Abstract
Hot flashes (HFs) are a rapid and exaggerated heat dissipation response, consisting of profuse sweating, peripheral vasodilation, and feelings of intense, internal heat. They are triggered by small elevations in core body temperature (Tc) acting within a greatly reduced thermoneutral zone, i.e., the Tc region between the upper (sweating) and lower (shivering) thresholds. This is due in part, but not entirely, to estrogen depletion at menopause. Elevated central sympathetic activation, mediated through α2-adrenergic receptors, is one factor responsible for narrowing of the thermoneutral zone. Procedures which reduce this activation, such as paced respiration and clonidine administration, ameliorate HFs as will peripheral cooling. HFs are responsible for some, but not all, of the sleep disturbance reported during menopause. Recent work calls into question the role of serotonin in HFs.
Keywords: Hot flashes, Menopause, Thermoregulation, Sleep
Introduction
Hot flashes (HFs) are the most common symptom of the climacteric and are reported as feelings of intense warmth along with sweating, flushing, and chills. Sweating is generally reported in the face, neck and chest. HFs usually last for 1 to 5 minutes, with some lasting as long as an hour [1]. The median duration of symptoms is about four years, with some lasting as long as 20 years [2]. In one U.S. study, 87% of the women reported daily HFs and about a third of those reported more than 10 per day [1]. There is some racial and ethnic variation of HFs with Caucasian women reporting the highest prevalence and Japanese and Chinese women reporting the lowest [3].
1. Physiologic events of the hot flash
Peripheral vasodilation, demonstrated by elevated skin blood flow and temperature, occurs during HFs in all areas that have been studied (Fig. 1). Skin temperature increases in the digits, face, arms, chest, abdomen, back, and legs [4–8] and blood flow in these areas are elevated, as well [6–8].
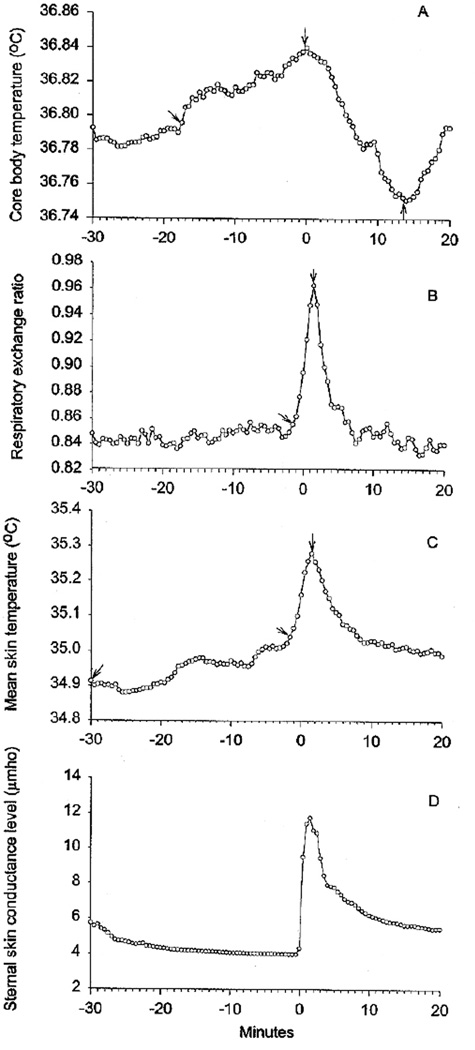
Fig. 1.
(A), Core body temperature (means) during menopausal hot flashes. (B), Respiratory exchange ratio (means) during hot flashes. (C), Mean skin temperature (means) during hot flashes. (D), Sternal skin conductance (means) during hot flashes. Time 0 is the beginning of the sternal skin conductance response. Intervals between arrows are significantly different from each other at P<.05, Duncan’s test.
Sweating and skin conductance, an electrical measure of this, also increases during HFs (Fig. D. 1). Molnar [5] determined the whole body sweat rate to be about 1.3 g/min in one subject. We measured sweating and skin conductance from the sternum at the same time in 14 women [4]. We found a close temporal correspondence between both measures, which were significantly elevated. Measureable sweating occurred in 90% of the HFs.
Core body temperature (Tc) also increases prior to HFs. We measured Tc and sternal skin conductance during 77 HFs in 10 menopausal women who reported frequent symptoms [9]. We found small but significant Tc elevations before the majority of HFs and replicated these findings in two subsequent studies [4,10].
The Tc elevations could be caused by increased metabolic rate (heat production) and/or peripheral vasoconstriction (decreased heat loss). We did find significant increases in metabolic rate (Fig. B. 1), but they occurred at the same time as the peripheral vasodilation and sweating; peripheral vasoconstriction did not occur. Therefore, the Tc elevations are not caused by metabolic rate elevations. Small increases in heart rate, about 7–15 beats/min do occur along with the metabolic rate increases [5,6].
