. HbA1c is also now recommended for use in diagnosis of diabetes. Nonetheless, HbA1c has important limitations: it does not change rapidly in response to changes in treatment and a number of conditions affect the validity of the test result (e.g., anemia, altered red cell lifespan, transfusion, kidney disease, liver disease, and abnormal forms of hemoglobin).
Fructosamine (glucose bound to circulating serum proteins, mainly albumins but also globulins and other proteins) and glycated albumin (glucose bound specifically to albumin) reflect glycemic control over the previous 2–4 weeks, reflecting the turnover of plasma proteins. Both fructosamine and glycated albumin can be measured in serum or plasma and may be useful for assessment of hyperglycemia in settings where HbA1c testing is problematic or not available.
There is growing interest and use of nontraditional biomarkers of hyperglycemia such as fructosamine and glycated albumin but limited data linking these tests to long-term outcomes. We have previously shown that fructosamine and glycated albumin are strongly associated with microvascular complications and may add prognostic information to HbA1c in the general population 1. Recent statements from professional diabetes and laboratory organizations have suggested the utility of these biomarkers, but have cited their unclear prognostic value as a barrier to their use and interpretation 2–6. The primary objective of this study was to characterize the associations of fructosamine and glycated albumin with risk of coronary heart disease, ischemic stroke, heart failure and total mortality in the community. We evaluated associations overall and compared associations among persons with and without a history of diabetes. A secondary objective was to compare risk factor associations for elevated levels of fructosamine and glycated albumin to HbA1c in persons without a history of diagnosed diabetes.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort of originally 15,792 persons from four U.S. communities: Forsyth, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. The first examination took place from 1987 to 1989, with three follow-up visits occurring approximately every three years. A fifth examination was completed from 2011 to 2013. The second examination took place from 1990 to 1992, was attended by 14,348 participants, and is the baseline for the present study. For the purposes of this study, we excluded persons who had a history of cardiovascular disease at baseline, were missing variables of interest, or who were fasting for fewer than 8 hours at the time of the examination. The final study sample included 11,104 participants (762 with a history of diabetes).
Institutional review boards at all institutions reviewed the study, and written informed consent was obtained from all participants.
Measurement of fructosamine and glycated albumin
Fructosamine (Roche Diagnostics Corp, Indianapolis, IN, USA) and glycated albumin (Lucia GA-L, Asahi Kasei Corp, Tokyo, Japan) were measured in 2012–2013 from serum samples, using a Roche Modular P800 system. Samples had been stored at −70°C since collection during the baseline examination in 1990 to 1992. For fructosamine, the inter-assay coefficients of variation (CVs) were 3.2% at a concentration of 212.6 umol/L and 2.5% at a concentration of 856.7 umol/L%. For glycated albumin, the inter-assay CVs were 2.3% at a concentration of 1.579 g/dL and 2.8% at a concentration of 0.426 g/dL. Previous studies have demonstrated the stability of these analytes in long-term stored samples7–9.
Ascertainment of Incident Cardiovascular Events
The ascertainment of cardiovascular events and deaths has been detailed previously 10–12. Briefly, all hospitalizations are reported annually or semi-annually (since 2012) by participants or their proxies and also identified by community-wide surveillance of local hospital discharge indices. Trained personnel abstract hospital records related to possible cardiovascular events. Deaths were also ascertained from state records and linkage to the National Death Index. Silent myocardial infarctions were identified from electrocardiograms conducted at the study examinations. We defined incident coronary heart disease events as the first occurrence of a validated definite or probable hospitalized myocardial infarction, a death from coronary heart disease, or electrocardiographic evidence of a silent myocardial infarction (during the study visits). We also examined definite or probable ischemic stroke. Heart failure was defined by a hospitalization with an ICD-9 code of 428 or death with an underlying cause of ICD-9 428 or ICD-10 I50 13. Participants who had a non-cardiac death were censored at the time of death in analyses of incident cardiovascular events.
Other variables of interest
All measurements were conducted at baseline unless stated otherwise. Diagnosed diabetes was defined on the basis of a self-reported physician diagnosis or current use of diabetes drugs. Serum glucose was measured using the hexokinase method. HbA1c was measured from stored whole blood samples using HPLC instruments standardized to the Diabetes Control and Complications Trial assay 14. Body mass index, blood pressure, and lipid concentrations were assessed using published methods 15–20. Hypertension was defined as a systolic blood pressure of 140 mm Hg or higher, or diastolic blood pressure of 90 mm Hg or higher from the mean of the second and third readings at the examination; or the current use of hypertension medication. Participants self-reported their educational level (at visit 1 only), alcohol use, and smoking status.
Statistical Analyses
We calculated summary statistics to characterize the baseline population overall and within the populations of persons with and without diagnosed diabetes stratified by concentrations of fructosamine and glycated albumin at baseline. Because there are no established clinical cutpoints for either fructosamine or glycated albumin, we used cut-points pegged to clinical categories of HbA1c. We divided the population of persons without diagnosed diabetes into three groups defined by the 70th and 96th percentiles of each biomarker (≤70th, 71st to 96th, and higher than the 96th percentile). These cut-points were used for equivalence to the clinical (diagnostic) values of HbA1c 5.7% (the 70th percentile) and 6.5% (the 96th percentile). In persons with diagnosed diabetes, we divided the population into two groups, above and below the 38th percentile (equivalent to an HbA1c value of 7%). In persons without diagnosed diabetes at baseline, we used multivariable logistic regression models to compare associations of traditional diabetes risk factors with elevated values of fructosamine, glycated albumin, and HbA1c where “elevated” was defined as values above the 96th percentile (equivalent to an HbA1c value of 6.5%).
Adjusted hazard ratios and their corresponding confidence intervals were estimated using Cox proportional hazards models. Our main analyses were conducted with fructosamine, glycated albumin, and HbA1c modeled using diabetes-specific categories using the cut-points as described above. P-values-for-trends across all the categories were calculated by modeling the category medians as a continuous variable. The two core models were specified as follows: Model 1 was adjusted for age, sex, body mass index, (body mass index)2, race-field center; Model 2 was adjusted for all variables in Model 1 plus LDL-cholesterol, HDL-cholesterol, triglycerides, waist-to-hip ratio, systolic blood pressure, blood pressure-lowering medication use, parental history of diabetes, education, drinking status, smoking status, and physical activity level. We also conducted analyses additionally adjusting for all variables in Model 2 plus HbA1c. We verified that the proportional hazards assumption was met using log-log plots.
