Abstract
Parkinson’s disease (PD) is a multifactorial and clinically complex age-related movement disorder. The cause of its most common form (sporadic PD, sPD) is unknown, but one prominent causal factor is mitochondrial dysfunction. Although several genetic- and toxin-based models have been developed along the last decades to mimic the pathological cascade of PD, cellular models that reliably recapitulate the pathological features of the neurons that degenerate in PD are scarce.
We describe here the generation of cytoplasmic hybrid cells (or cybrids) as a cellular model of sPD. This approach consists on the fusion of platelets harboring mtDNA from sPD patients with cells in which the endogenous mtDNA has been depleted (Rho0 cells).
The sPD cybrid model has been successful in recapitulating most of the hallmarks of sPD, constituting now a validated model for addressing the link between mitochondrial dysfunction and sPD pathology.
Keywords: Cybrids, Cellular models, Rho cells, Mitochondria, mtDNA, Neurodegeneration, Parkinson’s disease, Mitochondrial impairment, Oxidative stress, Alpha-synuclein oligomers
1 Introduction
Parkinson’s disease (PD) is a complex and molecularly heterogeneous disease in which a multitude of factors may contribute to the pathological process. While the etiology involved in the development of PD is still unknown, 90–95 % of PD cases occur sporadically and correlate, at least in part, with mitochondrial dysfunction and oxidative stress [1, 2].
Advances in PD pathobiology study have been based on the availability of different types of experimental model systems expected to recapitulate the various forms of this disease. However, the majority of the models commonly used to study PD do not reliably describe some of the pathological features frequently found 416 in PD patient brains, which obviously dampens the relevance of some of the data obtained with these models to PD etiology.
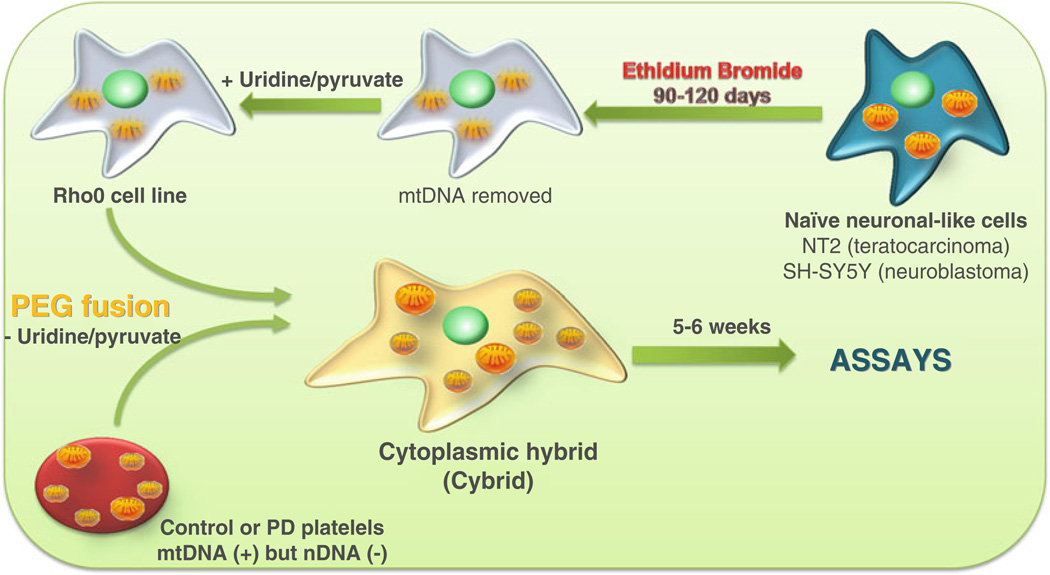
To circumvent these limitations, we have established a specific technical approach to address mitochondrial dysfunction as a common feature or, conceivably, the primary contributor to sporadic PD (sPD) pathology. sPD was modeled by creating cytoplasmic hybrid cell lines (cybrids) in which endogenous mitochondrial DNA (mtDNA) from sPD or control subject platelets is transferred to human neuronal-like cells (NT2 teratocarcinoma cells or SH-SY5Y neuroblastoma) after their complete depletion of endogenous mtDNA (Rho0 cells). After fusion, Rho0 cells repopulated with platelet-derived mitochondria are subjected to selection in order to eliminate non-fused and/or partially repopulated cells [3–5]. After multiple duplication cycles, cybrid cell lines with equivalent nuclear and environmental backgrounds are obtained and which differ from each other only in the source of their mitochondrial genome [3–5] (Fig. 1).