2. Objective measurement of hot flashes
Typically, diaries are used to assess treatment outcome in HF studies. However, there are several problems with these measures. Errors in compliance are major sources of bias [11]. Also, HFs occurring during sleep are not accurately reported because recall of these events is usually poor and many HFs do not produce awakenings [12]. Finally, placebo effects as large as 40–50% occur with self-reports [13]. Therefore, objective measures of HFs have been developed.
Increased skin conductance recorded from the sternum is presently the best objective marker of HFs. An increase in this measure ≥ 2µmho (electrical unit of conductance) within 30 sec corresponded with 95 [14], 90 [15], and 80% of reported HFs [16]. These results have been independently replicated [16]. Moreover, these results have been extended to men with HFs due to androgen depletion by GNRh agonists in the treatment of prostate cancer [17].
The skin conductance measure is also useful because it can be recorded outside the laboratory in daily life. Using the same recording methods with ambulatory monitors, we found an agreement of 85% between the skin conductance criterion and patient event marks [14]. A second study found an agreement of 77% [15].
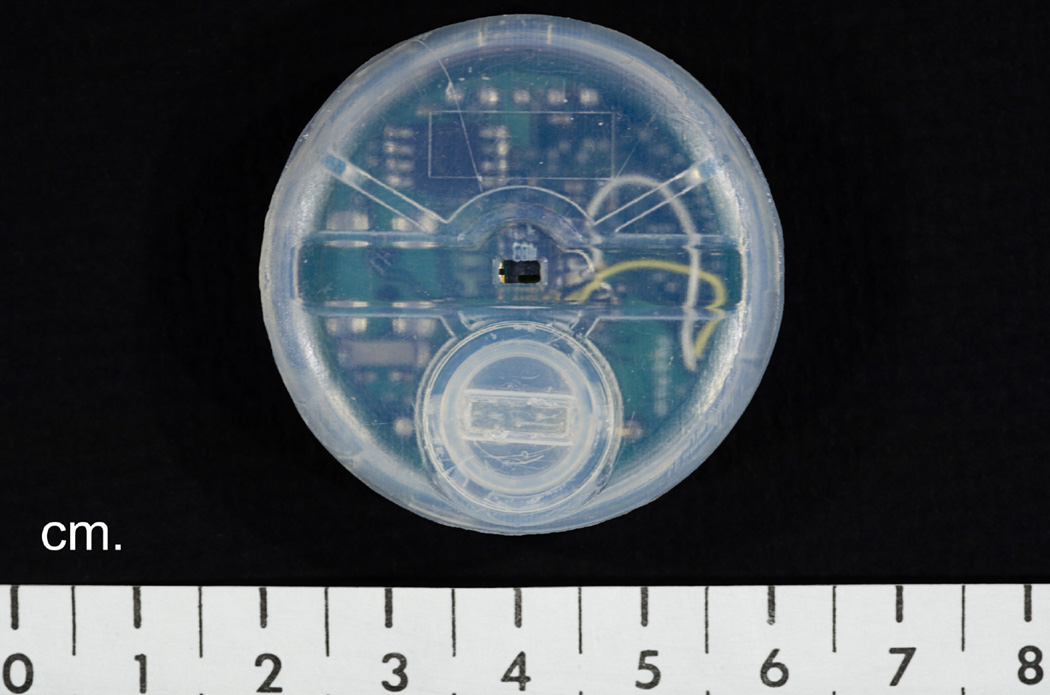
However, the major drawback of skin conductance recording is that it requires the use of electrodes and gel, which must be changed every 24h. Therefore, the author has invented a miniature, hygrometric HF recorder (Fig. 2), which requires neither electrodes nor gel [18]. This device will record all HFs for one month using a single hearing aid battery. It attaches to the skin with a double-sided sticky collar. A simple computer scoring program has been developed which will score one week of 24h data in <5 min.
Fig. 2.
Miniature hot flash recorder invented by the author.
In a recent study of patient satisfaction with the recorder, the author obtained positive responses from patients regarding the ease of use and appearance of the recorder [18,19].
3. Endocrinology of hot flashes
Since HFs occur in the vast majority of women having natural or surgical menopause, estrogens are clearly involved in their etiology. This is consistent with the fact that estrogen therapy virtually eliminates HFs. However, estrogen reduction alone does not explain the occurrence of HFs because there are no relationships between these symptoms and plasma, urinary, or vaginal [20] levels of estrogens, nor are there differences in plasma levels between women with and without HFs [9,20]. Additionally, clonidine reduces HF frequency but does not change estrogen levels [21], and prepubertal girls have low estrogen levels but no HFs.