To characterize the shape and compare the continuous associations of fructosamine, glycated albumin, and HbA1c with each of the outcomes in the overall population we fit linear and restricted cubic splines with four knots placed at the 5th, 35th, 65th, and 95th percentiles 21. Model discrimination with the addition of fructosamine, glycated albumin, or HbA1c to our core models (Model 1 and Model 2) was assessed using Harrel’s C-statistic 22. We also calculated the relative integrated discrimination improvement (IDI) statistic 23, 24 to assess the overall improvement in risk classification for the addition of fructosamine, glycated albumin, or HbA1c to the models. We conducted sensitivity analyses stratified by diabetes status. All reported p-values are two-sided, with p-value<0.05 indicating statistical significance. Statistical analyses were done using Stata/SE Version 13.1 (StataCorp, College Station, TX, USA).
RESULTS
Persons with higher fructosamine or glycated albumin values at baseline were more likely to be older, female, black, obese, hypertensive, and have a parental history of diabetes as compared to persons with lower values of fructosamine or glycated albumin (Table 1). The Pearson’s correlations of HbA1c with fructosamine or glycated albumin were high in the overall population: 0.81 and 0.85, respectively. Correlations were lower when the population was limited to those persons without diagnosed diabetes (eTable 1).
Table 1.
Study Population Characteristics* by Categories of Fructosamine and Glycated Albumin at Baseline in Persons without Diagnosed Diabetes (≤70th, 71–95th, >96th percentiles) and in Persons with Diagnosed Diabetes (≤38th, >38th percentiles), N=11,104
| No Diagnosed Diabetes | Diagnosed Diabetes | p-value for trend |
|||||
|---|---|---|---|---|---|---|---|
| Fructosamine | Total [89–706 umol/L] |
≤ 70th percentile [89–236.5 umol/L] |
71th – 96th percentile [236.6–266.7 umol/L] |
> 96th percentile [266.9–593.7 umol/L] |
≤ 38th percentile [158.5–270 umol/L] |
> 38th percentile [270.6–706 umol/L] |
|
| Fructosamine, umol/L | 236 (43.2) | 218 (12.9) | 247 (7.8) | 299 (54.7) | 238 (21.3) | 380 (90.3) | <0.001 |
| Glycated albumin, % | 13.4 (3.4) | 12.3 (1) | 13.6 (1.1) | 17.1 (5.1) | 13.7 (1.8) | 24.9 (7.4) | <0.001 |
| Hemoglobin A1c, % | 5.7 (1.1) | 5.4 (0.4) | 5.6 (0.5) | 6.7 (1.8) | 6.2 (0.8) | 9.2 (2) | <0.001 |
| Fasting glucose, mg/dL | 110 (34.8) | 102 (10.7) | 106 (14.7) | 144 (58.6) | 126 (29.8) | 229 (75) | <0.001 |
| Age, years | 56.6 (5.7) | 56.2 (5.6) | 57.3 (5.7) | 57.6 (5.8) | 57.7 (5.7) | 58 (5.8) | <0.001 |
| Female, % | 58.6 | 58.6 | 57.7 | 61.7 | 60.5 | 60.3 | 0.338 |
| Black, % | 23.4 | 17.4 | 30.5 | 49.9 | 30.2 | 48.6 | <0.001 |
| Body mass index, kg/m2 | 27.9 (5.4) | 28 (5.4) | 26.7 (4.8) | 28.3 (6.1) | 30.8 (6.5) | 31 (5.7) | <0.001 |
| Obese, % | 28.5 | 28.4 | 20.9 | 36.3 | 47.1 | 54.4 | <0.001 |
| Hypertension, % | 33.3 | 29.3 | 34.3 | 53.8 | 50.0 | 59.5 | <0.001 |
| Parental history of diabetes, % | 24.1 | 22.0 | 23.5 | 32.2 | 38.8 | 42.7 | <0.001 |
| No Diagnosed Diabetes | Diagnosed Diabetes | ||||||
| Glycated Albumin | Total [7.67- 51.53%] |
≤ 70th percentile [7.67–13.3%] |
71th – 96th percentile [13.31– 15.43%] |
> 96th percentile [15.46– 42.37%] |
≤ 38th percentile [7.91– 16.12%] |
> 38th percentile [16.13–51.53%] |
|
| Fructosamine, umol/L | 236 (43.2) | 221 (16.5) | 241 (16.6) | 287 (61.2) | 240 (25.1) | 379 (91.9) | <0.001 |
| Glycated albumin, % | 13.4 (3.4) | 12.1 (0.8) | 14.1 (0.5) | 18.1 (4.6) | 13.5 (1.5) | 24.9 (7.3) | <0.001 |
| Hemoglobin A1c, % | 5.7 (1.1) | 5.4 (0.4) | 5.6 (0.5) | 6.9 (1.7) | 6.1 (0.8) | 9.3 (2) | <0.001 |
| Fasting glucose, mg/dL | 110 (34.8) | 102 (10.5) | 105 (14.4) | 147 (57.9) | 125 (28) | 229 (74.5) | <0.001 |
| Age, years | 56.6 (5.7) | 56.2 (5.6) | 57.2 (5.8) | 58 (6) | 57.6 (5.7) | 58.1 (5.8) | <0.001 |
| Female, % | 58.6 | 56.0 | 64.8 | 61.9 | 59.9 | 60.7 | <0.001 |
| Black, % | 23.4 | 15.7 | 35.0 | 51.6 | 29.4 | 49.0 | <0.001 |
| Body mass index, kg/m2 | 27.9 (5.4) | 28 (5.2) | 26.7 (5.2) | 29.2 (6.3) | 30.6 (6.4) | 31.2 (5.7) | <0.001 |
| Obese, % | 28.5 | 28.0 | 21.4 | 40.1 | 45.0 | 55.6 | <0.001 |
| Hypertension, % | 33.3 | 30.7 | 31.7 | 46.8 | 50.3 | 59.2 | <0.001 |
| Parental history of diabetes, % | 24.1 | 21.8 | 24.7 | 29.8 | 37.0 | 43.8 | <0.001 |
Estimates are mean (standard deviation) or %. * Fructosamine and glycated albumin were divided into categories defined by the ≤70th, 71–96th, >96th percentiles in persons without diagnosed diabetes and by the ≤38th, >38th percentiles with diagnosed diabetes. These percentiles correspond to HbA1c categories of <5.7%, 5.7–6.4%, and >6.4% in persons without diagnosed diabetes, and HbA1c categories of <7%, ≥7% in persons with diagnosed diabetes