Fig. 1.
Generation of cybrid cell lines. Tumor or immortalized cell lines are grown in the presence of ethidium bromide, which efficiently eliminates functional mtDNA resulting in a Rho0 cell line. Rho0 cells are then fused with patient platelets, which contain mitochondria but not nuclei. This creates cytoplasmic hybrid (cybrid) cells that can be isolated and expanded. The expanded cybrid cell cultures are biochemically analyzed. Differences in function between cell lines mostly likely arise through differences in their mtDNA
Cybrids generated with mitochondria harvested from sPD patient platelets exhibit complex I deficiency similar to patient cells, showing direct inheritance of the biochemical abnormality through mitochondria [3, 5]. In addition to complex I activity deficiency, sPD cybrids present a number of other physiological alterations found in PD patient tissues, including: (1) redox deregulation, characterized by increased generation of reactive oxygen species (ROS) [6, 7] and by an increased content of oxidized proteins [7]; (2) loss of calcium homeostasis [8, 9]; (3) mitochondrial morphology abnormalities and alterations in the respiratory chain activity, such as increased proton leak and decreased maximum respiratory capacity [3, 5, 6, 10]; and (4) protein aggregation in the form of Lewy body-like inclusions, one of the histopathological hallmarks of PD [7, 11, 12]. We further found that this protein aggregation phenotype, mainly characterized by an altered alpha-synuclein oligomerization pattern, is a direct consequence of a decreased pro teolytic fl ux through autophagy due to alterations in the microtubule-directed trafficking driven by dysfunctional mitochondria [13, 14].
The evidence above demonstrates that sPD cybrids constitute a valuable cellular system which lets investigators address the impact of mtDNA mutations on a controlled nuclear background. Actually, comparison of cybrids derived from healthy individuals with those from sPD patients makes it possible to assess the effect of inherent PD-related changes in mitochondria over other cellular functions or processes.
Cybrid cell lines thus represent a breakthrough in cell biology research. They have been instrumental for dissecting molecular pathways of neurodegeneration resulting from specific genetic backgrounds and have already provided novel therapeutic targets and valuable insights for validation in more sophisticated models.
2 Materials
2.1 Reagents and Material
Histopaque-1077 sterile filtered, density: 1.077 g/mL.
Polyethylene glycol 1000 (PEG).
ACCUSPIN™ tubes sterile, 50 mL capacity.
Trypsin–EDTA solution cell culture tested.
Fetal bovine serum (FBS).
Fetal bovine serum (FBS), dialyzed.
Uridine powder, BioReagent, suitable for cell culture.
Sodium pyruvate cell culture tested.
Penicillin–streptomycin (10,000 U/mL).
Opti-MEM® reduced serum medium, powder.
Dulbecco’s Modified Eagle Medium, high glucose (DMEM).
SMEM Medium: MEM, suspension, no calcium, no glutamine (fusion medium).
2.2 Media
Opti-MEM growth medium (NT2 and cybrids growth medium): Opti-MEM containing 10 % heat-inactivated, non-dialyzed FBS, 1 % penicillin–streptomycin, 28.5 mM NaHCO3, and 8.4 mM HEPES, pH 7.3.
Opti-MEM Rho0 growth medium (NT2 Rho0 growth medium): Opti-MEM containing 10 % heat-inactivated, non-dialyzed FBS, 1 % penicillin–streptomycin, 28.5 mM NaHCO3, 8.4 mM HEPES, 200 µg/mL pyruvate, and 150 µg/mL uridine, pH 7.3.
Selection medium: Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose with l-glutamine, with pyridoxine hydrochloride, without sodium pyruvate and uridine, 1 % penicillin–streptomycin, and 10 % heat-inactivated, dialyzed FBS, pH 7.3.
Phosphate-buffered saline 10× (PBS): 1.37 M NaCl, 43 mM Na2 HPO4 anhydrous, 14 mM KH2 PO4 anhydrous, and 27 mM KCl, pH 7.3.
Dissociation medium: 136.8 mM NaCl, 8.17 mM Na2 HPO4, 1.47 mM KH2 PO4 anhydrous, 2.68 mM KCl, 0.43 mM EDTA, 400 µL fenol red, and 1 L H2 O, pH 7.3.
3 Methods
All these experimental procedures must be performed in a cell culture room under sterile conditions.
3.1 Creation and Culture of NT2 Rho0 Cells
A fairly large number of studies have used either human teratocarcinoma (NT2) or neuroblastoma (SH-SY5Y) Rho0 cell backgrounds to generate cybrid cell lines. In this protocol we used NT2 Rho0 cells.