Therefore, estrogen withdrawal is necessary but not sufficient to explain the occurrence of HFs. A temporal relationship was observed between HFs and luteinizing hormone (LH) pulses [22,23]. However, further work demonstrated that women with isolated gonadotropin deficiency had HFs but no LH pulses [24], and those with hypothalamic amenorrhea had LH pulses but no HFs. Also, HFs occur in women with LH suppression from GnRH compounds [25,26], in women with pituitary insufficiency and hypoestrogenism [27], and in hypophysectomized women, who have no LH pulses [28].
Subsequently, an opiate system was hypothesized in the etiology of HFs. Lightman [29] showed that an opiate antagonist reduced HF and LH pulse frequencies, although other research failed to replicate these results [30]. Thus, the evidence for opiate involvement in HFs is inconsistent.
Norepinephrine (NE) plays an important role in thermoregulation acting, in part, through α2-adrenergic receptors. Injected into the preoptic hypothalamus, NE causes heat dissipation responses followed by a decline in Tc [31]. Additionally, gonadal steroids modulate central NE activity [32]. Although plasma NE levels do not change during HFs [6,22], these do not represent levels in the brain [33].
We addressed these issues using pharmacologic probes. In a controlled, laboratory investigation [34], we showed that yohimbine, a α2-adrenergic antagonist that elevates brain NE [35], triggered HFs in symptomatic but not asymptomatic menopausal women, while clonidine, an α2 agonist, ameliorated them. Cadaver studies have shown that most α2 receptors in the human brain are inhibitory [36]. Blockade of these receptors with yohimbine would increase NE release, whereas clonidine would reduce it [37,38].
Furthermore, estrogens modulate brain adrenergic receptors [39,40]. Taken together, these data formed the basis of our theory that elevated brain NE, in conjunction with estrogen withdrawal, are part of the etiology of HFs.
4. Thermoregulation and hot flashes
Core body temperature (Tc) in homeotherms is regulated between an upper threshold for sweating and a lower threshold for shivering. Between these thresholds is a neutral zone within which major thermoregulatory responses (sweating, shivering) do not occur [41]. Small adjustments within the neutral zone are performed by changes in peripheral blood flow. According to this theory, the heat dissipation responses of the hot flash (sweating, peripheral vasodilation) would be provoked if Tc crossed the upper threshold. We already demonstrated Tc increases before most hot flashes [4,9,10]. We, therefore, chose to study the width of the thermoneutral zone in women with and without HFs.
Previous research showed that warm, ambient temperature and peripheral body heating could provoke HFs [14,15] suggesting that the upper threshold is lowered in women with HFs. We then demonstrated that the lower threshold is elevated in these women by inducing shivering while measuring Tc [43]. We then measured the upper and lower thresholds using ambient heating and cooling in women with and without HFs. We measured the thermoneutral zone to be 0.0°C in the symptomatic women and 0.4°C in the asymptomatic women [44]. We then replicated the Tc sweating threshold findings using exercise. When sweating thresholds were reached, all symptomatic but no asymptomatic women demonstrated objective and subjective HFs. Sweat rates in the former group were twice those of the later group.
Thus, we believe that hot flashes are triggered by Tc elevations acting within a greatly narrowed thermoneutral zone in postmenopausal women with HFs. A HF, consisting of sweating and peripheral vasodilation, is provoked when Tc reaches the upper threshold. Tc then declines, and when the lower threshold is crossed, shivering occurs. What biochemical mechanisms account for this?
Basic science investigations have found that increased brain norepinephrine (NE) narrows the width of the thermoneutral zone [31]. Conversely, clonidine lowers NE release, raises the sweating threshold, and reduces the shivering threshold. We therefore hypothesize that increased brain NE narrows the thermoneutral zone in menopausal women with HFs.
We determined the Tc sweating threshold in women with and without HFs during I.V. clonidine and placebo [45]. We showed that clonidine significantly elevated the sweating threshold compared to placebo in the women with HFs, whereas, the opposite occurred in the women without HFs. We therefore believe that clonidine reduces HFs by raising the Tc sweating threshold.
We then conducted a similar study to examine the mechanism through which estrogen ameliorates HFs [46]. Symptomatic menopausal women were randomly assigned to receive 1 mg/day 17 β-estradiol P.O. or placebo for 90 days. We found that the Tc sweating threshold was significantly elevated and HF frequency significantly ameliorated in the E2 but not the placebo group. Thus, estrogen ameliorates HFs by raising the Tc sweating threshold, but we do not know the precise mechanisms of this.