In persons without a diagnosis of diabetes, we conducted cross-sectional multivariable analyses to examine the independent correlates of elevated fructosamine or glycated albumin (i.e. values above the 96th percentile). These associations were compared to those for elevated HbA1c (i.e. values above the 96th percentile, ≥6.5%) (Table 2). We found that risk factor associations for elevated fructosamine or glycated albumin were, in general, relatively similar to risk factor associations observed for elevated HbA1c. In particular, older age, black race/ethnicity, hypertension, parental history of diabetes, and elevated liver markers (ALT, AST, or GGT) were consistently associated with elevated values of all three biomarkers of hyperglycemia. Current alcohol consumption was associated with lower (non-elevated) values of each biomarker. Low HDL-cholesterol and high triglycerides were associated with elevated biomarker levels, but these results were more robust for elevated HbA1c as compared to fructosamine or glycated albumin. Current smoking status was not associated with HbA1c but was associated with lower values of fructosamine or glycated albumin. Low kidney function (estimated glomerular filtration rate <60 ml/min/1.73m2) was significantly associated with elevated fructosamine; but the positive associations of eGFR with elevated glycated albumin and HbA1c were of smaller magnitude and not statistically significant. Anemia was positively associated with elevated glycated albumin. Substantial differences in associations were observed for body mass index and also C-reactive protein. Higher body mass index and C-reactive protein were strongly associated with elevated HbA1c but not with elevated fructosamine or glycated albumin. In additional analyses, there was evidence of non-linearity and Spearman’s correlations revealed modest inverse correlations of body mass index with glycated albumin (Spearman’s r = −0.15) and fructosamine (Spearman’s r = −0.14) in persons without diagnosed diabetes. This is in contrast to the positive correlation between HbA1c and BMI (Spearman’s r = 0.26).
Table 2.
Correlates of elevated levels (>96% vs ≤96%) of fructosamine, glycated albumin, or hemoglobin A1c (HbA1c) among persons without diagnosed diabetes, Adjusted* OR (95% CI)
| Fructosamine (> 266 umol/L) |
Glycated Albumin (> 15.46%) |
HbA1c (≥ 6.5%) |
||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| N with high levels | 515 | 514 | 350 | |
| Age, per SD | 1.31 (1.19, 1.43) | 1.38 (1.26, 1.51) | 1.22 (1.10, 1.36) | |
| Female (vs male) | 1.02 (0.84, 1.22) | 1.16 (0.96, 1.40) | 1.12 (0.89, 1.40) | |
| Black (vs white) | 3.98 (3.32, 4.77) | 4.57 (3.81, 5.49) | 4.62 (3.71, 5.74) | |
| Alcohol | ||||
| Current | 0.77 (0.62, 0.97) | 0.69 (0.55, 0.86) | 0.64 (0.49, 0.84) | |
| Former | 1.02 (0.80, 1.31) | 0.93 (0.73, 1.20) | 0.97 (0.72, 1.29) | |
| Never | 1 (reference) | 1 (reference) | 1 (reference) | |
| Smoking status | ||||
| Current | 0.62 (0.48, 0.81) | 0.72 (0.56, 0.92) | 0.91 (0.68, 1.22) | |
| Former | 0.97 (0.79, 1.19) | 0.93 (0.75, 1.15) | 1.06 (0.82, 1.37) | |
| Never | 1 (reference) | 1 (reference) | 1 (reference) | |
| Total Cholesterol ≥ 200 mg/dL |
1.58 (1.30, 1.92) | 1.10 (0.91, 1.33) | 1.48 (1.17, 1.86) | |
| Low HDL-cholesterol | 1.20 (0.99, 1.44) | 1.14 (0.94, 1.37) | 3.00 (2.40, 3.76) | |
| Triglycerides ≥ 150 mg/dL |
1.62 (1.32, 1.97) | 1.47 (1.20, 1.80) | 3.51 (2.80, 4.41) | |
| Body mass index, kg/m2 |
||||
| <25 | 1 (reference) | 1 (reference) | 1 (reference) | |
| 25–29 | 0.87 (0.70, 1.09) | 0.82 (0.65, 1.03) | 3.56 (2.23, 5.66) | |
| >30 | 1.08 (0.86, 1.36) | 1.16 (0.92, 1.46) | 10.60 (6.79, 16.55) | |
| C-reactive protein, mg/L |
||||
| <1 | 1 (reference) | 1 (reference) | 1 (reference) | |
| 1–3 | 0.84 (0.66, 1.08) | 0.83 (0.64, 1.07) | 3.39 (2.04, 5.62) | |
| >3 | 1.03 (0.81, 1.31) | 1.21 (0.95, 1.54) | 7.55 (4.64, 12.28) | |
| Anemia | 1.01 (0.77, 1.32) | 1.88 (1.49, 2.37) | 0.83 (0.59, 1.15) | |
| Hypertension | 1.84 (1.53, 2.22) | 1.26 (1.04, 1.52) | 2.29 (1.82, 2.87) | |
| Family hist diab | 1.45 (1.19, 1.77) | 1.41 (1.16, 1.73) | 1.89 (1.51, 2.38) | |
| eGFR < 60 ml/min/1.73 m2 |
3.18 (1.92, 5.28) | 1.80 (0.99, 3.28) | 1.69 (0.80, 3.55) | |
| High AST | 2.58 (2.01, 3.31) | 1.71 (1.29, 2.26) | 1.63 (1.17, 2.28) | |
| High ALT | 2.22 (1.75, 2.82) | 1.65 (1.27, 2.14) | 2.71 (2.07, 3.54) | |
| High GGT | 2.40 (1.86, 3.08) | 1.75 (1.33, 2.30) | 2.37 (1.77, 3.18) | |
Adjusted for age, race-center, and sex. Bold values indicate p-value <0.05.