Native NT2 human teratocarcinoma cells (Stratagene, La Jolla, CA) are expanded in Opti-MEM growth culture medium.
To eliminate endogenous mtDNA from the NT2 cells, supplement Opti-MEM growth medium with 25 ng/mL ethidium bromide (EtBr), 100 µg/mL pyruvate, 100 µg/mL uridine, 300 µM DL-2-amino-5-phosphonovaleric acid, and 20 µM 6,7-dinitroquinoxaline-2,3 (1H,4H)-dione (see Notes 1 and 2).
Incubate cells in this medium for 120 days. Replate cells approximately once per week and with medium changes every 2–3 days (see Note 3).
After 120 days propagate the treated cells in Opti-MEM growth medium supplemented with 25 ng/mL EtBr and pyruvate (200 µg/mL) and uridine (150 µg/mL), and do not add DL-2-amino-5-phosphonovaleric acid or 6,7-dinitroquinoxaline-2,3 (1H,4H)-dione.
At this point, cells are able to survive in higher EtBr concentrations. Incubate cells with the previous described Opti-MEM growth medium supplemented with increasing concentrations of EtBr until reaching 500 ng/mL EtBr during 3–4 months.
After 3–4 months, cells lose their mtDNA, and this is associated with loss of respiratory competence. The resulting cells, termed “Rho0,” become dependent on pyruvate and uridine for survival (see Note 4). NT2 Rho0 cells are then maintained and cultured in Opti-MEM Rho0 growth medium.
To corroborate Rho0 status in mtDNA-depleted cells, investigators can demonstrate an absence of functional electron transport chain activity, absence of oxygen utilization, pyruvate/uridine auxotrophy, lack of mtDNA amplification by PCR, and lack of mtDNA probe hybridization on Southern blotting.
3.2 Creation of Cybrid Cell Lines
Before starting the protocol previously warms all the necessary reagents at 37 °C: Histopaque; SMEM medium; Opti-MEM Rho0 growth medium; trypsin–EDTA (1:5); PBS (1×); and thaw PEG at 50 °C.
3.2.1 Preparation and Isolation of Platelets
Platelets are a particularly appropriate and ideal source for donor mtDNA transfer because they contain mitochondria (and hence mtDNA) but are anuclear.
Following provision of informed consent, 5 mL of fresh blood is collected through venipuncture in tubes containing acid– citrate–dextrose as an anticoagulant (see Notes 5–7).
-
Add 3 mL of Histopaque to a 12 mL Accuspin tube and spin Histopaque below the frit (30 s). Afterwards layer 5 mL of blood over Histopaque, and centrifuge for 10 min at 1,000 × g at room temperature.
At the end of the centrifugation, one should have something similar to what is shown in the Fig. 2.
Transfer the platelet-rich fraction to a sterilized 15 mL Falcon tube and add five volumes of SMEM (fusion medium), triturate “up and down,” and centrifuge at 1,700 × g for 15 min at room temperature. The final pellet contains the platelets.
Aspirate the supernatant and resuspend the pellet in 10 mL of SMEM (see Note 8).
Pellet platelets by spinning at 1,700 × g for 15 min at room temperature.
Fig. 2.
Schematic representation of the end result after centrifugation of anticoagulated venous blood layered onto Histopaque. During centrifugation, erythrocytes and granulocytes are aggregated by polysucrose and rapidly sediment, whereas lymphocytes and other mononuclear cells such as platelets remain at the plasma–Histopaque interface
3.2.2 Culture and Preparation of NT2 Rho0 Cells for Fusion
Use cells in T75-cm2 flasks with 80 % confluence. Per fusion (per cybrid) use about two million cells.
Remove the complete Opti-MEM Rho0 growth medium by aspiration.
Wash the Rho0 cells with 5 mL of PBS (1×) and remove it by aspiration.
Add 2 mL of 1:5 trypsin–EDTA and incubate for 2 min in a humidified 95 % air/5 % CO2 incubator at 37 °C (see Note 9).
Strike the side of the fl ask with the palm of the hand several times to completely dislodge sheets of cells. If dislodged sheets of cells do not become visible at this time, prolong the incubation period by 1 min increments for no more than a total of 7 min (see Note 10).
Immediately add 8 mL of complete Opti-MEM Rho0 growth medium to inhibit trypsin–EDTA.
Break up any clumps of cells by pipetting the cell suspension up and down ~10 times with a 5 mL pipette.