5. Circadian rhythm of hot flashes
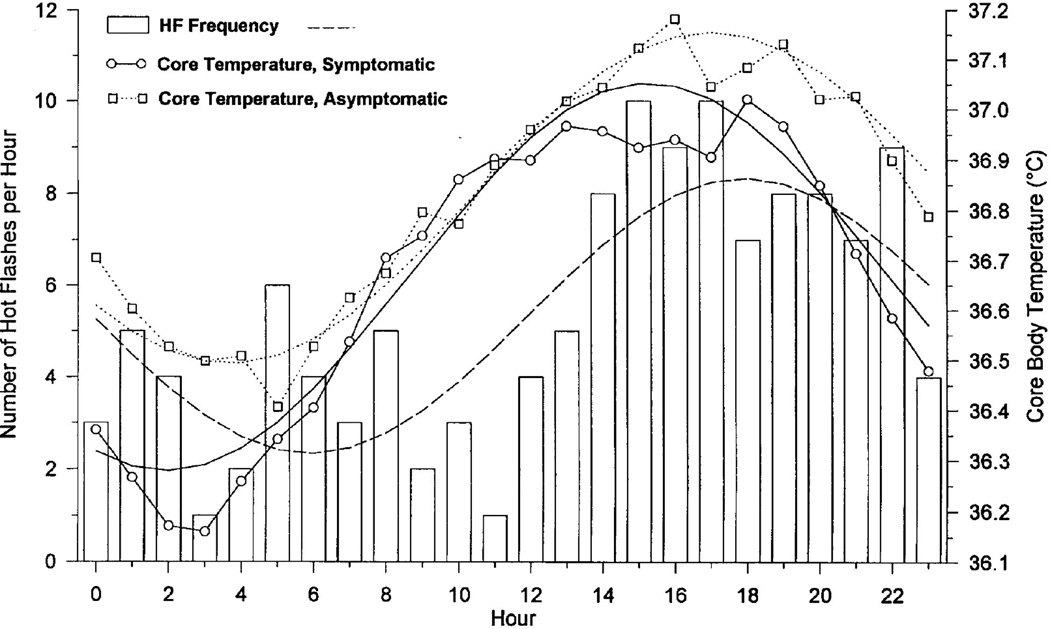
Given this mechanism, we sought to determine if HF occurrence was related to the Tc circadian rhythm. Using 24h ambulatory monitoring, we recorded sternal SCL to detect HFs, ambient temperature, skin temperature, and Tc (ingested radiotelemetry pill [9]. Cosinor analysis revealed an HF circadian rhythm with a peak at 1825h (Fig. 3). The majority of HFs were preceded by Tc elevations (P<.05). HFs began at significantly higher Tc levels (36.82 ± 0.04°C) compared with all nonflash periods (36.70 ± 0.005°C). We then replicated these findings in symptomatic women with breast cancer using a whole-room calorimeter [47].
Fig. 3.
Hot flash frequency and Tc during 24h. Hot flash frequency in 10 symptomatic women (bars); best-fit cosine curve for hot flash frequency (dashed line); 24h Tc data for 10 symptomatic women (○) with best fit cosine curve (solid line); 24h Tc data in 6 asymptomatic women (□) with best-fit cosine curve (dotted line).
6. Imaging studies
We were interested to determine the brain areas associated with the physiologic and phenomenological aspects of the HF and employed functional magnetic resonance imaging (fMRI) to do this. In the first study, we used symptomatic menopausal women and asymptomatic amenorrheic women and induced HFs and sweating (measured with sternal SCL) in the scanner [48]. Significant areas of activation in the symptomatic women included the insular and the anterior cingulate cortex. Sweating in the amenorrheic women was associated with activation in the anterior cungilate and superior frontal gyrus. We believe the insular activation is associated with the “rush of heat” described during menopausal HFs.
In a second investigation [49], we sought to determine the temporal sequencing of the neuronal events underlying the HF. Methods were similar to those described above. We performed fMRI in a group of postmenopausal women to measure neuronal activity in the brainstem, insular and prefrontal cortex around the onset of an HF (detected using synchronously acquired skin conductance responses). Rise in brainstem activity occurred before the detectable onset of an HF. Insular and prefrontal activity trailed activity in the brainstem, appearing following HF onset. Pre-HF brainstem responses may reflect the functional origins of internal thermoregulatory events such as HFs. By comparison, insular and prefrontal activity may be associated with the phenomenological correlates of HFs.
7. Hot flashes (HFs) and sleep
Although most epidemiologic studies have found increased reports of sleep disturbance at menopause [50], this has not been found in most laboratory studies [51]. A study in our laboratory [52] found no differences among age-matched premenopausal women, postmenopausal symptomatic women, and postmenopausal asymptomatic women on any sleep measure, performance test, or questionnaire measure. Additionally, hot flashes did not appear to trigger awakenings or arousals based on analysis of whole-night data.