Low HDL: HDL-cholesterol <40 mg/dL for men, <50 mg/dL for women
Anemia: hemoglobin <13 g/dL for men, <12 g/dL for women
High AST, ALT, and GGT are based on sex-specific 95th percentiles:
High AST: ≥32 U/L for men, ≥28 U/L for women
High ALT: ≥29 U/L for men, ≥23 U/L for women
High GGT: ≥70 U/L for men, ≥52 U/L for women
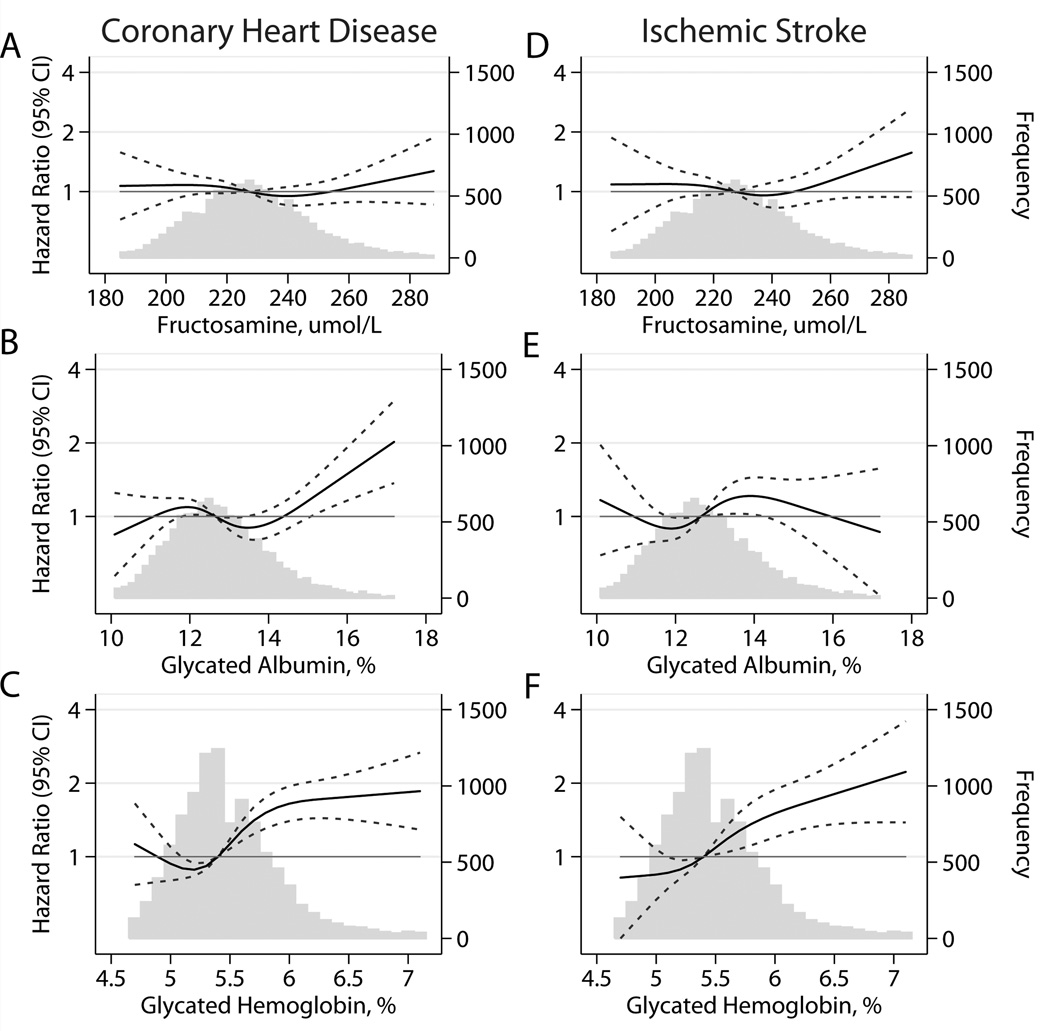
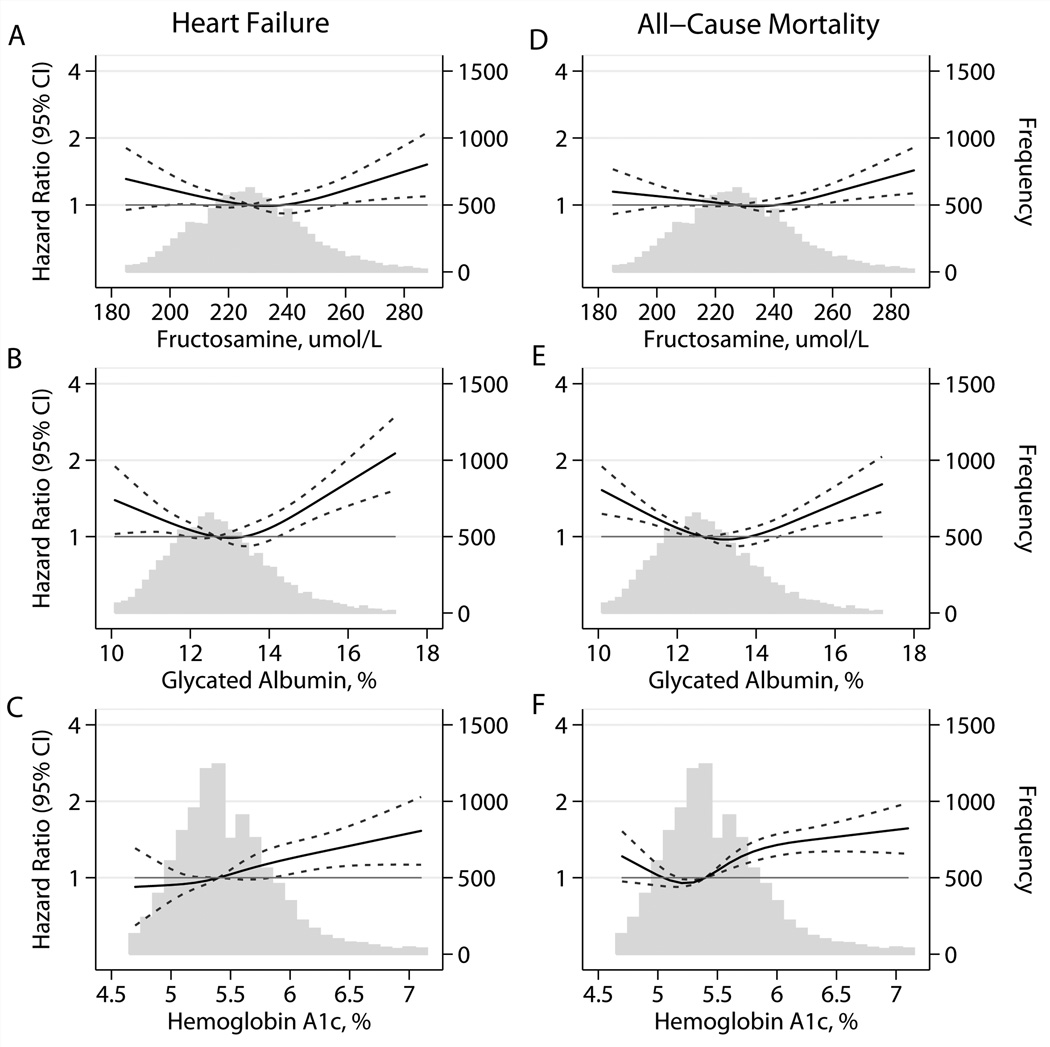
During over two decades of follow-up there were 1096 new cases of coronary heart disease, 605 of ischemic stroke, 1432 of heart failure, and 2860 deaths. In both persons with and without diagnosed diabetes, there was a higher risk of coronary heart disease, stroke, health failure, and death among persons with elevated fructosamine and/or elevated glycated albumin (Table 3 and Figures 1 & 2). In general, the associations were of similar magnitude to the associations observed for HbA1c (Figures 1 & 2), except for stroke, which appeared to have a stronger association with HbA1c (Figure 1, Panels D–F). There was also some evidence for a higher risk of events at very low values of fructosamine and glycated albumin, especially for heart failure and all-cause mortality (Figure 2), but some of the confidence intervals in the very low range were wide and overlapped the null value of 1. Similar results were also obtained when fructosamine or glycated albumin were modeled using linear splines (eFigure 1). When we modeled the associations separately for persons with or without diagnosed diabetes, we observed largely similar results but with generally more linear risk associations observed in persons with diagnosed diabetes (eFigures 2 and 3). The associations of fructosamine and glycated albumin with events were attenuated but remained statistically significant even after adjustment for HbA1c, with the exception of ischemic stroke (eTable 2).
Table 3.
Adjusted Hazard Ratios (95% confidence intervals) of Baseline Categories* of Fructosamine and Glycated Albumin with Incident Coronary Heart Disease, Ischemic Stroke, Heart Failure, and Death
| Coronary Heart Disease (1096 events) |
Ischemic Stroke (605 events) |
||||||
|---|---|---|---|---|---|---|---|
| Fructosamine HR (95% CI) |
Glycated Albumin HR (95% CI) |
HbA1c HR (95% CI) |
