Abstract
Objectives
Transdermal alcohol monitoring is used extensively in forensic settings to identify whether individuals have violated court-ordered mandates to abstain from drinking. Despite widespread use in that setting, comparatively few studies have explored the clinical utility of transdermal alcohol monitoring. Furthermore, of the few studies conducted, most have relied on the forensically established conservative criteria to identify whether or not a drinking episode has occurred. Here, we explore how transdermal alcohol monitoring data can be used to estimate more clinically meaningful parameters relevant to clinical treatment programs.
Methods
We developed a procedure to use transdermal data to objectively estimate the number of standardized drinks an individual has consumed. Participants included 46 men and women who consumed 1 to 5 beers within 2 hours in the laboratory on separate days while wearing devices to monitor transdermal alcohol concentrations (TAC).
Results
A mathematical model was derived to estimate the number of standardized alcohol drinks consumed, which included a number of variables (time-to-peak TAC, area under the TAC curve, and sex). The model was then validated by applying it to data from a separate study. Our results indicate that transdermal alcohol devices can be used to estimate the number of standard drinks consumed.
Conclusions
Objective methods characterizing both the level of intoxication achieved and the number of drinks consumed, such as transdermal alcohol monitoring, could be useful in both research and treatment settings.
Keywords: transdermal alcohol monitoring, breath alcohol concentration, standard alcohol drinks, alcohol treatment
Transdermal alcohol monitors are often used in forensic settings to detect alcohol use among offenders1, yet their clinical utility has not been fully explored. These devices are secured on offenders’ ankles and detect levels of alcohol excreted through skin; removal or tampering is prevented by sensors that monitor temperature and skin reflectivity2. In 2012, over 200,000 individuals in 49 states were wearing these devices3. In forensic circumstances, transdermal alcohol monitors are used to provide a dichotomous pass/fail metric of whether or not drinking has occurred. Furthermore, conservative criteria are used in such settings to assure the stringent legal requirement for a high degree of specificity of findings. Thus, only heavy drinking may be recognized under these conservative criteria. While the use of conservative criteria understandably gives the person wearing the monitor the benefit of the doubt3,4, these criteria may not be as useful in research-oriented or therapeutic settings, where quantifying the amount of drinking is relatively more important.
Two clinical studies have demonstrated the potential utility of transdermal alcohol monitoring to reduce problematic patterns of drinking5,6. These studies have relied on criteria that closely align with those used in forensic settings5–7. Few investigators have evaluated how transdermal alcohol monitoring data could be used to extract clinically useful information, such as quantifying the amount of drinking and what criteria should be used to identify such drinking.
We have conducted a series of studies to determine how data gathered through transdermal alcohol monitoring can be used to estimate meaningful variables for use in clinical treatment programs. Clinically meaningful variables include peak intoxication achieved and the number of standard units of alcohol consumed because these measures are used to define drinking patterns and behaviors. For example, heavy drinking is defined as ≥ 4 drinks for women and ≥ 5 drinks for men during a single day, or ≥ 7 drinks in a week for women and ≥ 14 drinks for men in a week8. Binge drinking is defined as an intermittent pattern of drinking at levels that produce a blood alcohol concentration of ≥ 0.08%; typically, this means 5 drinks for men and 4 drinks for women consumed within 2 hours8, 9.
Recently, we reported our development of methods to use transdermal alcohol concentration (TAC) data to estimate levels of peak intoxication (peak breath alcohol), which is a more clinically pertinent variable than either TAC values or the dichotomous indication of whether or not drinking is detected10,11. In fact, that model accurately predicted peak breath alcohol concentration (BrAC), accounting for 76% of the variance. In the current study, we demonstrate that TAC data also can be used to estimate another clinically relevant variable, the number of (standard) drinks consumed.