A subsequent study analyzed this last issue in greater depth by analyzing data by halves of the night [53]. This was done because there is more rapid eye movement (REM) sleep in the second half of the night. It has been shown that REM sleep suppresses thermoregulatory effector responses, such as sweating and peripheral vasodilation, which constitute HFs. Indeed, it was found that HFs in the second half of the night occurred after the awakenings and arousals, whereas, those in the first half of the night preceded them and could, therefore, trigger them.
This temporal relationship was replicated in a recent laboratory study of 102 women, 44–56 years of age, who complained of poor sleep [54]. Fifty-three percent of the women had apnea, restless legs, or both. The best predictors of objective sleep quality (laboratory sleep efficiency) were apneas, periodic limb movements, and arousals (R2 = 0.44, P <0.0001). The best predictors of subjective sleep quality (Pittsburgh Sleep Quality Index global score) were the Hamilton anxiety score and the number of hot flashes in the first half of the night (R2 = 0.19, P <0.001). It is, therefore, possible that anxiety mediates some reports of poor sleep.
These results may explain the difference between our first laboratory study which did not analyze data by halves of the night [51], and self-report studies of increased sleep disturbance at menopause [50]. Our findings also emphasize the importance of detecting primary sleep disorders, such as apnea and periodic limb movements which are highly disruptive of sleep and can have serious medical consequences.
8. Treatment
a. Hormone therapy (HT)
Hormone therapy is clearly the most effective treatment for HFs and is the only FDA-approved indicator for this symptom. However, many women are presently unwilling to take HT due to concerns about risks. Estrogens and progestogens are discussed in detail elsewhere in this issue.
b. Nonhormonal Treatments
The risks of HT have prompted research on nonhormonal treatments.
8.2.1 Behavioral treatment for hot flashes
Because elevated sympathetic activation is involved in the etiology of HFs, relaxation-based procedures have been used to treat them. In one investigation [55], postmenopausal women with frequent HFs were randomly assigned to receive six weekly sessions of progressive muscle relaxation and slow, deep breathing (paced respiration) or α-wave electroencephalographic (EEG) biofeedback (placebo control procedure). The relaxation procedure significantly reduced both objective symptoms recorded in the laboratory and diary-recorded HF frequency by about 50% compared with the control procedure. A second study was performed in which a group of subjects received slow deep breathing alone, a second group received muscle relaxation exercise alone, and a third group received α-wave EEG biofeedback [55]. Treatment outcome was assessed by ambulatory monitoring of sternal skin conductance responses, which were used to define HFs. Only the paced respiration group showed a significant decline (50%) in HF frequency. There were no significant changes shown in the two other groups.
In the next study, 24 symptomatic postmenopausal women were randomly assigned to receive paced-respiration (n=12) or α-wave EEG biofeedback (n=12), the control condition [56]. Treatment outcome was again assessed by ambulatory monitoring of sternal skin conductance. The paced respiration group showed a significant decline in HF frequency (again about 50%) compared with no change in the control group. The last controlled study [56] randomly assigned symptomatic postmenopausal women to receive relaxation response training (paced respiration plus mental focusing), a reading control group, or no treatment. The relaxation response group showed a significant reduction in HF intensity, but not frequency. There was no significant change in the other groups. Thus, we conclude that paced respiration training produces a significant decline in HF frequency and, perhaps, intensity. There are no known harmful effects.
8.2.2 Clonidine
As noted earlier, clonidine ameliorates HFs by widening the thermoneutral zone. Two small placebo-controlled studies found that clonidine P.O. reduced HF frequency by 46% and transdermal clonidine reduced it by 80% [58]. Two studies of breast cancer survivors receiving tamoxifen showed smaller, but significant reductions in HF frequency for oral [59] and transdermal clonidine [60] compared with placebo. Side effects of clonidine include hypotension, dry mouth, and sedation [61].
8.2.3 Serotonergic agents
Work described above has implicated elevated brain NE in the reduction of the thermoneutral zone in symptomatic postmenopausal women. Basic science investigations have shown that NE and serotonin (5-HT) tend to work in opposite fashion [31]. These observations suggested that compounds that increase the availability of 5-HT in brain might ameliorate HFs.
Several studies have found effectiveness for some antidepressants in the treatment of HFs. Paroxetine, an SSRI, decreased HF composite scores by 62% (12.5 mg/day) and 65% (25.0 mg/day) in 165 women reporting 2–3 HFs/day [62]. The placebo response rate was 37.8%. Fluoxetine is another selective 5HT reuptake inhibitor used to treat HFs. A study of women with breast cancer found a reduction in HF frequency of about 20% compared to placebo [63]. Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, has also shown some positive results in the treatment of HFs. In a study of 229 women, this drug reduced HF scores by 60% from baseline at 75 and 150 mg/day and 37% at 37.5 mg/day compared with 27% for placebo [64]. Side effects of these antidepressants include insomnia, sleepiness, and dry mouth.