Fructosamine HR (95% CI) |
Glycated Albumin HR (95% CI) |
HbA1c HR (95% CI) |
||
| No Diagnosed Diabetes | |||||||
| ≤ 70th percentile | 1.11 (0.95, 1.30) | 1.08 (0.92, 1.28) | 0.64 (0.55, 0.75) | 1.06 (0.85, 1.31) | 0.87 (0.71, 1.07) | 0.74 (0.61, 0.91) | |
| 71th–96th | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| > 96th percentile | 1.33 (0.98, 1.81) | 1.61 (1.21, 2.15) | 1.17 (0.89, 1.53) | 1.93 (1.37, 2.71) | 1.46 (1.02, 2.07) | 1.79 (1.31, 2.45) | |
| Diagnosed Diabetes | |||||||
| ≤ 38th percentile | 1.89 (1.37, 2.60) | 2.08 (1.52, 2.83) | 1.47 (1.10, 1.96) | 1.37 (0.86, 2.19) | 1.22 (0.77, 1.93) | 1.18 (0.76, 1.83) | |
| > 38th percentile | 3.41 (2.72, 4.28) | 3.29 (2.60, 4.16) | 2.63 (2.13, 3.26) | 2.97 (2.20, 4.01) | 2.51 (1.87, 3.38) | 2.60 (1.96, 3.45) | |
| p-value-for-trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Heart Failure (1432 events) |
Death (2860 events) |
||||||
| Fructosamine HR (95% CI) |
Glycated Albumin HR (95% CI) |
HbA1c HR (95% CI) |
Fructosamine HR (95% CI) |
Glycated Albumin HR (95% CI) |
HbA1c HR (95% CI) |
||
| No Diagnosed Diabetes | |||||||
| ≤ 70th percentile | 1.08 (0.94, 1.24) | 1.00 (0.87, 1.15) | 0.82 (0.72, 0.94) | 1.02 (0.93, 1.12) | 1.13 (1.03, 1.25) | 0.81 (0.74, 0.89) | |
| 71th–96th | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| > 96th percentile | 1.32 (1.02, 1.71) | 1.38 (1.08, 1.78) | 1.24 (0.98, 1.56) | 1.30 (1.08, 1.57) | 1.53 (1.28, 1.83) | 1.27 (1.07, 1.51) | |
| Diagnosed Diabetes | |||||||
| ≤ 38th percentile | 1.64 (1.25, 2.15) | 1.58 (1.21, 2.07) | 1.26 (0.96, 1.65) | 1.69 (1.39, 2.05) | 1.80 (1.48, 2.19) | 1.32 (1.09, 1.60) | |
| > 38th percentile | 3.15 (2.60, 3.83) | 3.00 (2.47, 3.65) | 2.96 (2.47, 3.54) | 2.10 (1.80, 2.45) | 2.33 (1.99, 2.73) | 2.05 (1.77, 2.38) | |
| p-value-for-trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Fructosamine and glycated albumin were divided into categories defined by the ≤70th, 71–96th, >96th percentiles in persons without diagnosed diabetes and by the ≤38th, >38th percentiles with diagnosed diabetes. These percentiles correspond to HbA1c categories of <5.7%, 5.7–6.4%, and >6.4% in persons without diagnosed diabetes, and HbA1c categories of <7%, ≥7% in persons with diagnosed diabetes.