Methods
Participants and Inclusion Criteria
For this report, we included 11 male and 10 female participants from our most recent study11 and 10 male and 11 female participants from an earlier study10. All were 21 to 47 years of age; reported consuming alcohol on one to four days per week; and were recruited through newspaper, radio, and television advertisements. Those responding to community advertisements underwent an initial phone screen to answer questions about their health and drinking behavior to determine eligibility. Respondents who met study criteria were invited to the clinic for written informed consent and detailed study screening to confirm eligibility. Eligibility screening included a detailed substance use history, assessment of alcohol consumption within the prior month, a psychiatric screening using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders: Research Version, Non-Patient Edition (SCID-I/NP)12, urinalysis, medical history, and a physical examination. We excluded respondents with a current or past Axis I psychiatric diagnosis, a medical condition that would be exacerbated by alcohol, or who were pregnant or had a history of substance dependence. Respondents also were excluded if they screened positive for drugs of abuse (cocaine, opiates, methamphetamines, barbiturates, benzodiazepines, and THC) or if, during the past month, they did not report drinking at least 5 standard drinks for men or 4 for women within a 2-hour period. The Institutional Review Board at The University of Texas Health Science Center at San Antonio reviewed and approved the experimental protocol. Participants received $65-$70 per day for their participation.
Procedures
Study design
Participants were instructed to fast after midnight each day and not to drink alcohol outside the laboratory until study completion. Upon arrival at the laboratory (7:30 a.m.), participants provided a urine sample for drug and pregnancy testing and alcohol-free breath samples. All participants were fitted with a Secure Continuous Remote Alcohol Monitor (SCRAM-II; Alcohol Monitoring Systems, Inc., Denver, CO) ankle bracelet before alcohol administration. They then consumed an alcohol dose designated for that testing day, and both TAC and BrAC were monitored repeatedly throughout the day. BrAC was measured using Dräger Alcotest 6810 portable breathalyzers (Dräger Safety Diagnostics, Inc., Irving, TX). A meal was provided after BrAC levels reached 0.000 or 4:00 p.m. at the latest. Participants remained in the laboratory until their TAC readings fell to ≤ 0.005 g/dl, which was reached within 3 hours after BrAC fell to 0.0% (usually by 7 p.m. after the higher doses).
Alcohol administration
Twelve-ounce Corona beers (Grupo Modelo S.A.B. de C.V., Mexico City, Mexico; 4.6% alcohol by volume) were administered to participants by research staff. Each was the equivalent of 0.92 standard units of alcohol. Participants from both studies consumed one beer on the first day, and their intake increased by one beer on each subsequent day, ending with a maximum of 5 beers (or 4.6 total standard units). Drink administration varied between the two studies that our participants were drawn from. In the first study10, women drank up to 4 beers at the rate of one every 30 minutes, and men drank up to 5 beers at the rate of one every 24 minutes. In the second study11, men and women consumed the same amount of beer (up to 5) at the same rate (one every 24 minutes) so that sex-related differences in BrAC and TAC readings could be observed. Participants in both studies were required to complete each beer within 10 minutes.
Transdermal alcohol concentration monitoring
Each participant was fitted with a SCRAM-II device on his or her ankle. The SCRAM-II measured TAC every 30 minutes until the device was removed; results were downloaded daily. Infrared signals and temperature were also recorded to validate the readings and to ensure that no tampering or device disruption occurred. For current analyses, TAC data included peak TAC (the highest TAC value recorded during a drinking episode), time-to-peak TAC (the time in minutes from the last 0.0 g/dl TAC recording to the peak TAC recording in a drinking episode), and the area under the TAC curve (AUC).
Data Analysis
TAC data from 11 male and 10 female participants, given 1 to 5 beers to drink under controlled laboratory conditions in our most recent study11, were used to develop an equation to estimate the number of standard drinks of alcohol consumed. The independent predictive validity of the derived equation was demonstrated by applying it to data from 10 male and 11 female participants in an earlier study10.
Model derivation began by considering how the primary TAC level data, including the peak TAC observed and TAC area-under-the-curve (AUC) parameters, varied as a function of the number of standard drinks of alcohol consumed, and whether or not there were sex differences in these relationships. Utilizing statistical software from SAS Release 9.3 (SAS Institute, Inc., Cary, NC), fixed-factor, mixed-model ANOVAs were tested by the PROC MIXED program using between-within degrees of freedom with unstructured covariance matrixes and random intercept assumptions. Since the peak and AUC variables were highly correlated with each other (both Pearson’s and Spearman’s r) and their colinearity would preclude the use of both in an estimation model, we considered whether the TAC-AUC might better reflect total body-burden exposure to alcohol than the peak TAC alone. Since AUC had the highest correlation with standard units consumed and systematic consideration of the peak and AUC parameters alone or in combination showed that TAC-AUC was superior as a predictor of standard units, all further model development used the AUC parameter and not the peak TAC parameter.