A recent study of 205 women given 10–20 mg/day of escitalopram found small but significant reductions in reported HF frequency and severity [65]. However, another recent study using an objective outcome measure (ambulatory HF recording) found no such effects using the same doses [66]. A similar investigation, using 5-HT, the immediate precursor of 5-HT, also found no significant therapeutic effects [67]. Given the mechanisms described above, a reduction in brain 5-HT should worsen HFs. However, a recent study performed this using acute tryptophan depletion and objective HF recordings. There were no significant effects whatsoever upon HFs. Taken together, these studies raise serious questions regarding the role of 5-HT in HFs.
8.2.4 Isoflavones and botanical compounds
Isoflavones or phytoestrogens have estrogenic properties and are found in soy products and red clover. Black cohosh is another plant-derived compound used to treat hot flashes. A recent controlled trial by Newton et al. found no significant effects, whatsoever, for black cohosh [65]. A recent review of 22 clinical trials, 12 on soy and 10 on other botanicals, showed no consistent improvement of HFs relative to placebo [66].
8.2.5 Gabapentin
Gabapentin is an anticonvulsant of unknown mechanism, which was accidentally found to reduce HFs in some women. A recent review found that gabapentin reduced HF frequency by 45–71% in four clinical trials [68]. The most common adverse effects were somnolence, ataxia, and dizziness.
Acknowledgement
This work was supported by NIH Merit Award, R37-AG05233 and by R01-MH63089.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Dr. Freedman is the President and CEO of Biomedical Monitors, LLC, the manufacturer of the hot flash recorder described herein.
References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann. N Y Acad. Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BM, Voda A, Groseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res. Nurs. Health. 1985;8:261–268. doi: 10.1002/nur.4770080308. [DOI] [PubMed] [Google Scholar]
- 3.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am. J. Pub. Health. 2006;96:1–10. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flushes. Fertil. Steril. 1998;70:1–6. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 5.Molnar GW. Body temperature during menopausal hot flashes. J. Appl. Physiol.: Respir. Environ. Exercise Physiol. 1975;38:499–503. doi: 10.1152/jappl.1975.38.3.499. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg F, Cote LJ, Linkie DM, Dyrenfurth I, Downey JA. Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas. 1984;6:31–43. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 7.Tataryn IV, Lomax P, Bajorek JG, Chesarek W, Meldrum DR, Judd HL. Postmenopausal hot flushes: a disorder of thermoregulation. Maturitas. 1980;2:101–107. doi: 10.1016/0378-5122(80)90043-2. [DOI] [PubMed] [Google Scholar]
- 8.Ginsburg J, Swinhoe J, O’Reilly B. Cardiovascular responses during the menopausal hot flush. Br. J. Obstet. Gynaecol. 1981;88:925–930. doi: 10.1111/j.1471-0528.1981.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J. Clin. Endocrinol. Metab. 1995;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 10.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil. Steril. 1996;65:1141–1144. [PubMed] [Google Scholar]
- 11.Takarangi MK, Garry M, Loftus EF. Dear diary, is plastic better than paper? I can’t remember. Comment on Green, Bolger, Shrout, and Reis. Psychol. Methods. 2006;11:119–122. doi: 10.1037/1082-989X.11.1.119. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR. Objective or subjective measurement of hot flashes in clinical trials: Quo vadis. Maturitas. 2010;67:99–100. doi: 10.1016/j.maturitas.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 15.Freedman RR, Woodward S, Norton D. Laboratory and ambulatory monitoring of menopausal hot flushes: comparison of symptomatic and asymptomatic women. J. Psychophysiol. 1992;6:162–166. [Google Scholar]
- 16.de Bakker IPM, Everaerd W. Measurement of menopausal hot flushes: validation and cross-validation. Maturitas. 1996;24:87–98. doi: 10.1016/0378-5122(96)01046-8. [DOI] [PubMed] [Google Scholar]
- 17.Hanisch LJ, Palmer SC, Donahue A, Coyne JC. Validation of sternal skin conductance for detection of hot flashes in prostate cancer survivors. Psychophysiol. 2007;44:189–193. doi: 10.1111/j.1469-8986.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RR, Wasson S. Miniature, hygrometric hot flash recorder. Fertil. Steril. 2007;88:494–496. doi: 10.1016/j.fertnstert.2006.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RR. Patient satisfaction with miniature, ambulatory, postmenopausal hot flash recorder. The Open Medical Devices Journal. 2009;1:1–2. [Google Scholar]