Models adjusted for age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2, LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), waist-to-hip ratio, systolic blood pressure (mmHg), blood pressure-lowering medication use (yes, no), parental history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), physical activity index
Abbreviations: CI, confidence interval; HR, odds ratio.
P-values-for-trends across all the categories were calculated by modeling the category medians as a continuous variable in the model.
Figure 1.
Adjusted* HRs (95% CIs) for baseline fructosamine, glycated albumin, and hemoglobin A1c (HbA1c) with incident coronary heart disease and ischemic stroke. *Adjusted HRs are from Cox proportional hazards models. Baseline fructosamine, glycated albumin, and HbA1c were modeled using restricted cubic splines (solid lines) with knots at the 5th, 35th, 65th, and 95th percentiles. Models are centered at the 50th percentile of each marker and are adjusted for age (years), race-center, sex (male, female), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), body mass index (kg/m2), body mass index squared, waist-to-hip ratio, mean systolic blood pressure (mmHg), blood pressure–lowering medication use (yes, no), diabetes (yes, no), parental history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), and physical activity index (score). Frequency histograms (gray bars) are shown for each biomarker, and are truncated at the 95th percentile. 95% confidence intervals are shown by the dotted lines.
Figure 2.
Adjusted* HRs (95% CI) for baseline fructosamine, glycated albumin, and hemoglobin A1c with incident heart failure and all-cause mortality. *Adjusted HRs are from Cox proportional hazards models. Baseline fructosamine, glycated albumin, and HbA1c were modeled using restricted cubic splines (solid lines) with knots at the 5th, 35th, 65th, and 95th percentiles. Models are centered at the 50th percentile of each marker and are adjusted for age (years), race-center, sex (male, female), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), body mass index (kg/m2), body mass index squared, waist-to-hip ratio, mean systolic blood pressure (mmHg), blood pressure–lowering medication use (yes, no), diabetes (yes, no), parental history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), and physical activity index (score). Frequency histograms (gray bars) are shown for each biomarker, and are truncated at the 95th percentile. 95% confidence intervals are shown by the dotted lines.
Table 4 presents the relative IDI and C-statistics comparing models with and without the different glycemic markers. We observed similar and statistically significant improvements in the C-statistics when fructosamine, glycated albumin, or HbA1c were added to Model 1 or Model 2. The IDI statistics were highly consistent with the C-statistic results.
Table 4.
C-statistics and Relative Integrated Discrimination Improvement (IDI) Statistics for the Addition of HbA1c, Fructosamine, or Glycated Albumin to Adjusted Cox Proportional Hazards Models of Risk of Coronary Heart Disease, Ischemic Stroke, Heart Failure, or Death
| C-statistic |
IDI |
||||
|---|---|---|---|---|---|
| C-statistic | Difference in c- statistic from reference model |
p-value for difference |
Relative IDI | p-value | |
| Coronary Heart Disease |
|||||
| Model 1 | 0.6902 | (reference) | - | - | - |
| Model 1 + HbA1c | 0.7316 | 0.0414 | < 0.0001 | 0.2151 | < 0.0001 |
| Model 1 + Fructosamine | 0.7088 | 0.0186 | < 0.0001 | 0.0747 | 0.0002 |
| Model 1 + Glycated albumin | 0.7081 | 0.0178 | < 0.0001 | 0.0874 | 0.0003 |
| Model 2 | 0.7717 | (reference) | - | ||
| Model 2 + HbA1c | 0.7854 | 0.0137 | < 0.0001 | 0.0685 | 0.0002 |
| Model 2 + Fructosamine | 0.7792 | 0.0075 | 0.0003 | 0.0372 | 0.0165 |
| Model 2 + Glycated albumin | 0.7789 | 0.0073 | 0.0009 | 0.0410 | 0.0156 |
| Ischemic Stroke |
|||||
| Model 1 | 0.6868 | (reference) | - | - | - |
| Model 1 + HbA1c | 0.7289 | 0.0421 | < 0.0001 | 0.5737 | < 0.0001 |
| Model 1 + Fructosamine | 0.7097 | 0.0229 | < 0.0001 | 0.2953 | < 0.0001 |
| Model 1 + Glycated albumin | 0.7095 | 0.0227 | < 0.0001 | 0.3307 | < 0.0001 |
| Model 2 | 0.7419 | (reference) | - | - | - |
| Model 2 + HbA1c | 0.7641 | 0.0222 | < 0.0001 | 0.2553 | < 0.0001 |
| Model 2 + Fructosamine | 0.7558 | 0.0139 | < 0.0001 | 0.1468 | 0.0002 |
| Model 2 + Glycated albumin | 0.7570 | 0.0151 | < 0.0001 | 0.1676 | < 0.0001 |
| Heart Failure |
|||||
| Model 1 | 0.7012 | (reference) | - | - | - |
| Model 1 + HbA1c | 0.7240 | 0.0228 | < 0.0001 | 0.1711 | < 0.0001 |
| Model 1 + Fructosamine | 0.7176 | 0.0164 | < 0.0001 | 0.0840 | 0.0001 |
| Model 1 + Glycated albumin | 0.7200 | 0.0188 | < 0.0001 | 0.1093 | < 0.0001 |
| Model 2 | 0.7548 | (reference) | - | - | - |
| Model 2 + HbA1c | 0.7673 | 0.0125 | < 0.0001 | 0.0643 | 0.0004 |
| Model 2 + Fructosamine | 0.7654 | 0.0106 | < 0.0001 | 0.0404 | 0.0043 |
| Model 2 + Glycated albumin | 0.7666 | 0.0118 | < 0.0001 | 0.0501 | 0.0002 |
| Death |
|||||
| Model 1 | 0.6854 | (reference) | - | - | - |
| Model 1 + HbA1c | 0.7005 | 0.0151 | < 0.0001 | 0.0820 | < 0.0001 |
| Model 1 + Fructosamine | 0.6939 | 0.0085 | < 0.0001 | 0.0268 | 0.0004 |
| Model 1 + Glycated albumin | 0.6963 | 0.0108 | < 0.0001 | 0.0467 | < 0.0001 |
| Model 2 | 0.7306 | (reference) | - | - | - |
| Model 2 + HbA1c | 0.7377 | 0.0070 | < 0.0001 | 0.0343 | 0.0001 |
| Model 2 + Fructosamine | 0.7348 | 0.0041 | < 0.0001 | 0.0161 | 0.0064 |
| Model 2 + Glycated albumin | 0.7358 | 0.0052 | < 0.0001 | 0.0193 | 0.0007 |
Model 1: age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2
Model 2: Model 1 + LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), waist-to-hip ratio, systolic blood pressure (mmHg), blood pressure-lowering medication use (yes, no), parental history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), physical activity index
Each marker is modeled using cubic splines with 4 knots (at the 5th, 35th, 65th, and 95th percentiles)
DISCUSSION
The acceptance of new measures of hyperglycemia is partly dependent on establishing their association with long-term outcomes. Furthermore, the comparison of risk factor associations for elevations in non-traditional versus traditional biomarkers of hyperglycemia is important for the interpretation of these biomarkers in the general population and the clinic. Previous studies have demonstrated strong associations between fructosamine and glycated albumin with microvascular conditions 1, 25, 26, with associations of similar magnitude to those observed for HbA1c. Data on risk associations using modern assays of fructosamine and glycated albumin with cardiovascular disease are sparse; and previous studies have been limited by cross-sectional designs or prospective studies with small numbers of cardiovascular events 25, 26. In the present study, we found that both fructosamine and glycated albumin were associated with coronary heart disease, ischemic stroke, heart failure, and death, with patterns of association similar to those observed for HbA1c. Even after adjustment for HbA1c, fructosamine and glycated albumin remained significantly associated with vascular outcomes and death. Indeed, improvements in the C-statistic and IDI when fructosamine, glycated albumin, or HbA1c were added to our fully adjusted model were all similar and statistically significant. Our results inform the prognostic value of fructosamine and glycated albumin.