Count an aliquot of the cell suspension in a hemocytometer by diluting the suspension 1:2 with trypan blue solution, ensuring that the cells are resuspended properly. Count the aliquot immediately to determine accurate ratios of live to dead cells.
In two 50 mL Falcon tubes, transfer two million of cells to each (one for the cybrid creation and one for the “mock fusion”), and centrifuge at 200 × g for 5 min at room temperature to pellet the cells (see Note 11).
Aspirate the supernatant and resuspend the pellet in SMEM. For each two million cells, add 1 mL of SMEM.
3.2.3 Transfer of Human Platelet Mitochondria to NT2 Rho0 Cells
Heat PEG to about 50 °C in order to melt, while liquid moves around 20 mL to a glass tube in order to be autoclaved (see Note 12).
Melt the autoclaved PEG and prepare several 15 mL Falcon tubes with 1 mL of PEG.
Weight the amount of PEG in each tube. These aliquots of PEG can be stored at 4 °C for next use.
While harvesting and centrifuging the Rho0 cells, melt one aliquot of PEG. After it melts add SMEM to the PEG in a 1:1 weight–volume solution and triturate (see Note 13).
The unadjusted PEG mixture pH should be around 6, and one wants the pH to be 7.2. Use a dilute sterile NaOH solution to bring the pH to 7.2.
At this point one should have one 15 mL Falcon tube with a platelet pellet; two 50 mL Falcon tubes with two million Rho0 cells resuspended in 1 mL of SMEM; and a 15 mL Falcon tube with the PEG–SMEM mix solution.
Prepare two T75-cm2 fl asks with 10 mL each of fresh Opti-MEM Rho0 growth medium (one for the real fusion (cybrid) and one for the “mock fusion”).
Pipette 1 mL of Rho0 cell solution on top of the platelet pellet. One will have two million Rho0 cells in SMEM on top of the platelet pellet. At this point do not resuspend.
Centrifuge at 200 × g for 5 min at room temperature to pellet the Rho0 cells on top of the platelets pellet. Centrifuge also the 50 mL Falcon tube with two million Rho0 cells in SMEM for the “mock fusion.”
Carefully aspirate the supernatant.
Pipette 150 µL of PEG-SMEM diluted solution onto the pellet. Resuspend the pellets for about 60–70 s by triturating up and down. Then quickly transfer the suspensions into T75-cm2 fl asks filled with Opti-MEM Rho0 growth medium (see Note 14).
Concomitantly perform “mock fusions,” in which Rho0 cells but no platelets are suspended for 60–70 s in PEG–SMEM solution and transferred into a T75-cm2 flask filled with Opti-MEM Rho0 growth medium.
Next day change growth medium.
The cells are allowed to recover for 1 week in Opti-MEM Rho0 growth medium. Change the medium every 2–3 days thereafter. Do not divide the cells during the week after the fusion unless they become 100 % confluent.
3.2.4 Selection of Rho0 Cells Repopulated with Mitochondria
Following fusion, the Rho0 cells repopulated with exogenous platelet mitochondria are selected by culturing in medium lacking uridine and pyruvate and supplemented with 10 % dialyzed heat-inactivated FBS. These conditions were designed so that only aerobically competent cells, i.e., cells successfully fused with exogenous mitochondria, could survive. By removing pyruvate and uridine supplementation from the culture medium, untransformed cells are removed, while the transformed individual cybrid cells expand.
In order to make the selection medium dialyzed, FBS was thawed overnight at 4 °C, warmed to 37 °C, and then heated to 56 °C for 30 min for heat inactivation.
One week after fusion, change from Opti-MEM Rho0 cell growth medium to selection medium. Unfused cells will die and the colonies of fused cells will grow.
Over the following 6 weeks, change the selection medium according to the color of the medium, or divide the cells according to the percentage of confluence. When the cells are 100 % confluent, divide them. Even if the “mock fusion” flask is not confluent, one should go ahead and divide them anyway. During 6 weeks the selection medium should allow complete die-off of Rho0 cells from mock fusions.
After selection is complete the resultant cybrid lines are maintained in Opti-MEM growth medium in a 95 % humidified air/5 % CO2 incubator at 37 °C.
The handling of the cybrid cells is the same as described in Subheading 3.2.2.
The end result is a unique “cybrid cell line” that contains and expresses the nuclear genes of the original Rho0 cell line and the mitochondrial genes of the platelet donor.
Footnotes
The response of cells to mtDNA replication inhibitor, EtBr, is cell line dependent suggesting that sensitivity to EtBr treatment may be cell type specific and therefore must be titrated for each cell type [15, 16].