- 20.Askel S, Schomberg DW, Tyrey L, Hammond CB. Vasomotor symptoms, serum estrogens, gonadotropin levels in surgical menopause. Am. J. Obstet. Gynecol. 1976;126:165–169. doi: 10.1016/0002-9378(76)90270-2. [DOI] [PubMed] [Google Scholar]
- 21.Schindler AE, Muller D, Keller E, Goser R, Runkel F. Studies with clonidine (Dixarit) in menopausal women. Arch. Gynecol. 1979;227:341–347. doi: 10.1007/BF02109923. [DOI] [PubMed] [Google Scholar]
- 22.Casper RF, Yen SSC, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science. 1979;204:823–825. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]
- 23.Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. LH, FSH, and skin temperature during menopausal hot flush. J. Clin. Endocrinol. Metab. 1979;49:152–154. doi: 10.1210/jcem-49-1-152. [DOI] [PubMed] [Google Scholar]
- 24.Gambone J, Meldrum DR, Laufer L, Chang RJ, Lu JKH, Judd HL. Further delineation of hypothalamic dysfunction responsible for menopausal hot flashes. J. Clin. Endocrinol. Metab. 1984;59:1097–1102. doi: 10.1210/jcem-59-6-1097. [DOI] [PubMed] [Google Scholar]
- 25.Casper RF, Yen SSC. Menopausal flushes: effect of pituitary gonadotropin desensitization by a potent luteinizing hormone releasing factor agonist. J. Clin. Endocrinol. Metab. 1981;53:1056–1058. doi: 10.1210/jcem-53-5-1056. [DOI] [PubMed] [Google Scholar]
- 26.DeFazio J, Meldrum DR, Laufer L, et al. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J. Clin. Endocrinol. Metab. 1983;56:445–448. doi: 10.1210/jcem-56-3-445. [DOI] [PubMed] [Google Scholar]
- 27.Meldrum DR, Erlik Y, Lu JKH, Judd HL. Objectively recorded hot flushes in patients with pituitary insufficiency. J. Clin. Endocrinol. Metab. 1981;52:684–687. doi: 10.1210/jcem-52-4-684. [DOI] [PubMed] [Google Scholar]
- 28.Mulley G, Mitchell RA, Tattersall RB. Hot flushes after hypophysectomy. BMJ. 1977;2:1062. doi: 10.1136/bmj.2.6094.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffcoate SL. Climacteric flushing: clinical and endocrine response to infusion of naloxone. Br. J. Obstet. Gynaecol. 1981;88:919–924. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 30.DeFazio J, Vorheugen C, Chetkowski R, Nass T, Judd HL, Meldrum DR. The effects of naloxone on hot flashes and gonadotropin secretion in postmenopausal women. J. Clin. Endocrinol. Metab. 1984;58:578–581. doi: 10.1210/jcem-58-3-578. [DOI] [PubMed] [Google Scholar]
- 31.Brück K, Zeisberger E. Adaptive changes in thermoregulation and their neuropharmacological basis. In: Schönbaum E, Lomax P, editors. Thermoregulation: Physiology and Biochemistry. New York: Pergamon; 1990. pp. 255–307. [Google Scholar]
- 32.Insel PA, Motulsky HJ. Physiologic and pharmacologic regulation of adrenergic receptors. In: Insel PA, editor. Adrenergic Receptors in Man. New York: Marcel Dekker; 1987. pp. 201–236. [Google Scholar]
- 33.Kopin IJ, Blombery P, Ebert MH, et al. Disposition and metabolism of MHPG-CD3 in humans: plasma MHPG as the principal pathway of norepinephrine metabolism and as an important determinant in CSF levels of MHPG. In: Usdin E, et al., editors. Frontiers in Biochemical and Pharmacological Research in Depression. New York: Raven Press; 1984. pp. 57–68. [PubMed] [Google Scholar]
- 34.Freedman RR, Woodward S, Sabharwal SC. Adrenergic mechanism in menopausal hot flushes. Obstet. Gynecol. 1990;76:573–578. [PubMed] [Google Scholar]
- 35.Goldberg M, Robertson D. Yohimbine: a pharmacological probe for study of α2-adrenoceptor. Pharmacol. Rev. 1983;35:143–180. [PubMed] [Google Scholar]
- 36.Sastre M, Garcia-Sevilla JA. Density of alpha-2A adrenoceptors and Gi proteins in the human brain: ratio of high-affinity agonist sites to antagonist sites and effect of age. J. Pharmacol. Exp. Ther. 1994;269:1062–1072. [PubMed] [Google Scholar]
- 37.Starke K, Gothert M, Kilbringer H. Modulation of neurotransmitter release of presynaptic autoreceptors. Physiol. Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 38.Charney DS, Heninger GR, Sternberg DE. Assessment of α2-adrenergic autoreceptor function in humans: effects of oral yohimbine. Life Sci. 1982;30:2033–2041. doi: 10.1016/0024-3205(82)90444-1. [DOI] [PubMed] [Google Scholar]
- 39.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm. Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- 40.Ansonoff MA, Etgen AM. Receptor phosphorylation mediates estradiol reduction of alpha 2-adrenoceptor coupling to G protein in the hypothalamus of female rats. Endocrine. 2001;14:165–174. doi: 10.1385/ENDO:14:2:165. [DOI] [PubMed] [Google Scholar]