Interestingly, we observed J-shaped associations of fructosamine and glycated albumin with some of the outcomes examined here, particularly mortality. A J-shaped association of HbA1c with risk of vascular endpoints and mortality has been observed in a number of different populations 27–31. Previous studies have suggested that low normal values of HbA1c may be associated with an increased risk of cardiovascular disease or death as a result of subclinical illness and/or possibly liver dysfunction. Indeed, it has been shown that persons with low-normal HbA1c have a high prevalence of elevated liver markers and hepatitis C (11.1% hepatitis C seropositivity in persons with HbA1c <4% compared to around 2% in the rest of the US adult population) 30 and an elevated risk of hospitalization for liver-related conditions 32. The elevated risk of cardiovascular disease and death at low-normal values of fructosamine and glycated albumin is consistent with previous studies of HbA1c and deserves further examination.
The correlations of HbA1c with fructosamine and glycated albumin in the overall population were high. The lower correlations (especially Spearman’s correlations) in persons without a history of diabetes reflects discordance of HbA1c with fructosamine or glycated albumin in ranking individuals by glycemic status within the normal range, possibly because of the very low short-term variability of HbA1c in persons without diabetes. In persons without a history of diabetes, we found that cross-sectional risk factor associations for elevated fructosamine or glycated albumin were generally similar to those observed for elevated (diagnostic) values of HbA1c but with the clear exception of body mass index, and also C-reactive protein. The nonlinear associations of body mass index and C-reactive protein with fructosamine and glycated albumin have been shown in a number of previous studies 33–41, but the mechanisms remain unexplained. It is possible that protein metabolism is altered in obese adults and may be affected by inflammation. C-reactive protein is known to be highly associated with body mass index and is lowered by weight loss 42–44. In additional to alterations in protein metabolism, other conditions that may affect the interpretation of fructosamine and glycated albumin test results include liver disease, hyperuricemia, acute illness or infection, and thyroid dysfunction 25. Nonetheless, the overall similarity of major diabetes risk factor associations for elevated HbA1c, fructosamine, and glycated albumin is reassuring and suggests that, in general, elevations in fructosamine and glycated albumin are largely being driven by the same pathophysiological processes that act to raise blood glucose concentrations over time.
Important limitations that should be considered when interpreting our results include the single baseline measurements of fructosamine, glycated albumin, and HbA1c. We also did not have contemporaneous 2-hour glucose measurements for comparison. Strengths of the study include the large community-based sample, active surveillance for cardiovascular events and deaths, and high number of incident events and deaths during the over 20 years of follow-up. This study also benefited from the rigorous data collection and measurement of covariates in ARIC and we implemented an array of statistical models to fully explore possible non-linear associations of fructosamine and glycated albumin with risk factors and clinical outcomes in our data. We also demonstrated excellent laboratory performance of the fructosamine and glycated albumin assays used here (intra-assays CVs of 3% or less). Our experience in the laboratory has been that fructosamine and glycated albumin are all highly stable and we have been able to conduct these tests with high reliability and validity in long-term stored samples in multiple studies 7, 9, 45, 46. Further, investigators of the DCCT/EDIC Study have demonstrated robust associations of the same glycated albumin assay with outcomes in a type 1 diabetes population 26 and demonstrated excellent stability in samples stored for 19–23 years 8.
In conclusion, we found that fructosamine and glycated albumin were associated with vascular outcomes and mortality in the community and that these associations were similar to those observed for HbA1c. Our results add to growing evidence for the utility of fructosamine or glycated albumin in settings where HbA1c testing cannot be used or where its interpretation is problematic.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions. Reagents for the fructosamine, liver enzyme, and C-reactive protein assays were donated by Roche Diagnostics. Reagents for the glycated albumin assays were donated by the Asahi Kasei Corporation.
Funding Sources: This research was supported by NIH/NIDDK grant R01 DK089174 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Disclosures: None.