Model development then considered adding the sex and time-to-peak parameters previously identified as predictors of peak BrAC11, followed by use of the 2 and 3 factor interactions of these parameters to estimate the number of standard drink units (eUnits). Systematic model building utilized SAS PROC REG software with the “Forward Step” option in a multiple regression process to consider first AUC alone, and then included the main effects of sex and time-to-peak. Because PROC REG found that all three parameters were significant and should be retained in the 3-factor model, we then explored the possible addition of the 2 and 3 factor interactions, again using the Forward Step process of PROC REG to empirically decide which factors to retain in the final model. At this stage we also considered the inclusion of a quadratic term for AUC because of observations of a non-linear trend in this parameter. Due to the mixed model aspects of our study design (i.e., sex is a between-subject comparison, but units consumed is a within-subject comparison), each stepwise model derived by PROC REG was evaluated in a fixed-factors ANOVA tested by PROC MIXED using between-within degrees of freedom, with unstructured covariance matrixes and random intercept. Akaike Information Criterion (AIC)13 and marginal R2 was calculated to summarize the amount of variance in the actual number of units consumed explained by the fixed factors in the mixed-effects model14.
Results
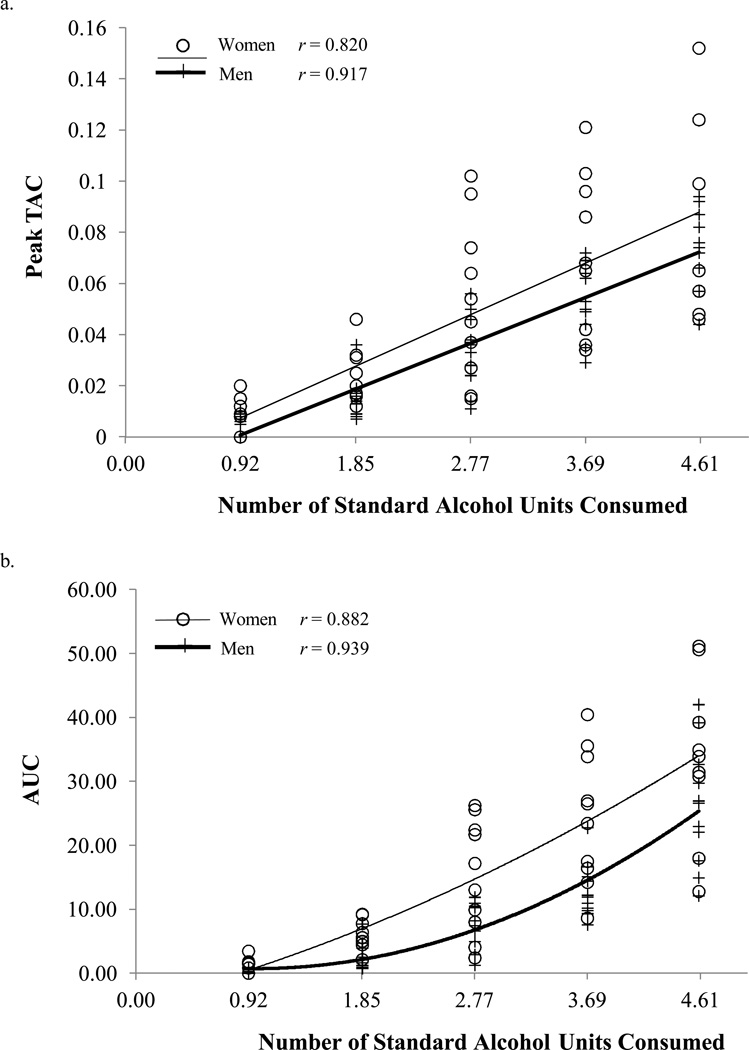
Male and female participants did not differ in any demographic variables or their alcohol use history, but there were sex-related differences in height, weight, and BMI10,11. TAC parameters of both peak TAC [F(4, 75) = 87.88, p < 0.0001] and AUC [F(4, 75) = 94.06, p < 0.0001] increase as a function of the standard units of alcohol consumed (Figure 1). For peak TAC (Figure 1a), the main effect of sex was not significant [F(1, 19) = 2.88, p = 0.11], and there was no significant interaction between sex and standard units [F(4, 4) = 1.21, p = 0.31]. However, AUC (Figure 1b) showed significant sex-related differences [F(1, 19) = 8.93, p = 0.008] and a sex-by-units interaction [F(4, 4) = 3.17, p = 0.02]. Specifically, women (M = 15.63, SD = 14.28) had higher AUC than men (M = 9.91, SD = 10.42). Post-hoc contrasts showed that women had significantly higher AUC than men at 1.85, 2.77, and 3.69 standard units (all p < 0.03), but not at the lowest (i.e., 0.92 standard units) or the highest (i.e., 4.6 standard units) level of drinking (all p > 0.15). This effect appeared as a significant quadratic trend [t(80) = −5.55, p < 0.0001] in the relationship of standard units to AUC.
Figure 1.
(a) Peak TAC and (b) area under the TAC curve for each standard unit of alcohol consumed among male and female participants (note that each beer was slightly less than one standard unit of alcohol). Each symbol (open circle and plus sign) is a single measure per participant.
Model Derivation to Estimate Standard Units
We began model derivation by comparing Pearson and Spearman inter-correlations of the peak, AUC, and time-to-peak TAC parameters with each other and with standard units consumed. All these correlations were significant, but peak TAC and AUC were the most highly linked (Pearson’s r = 0.95). Time-to-peak was less well correlated with the peak TAC and AUC parameters (r = 0.78 and r = 0.82, respectively), although it still was related to units consumed (r = 0.85). We then considered both AUC and peak-TAC in a Forward Step multiple regression analysis using PROC REG software. AUC was significant (p < 0.01), while the independent contribution of peak TAC was not (p > 0.05), so we elected to utilize AUC as the first step in model construction. The three essential steps of model construction are summarized in Table 1.
Table 1.
Parameter estimates in model formulations
| Model | Formulation | AIC | Marginal R2 |
|---|---|---|---|
| 1 | 1.6952 + 0.08861 * AUC | 229.5 | 0.66 |
| 2 | 0.3428 + 0.01043 * time-to-peak TAC + 0.04309 * AUC + 0.6347 * Sex | 197.1 | 0.77 |
| 3 | 0.6990 + 0.006317 * time-to-peak TAC + 0.09735 * AUC – 0.00097 * AUC2 + 0.08492 * AUC * Sex – 0.00223 * AUC2 * Sex | 194.0 | 0.82 |
Note. Beta-weighted parameter estimates found in Model Formulations tested by SAS PROC MIXED with Akaike Information Criterion (AIC) and R2 associated with each of three models.