- 41.Savage MV, Brengelmann GL. Control of skin blood flow in the neutral zone of human body temperature regulation. J. Appl. Physiol. 1996;80:1249–1257. doi: 10.1152/jappl.1996.80.4.1249. [DOI] [PubMed] [Google Scholar]
- 42.Kronenberg F, Barnard RM. Modulation of menopausal hot flashes by ambient temperature. J. Therm. Biol. 1992;17:43–49. [Google Scholar]
- 43.Freedman RR, Woodward S. Altered shivering threshold in postmenopausal women with hot flashes. Menopause. 1995;2:163–168. [Google Scholar]
- 44.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am. J. Obstet. Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 45.Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not in asymptomatic postmenopausal women. Fertil. Steril. 2000;74:20–23. doi: 10.1016/s0015-0282(00)00563-x. [DOI] [PubMed] [Google Scholar]
- 46.Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil. Steril. 2002;77:487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004;11:375–381. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 48.Freedman RR, Benton MD, Genik RJ, II, Graydon FX. Cortical activation during menopausal hot flashes. Fertil. Steril. 2006;85:674–678. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, Gompel A, Hickey M, Hunter MS, Lobo RA, Lunsden MA, MacLennan AH, Maki P, Palacios S, Shah D, Villaseca P, Warren M. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14:515–528. doi: 10.3109/13697137.2011.608596. [DOI] [PubMed] [Google Scholar]
- 50.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 51.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 52.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil. Steril. 2004;82:138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576–583. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- 54.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 55.Freedman RR. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am. J. Obstet. Gynecol. 1992;167:436–439. doi: 10.1016/s0002-9378(11)91425-2. [DOI] [PubMed] [Google Scholar]
- 56.Irvin JH, Domar AD, Clark C, Zuttermeister PC, Friedman R. The effects of relaxation response training on menopausal symptoms. J. Psycho. Obstet. Gynecol. 1996;17:202–207. doi: 10.3109/01674829609025684. [DOI] [PubMed] [Google Scholar]
- 57.Nagamani M, Kelver ME, Smith ER. Treatment of menopausal hot flashes with transdermal administration of clonidine. Am. J. Obstet. Gynecol. 1987;156:561–565. doi: 10.1016/0002-9378(87)90050-0. [DOI] [PubMed] [Google Scholar]
- 58.Pandya KJ, Raubertas RF, Flynn PJ, Hynes HE, Rosenbluth RJ, Kirshner JJ, Pierce HI, Dragalin V, Morrow GR. Oral clonidine in post-menopausal patients with breast cancer experiencing tamoxifen induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann. Intern. Med. 2000;132:788–793. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg RM, Loprinzi CL, O’Fallon JR, Veeder MH, Miser AWMH, Milliard JA, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J. Clin. Oncol. 1994;12:155–158. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 60.Laufer LR, Erlik Y, Meldrum DR, Judd HL. Effects of clonidine on hot flushes in postmenopausal women. Obstet. Gynecol. 1982;60:583–589. [PubMed] [Google Scholar]
- 61.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827–2834. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 62.Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA. Phase III evaluation of fluoxetine for treatment of hot flashes. J. Clin. Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 63.Loprinzi CL, Kugler JW, Sloan JA, Loprinzi C, Kugler J, Sloan J, Milliard J, La Vasseur B, Barton D, Novotny P, Dakhil S, Rodger K, Rummans T. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomized controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 64.Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, Carpenter JS, Anderson GL, Larson JC, Ensrud KE, Reed SD, Newton KM, Sherman S, Sammel MD, LaCroix AZ. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freedman RR, Kruger ML, Tancer ME. Escitalopram treatment of menopausal hot flashes. Menopause. 2011;18:893–896. doi: 10.1097/gme.0b013e31820ccae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freedman RR. Treatment of menopausal hot flashes with 5-hydroxytryptophan. Maturitas. 2010;65:383–385. doi: 10.1016/j.maturitas.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayes LP, Carroll DG, Kelley KW. Use of gabapentin for the management of natural or surgical menopausal hot flashes. Ann. Pharmacother. 2011;45:388–394. doi: 10.1345/aph.1P366. [DOI] [PubMed] [Google Scholar]