References
- 1.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279–288. doi: 10.1016/S2213-8587(13)70199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins POC-IT Guides: Alternative markers of glycemia: fructosamine, glycated albumin, 1,5-AG. [Google Scholar]
- 3.Joslin Diabetes Center: Home Blood Glucose (Sugar) Monitoring, Hemoglobin A1C Testing, and Fructosamine Tests. [Google Scholar]
- 4.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:e1–e47. doi: 10.1373/clinchem.2010.161596. [DOI] [PubMed] [Google Scholar]
- 5.Type 2 diabetes: national clinical guideline for management in primary and secondary care (update) 2008. [PubMed] [Google Scholar]
- 6.Diabetes UK Guide to Diabetes: Testing. [Google Scholar]
- 7.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648–1655. doi: 10.1373/clinchem.2012.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem. 2011;57:286–290. doi: 10.1373/clinchem.2010.150250. [DOI] [PubMed] [Google Scholar]
- 9.Shafi T, Sozio SM, Plantinga LC, Jaar BG, Kim ET, Parekh RS, Steffes MW, Powe NR, Coresh J, Selvin E. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36:1522–1533. doi: 10.2337/dc12-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke Incidence and Survival Among Middle-Aged Adults : 9-Year Follow-Up of the Atherosclerosis Risk in Communities (ARIC) Cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 11.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes. 2010;2:118–124. doi: 10.1111/j.1753-0407.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 16.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Operations manual no. 11: sitting blood pressure, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 19.Operations manual no. 2: cohort component procedures, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 20.Operations manual no. 10: clinical chemistry determinations, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 21.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Sr Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 25.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14:548. doi: 10.1007/s11892-014-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan DM, McGee P, Steffes MW, Lachin JM Group DER. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63:282–290. doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer N, Wright CS, Travier N, Cunningham CW, Hornell J, Pearce N, Jeffreys M. A New Zealand Linkage Study Examining the Associations Between A1C Concentration and Mortality. Diabetes Care. 2008;31:1144–1149. doi: 10.2337/dc07-2374. [DOI] [PubMed] [Google Scholar]
- 28.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care. 2009;32:1440–1446. doi: 10.2337/dc09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carson AP, Fox CS, McGuire DK, Levitan EB, Laclaustra M, Mann DM, Muntner P. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3:661–667. doi: 10.1161/CIRCOUTCOMES.110.957936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emerging Risk Factors C, Di Angelantonio E, Gao P, Khan H, Butterworth AS, Wormser D, Kaptoge S, Kondapally Seshasai SR, Thompson A, Sarwar N, Willeit P, Ridker PM, Barr EL, Khaw KT, Psaty BM, Brenner H, Balkau B, Dekker JM, Lawlor DA, Daimon M, Willeit J, Njolstad I, Nissinen A, Brunner EJ, Kuller LH, Price JF, Sundstrom J, Knuiman MW, Feskens EJ, Verschuren WM, Wald N, Bakker SJ, Whincup PH, Ford I, Goldbourt U, Gomez-de-la-Camara A, Gallacher J, Simons LA, Rosengren A, Sutherland SE, Bjorkelund C, Blazer DG, Wassertheil-Smoller S, Onat A, Marin Ibanez A, Casiglia E, Jukema JW, Simpson LM, Giampaoli S, Nordestgaard BG, Selmer R, Wennberg P, Kauhanen J, Salonen JT, Dankner R, Barrett-Connor E, Kavousi M, Gudnason V, Evans D, Wallace RB, Cushman M, D'Agostino RB, Sr Umans JG, Kiyohara Y, Nakagawa H, Sato S, Gillum RF, Folsom AR, van der Schouw YT, Moons KG, Griffin SJ, Sattar N, Wareham NJ, Selvin E, Thompson SG, Danesh J. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311:1225–1233. doi: 10.1001/jama.2014.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aggarwal V, Schneider AL, Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. 2012;35:2055–2060. doi: 10.2337/dc11-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga M, Hirata T, Kasayama S, Ishizaka Y, Yamakado M. Body mass index negatively regulates glycated albumin through insulin secretion in patients with type 2 diabetes mellitus. Clin Chim Acta. 2014;438C:19–23. doi: 10.1016/j.cca.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Miyashita Y, Nishimura R, Morimoto A, Matsudaira T, Sano H, Tajima N. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract. 2007;78:51–55. doi: 10.1016/j.diabres.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, Morimoto A, Shirasawa T, Kawaguchi T, Tajima N. Glycated albumin is low in obese, non-diabetic children. Diabetes Res Clin Pract. 2006;71:334–338. doi: 10.1016/j.diabres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Koga M, Matsumoto S, Saito H, Kasayama S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J. 2006;53:387–391. doi: 10.1507/endocrj.k05-137. [DOI] [PubMed] [Google Scholar]
- 37.Woo J, Cockram C, Lau E, Chan A, Swaminathan R. Influence of obesity on plasma fructosamine concentration. Clin Chem. 1992;38:2190–2192. [PubMed] [Google Scholar]
- 38.Wang F, Ma X, Hao Y, Yang R, Ni J, Xiao Y, Tang J, Bao Y, Jia W. Serum glycated albumin is inversely influenced by fat mass and visceral adipose tissue in Chinese with normal glucose tolerance. PLoS One. 2012;7:e51098. doi: 10.1371/journal.pone.0051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga M, Saito H, Mukai M, Otsuki M, Kasayama S. Serum glycated albumin levels are influenced by smoking status, independent of plasma glucose levels. Acta Diabetol. 2009;46:141–144. doi: 10.1007/s00592-008-0072-5. [DOI] [PubMed] [Google Scholar]
- 40.Poon AK, Juraschek SP, Ballantyne CM, Steffes MW, Selvin E. Comparative associations of diabetes risk factors with five measures of hyperglycemia. BMJ Open Diabetes Res Care. 2014;2:e000002. doi: 10.1136/bmjdrc-2013-000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nouya AY, Nansseu JR, Moor VJ, Pieme CA, Noubiap JJ, Tchoula CM, Mokette BM, Takam RD, Tankeu F, Ngogang JY, Kengne AP. Determinants of fructosamine levels in a multi-ethnic Sub-Saharan African population. Diabetes Res Clin Pract. 2015;107:123–129. doi: 10.1016/j.diabres.2014.09.042. Epub 2014 Oct 24. [DOI] [PubMed] [Google Scholar]
- 42.Erlinger TP, Selvin E, Ridker PM. Effects of Adiposity and Weight Loss on C-reactive Protein C-Reactive Protein and Cardiovascular Disease. MediEdition Inc; 2006. [Google Scholar]
- 43.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 44.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 45.Juraschek SP, Steffes MW, Miller ER, 3rd, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265–2270. doi: 10.2337/dc12-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selvin E, Francis LM, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, Steffes MW. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34:960–967. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.