EtBr is a cationic mutagen capable of intercalating into DNA during replication. Because of its positive charge, it concentrates within negatively charged mitochondrial matrices. This allows determination of EtBr concentrations that block mtDNA replication without disrupting nuclear DNA replication [17].
Incubate cells with EtBr in the dark.
Cells depleted of their mitochondrial DNA become pyrimidine auxotrophic, and this can be overcome by supplementing the medium with uridine. This happens because dihydroorotate dehydrogenase enzyme activity, which is located within mitochondria, is dependent on electron transport chain function. Following mtDNA depletion dihydroorotate dehydrogenase is no longer able to catalyze the conversion of dihydroorotic acid to orotic acid. By supplementing cells with uridine, a pyrimidine pathway metabolite generated downstream of this synthetic step, this blocked intermediary step is overcome. Moreover, these cells are also auxotrophic for pyruvate. By deple ting mtDNA, oxidative phosphorylation does not occur, and this precludes NADH oxidation. By supplementing cells with pyruvate, pyruvate conversion to lactate by lactate dehydro genase is induced, thereby promoting the regeneration of NAD+ [17].
Acid–citrate–dextrose is better than EDTA as an anticoagulant for this application.
Acid–citrate–dextrose tubes containing blood need to be refrigerated if not used right away. After drawing the blood there is a 24 h period during which tubes can sit at room temperature, but beyond this point placing tubes on wet ice is preferred.
A fusion of platelets harvested from 5 mL of blood and 1 × 106 Rho0 cells typically yields up to several hundred individual transformed cybrid cells.
Requires forceful pipetting as the pellet tends to stay as a clump.
Previously dilute trypsin–EDTA 5× in dissociation medium.
Do not leave the cells unattended at this point as these cells are highly sensitive to overtrypsinization.
The “mock fusion” is generated using Rho0 cells but no platelets and serves as both a negative control and to monitor reversion of the Rho0 cell phenotype.
PEG should be prepared in advance.
Each day a cybrid fusion is performed, and use fresh PEG aliquots stored at 4 °C (without SMEM).
Do not let cells sit in PEG for more than 90 s total.
References
- 1.Schapira AH. Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ. 2007;14(7):1261–1266. doi: 10.1038/sj.cdd.4402160. [DOI] [PubMed] [Google Scholar]
- 2.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16:R183–R194. doi: 10.1093/hmg/ddm159. 16/R2/R183 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8(3):219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Jr, Davis RE. Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer’s disease and Parkinson’s disease. Ann N Y Acad Sci. 1999;893:176–191. doi: 10.1111/j.1749-6632.1999.tb07825.x. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40(4):663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 6.Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr, Bennett JP., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta. 1997;1362(1):77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 7.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Oxidative stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2009;11(3):439–448. doi: 10.1089/ars.2008.2247. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. J Neurochem. 1997;68(3):1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- 9.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Dysfunctional mitochondria uphold calpain activation: contribution to Parkinson’s disease pathology. Neurobiol Dis. 2010;37(3):723–730. doi: 10.1016/j.nbd.2009.12.011. S0969-9961(09) 00366-0 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Esteves AR, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons KE, Pahwa R, Burns JM, Cardoso SM, Swerdlow RH. Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. J Neurochem. 2010;113(3):674–682. doi: 10.1111/j.1471-4159.2010.06631.x. JNC6631 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Trimmer PA, Borland MK, Keeney PM, Bennett JP, Jr, Parker WD., Jr Parkinson’s disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem. 2004;88(4):800–812. doi: 10.1046/j.1471-4159.2003.02168.x. 2168 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Microtubule depolymerization potentiates alpha-synuclein oligomerization. Front Aging Neurosci. 2010;1:5. doi: 10.3389/neuro.24.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arduino DM, Esteves AR, Cardoso SM. Mitochondria drive autophagy pathology via microtubule disassembly: a new hypothesis for Parkinson disease. Autophagy. 2013;9(1):112–114. doi: 10.4161/auto.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arduino DM, Esteves AR, Cortes L, Silva DF, Patel B, Grazina M, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial metabolism in Parkinson’s disease impairs quality control autophagy by hampering microtubule-dependent traffic. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds309. dds309 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985;5(5):1163–1169. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 17.Khan SM, Smigrodzki RM, Swerdlow RH. Cell and animal models of mtDNA biology: progress and prospects. Am J Physiol Cell Physiol. 2007;292(2):C658–C669. doi: 10.1152/ajpcell.00224.2006. [DOI] [PubMed] [Google Scholar]