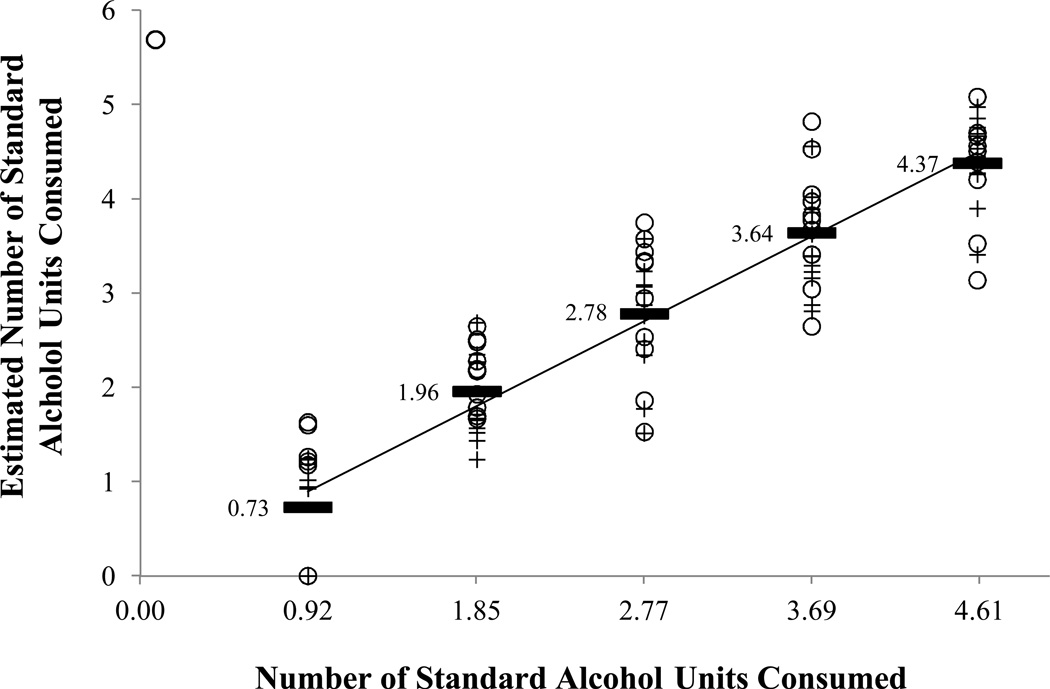
Model 1 shows the results of a simple model using AUC alone, which accounted for 66% of the variance in the number of standard units consumed. The next step was to include the main effects of sex (male = 1, female = 0) and time-to-peak in the analysis. The PROC REG Forward Step procedure found that time-to-peak was the most important factor for model inclusion, but that all three main effects were individually significant (all p < 0.006) and retained in the 3-factor model. Model #2 shows that the simple main effects model improved the AIC and accounts for 77% of the variance in estimating the number of standard units consumed. Though model simplicity is an important consideration, our findings of a non-linear trend in the AUC parameter and its interaction with sex forced us to consider adding both the AUC2 term and interactions with sex into the model. In consideration of a model including the three main effects, plus an AUC2 term, plus interactions of sex with the AUC and time-to-peak factors, the forward regression analysis retained the factors shown in Model #3. Each factor shown for Model #3 (Table 1) were individually significant contributors to a final model prediction of 82% of the variance in estimating the number of standard units consumed (all p < 0.03). The highly significant correlation between the estimated and actual number of standard units consumed is shown in Figure 2 (Spearman’s r = 0.92, p < 0.0001). No other combination of 2- or 3-factor interactions demonstrated any substantial improvement in the model.
Figure 2.
Associations between estimated and actual standard units of alcohol consumed. Each symbol (open circle; plus sign) is a single measure per participant. Mean estimated number of units are indicated by the solid bars.
Equation Validation
The proposed final model (Model #3 from Table 1) for estimating standard units of alcohol consumed was validated by using it to predict an independent set of data from our original report10. The equation accurately estimated the number of units of alcohol consumed, with an R2 = 0.80, thus validating its independent predictive validity in a different sample (data not shown).
Discussion
This study complements and extends our previous work using TAC data to estimate peak BrAC levels by developing a new mathematical model to accurately estimate the number of standard drinks consumed. The model that best estimated units of alcohol consumed included three parameters: time-to-peak TAC, AUC, and sex variables. We validated the model developed from data in one study11 to accurately predict the number of standard units of alcohol consumed in another independent study10. Taken together, these studies demonstrate that transdermal alcohol monitoring can continuously and non-invasively quantify both the peak BrAC achieved and the number of standard drinks consumed. These are important steps because they surpass the dichotomous criteria of pass/fail in forensic settings to provide quantitative data relevant for clinical settings. Such quantitative data are more in line with criteria defining problematic patterns of drinking8.
These outcomes are of substantial interest to clinicians and researchers alike. For example, TAC monitoring data can be beneficial in therapies that seek either reduction of drinking or abstinence but necessitate objective verification over self-report, such as with contingency management7. Alternatively, clinicians can utilize TAC monitoring data to give patients personalized feedback in commonly used terms (i.e. peak blood levels or numbers of drinks) regarding drinking behaviors.
Strengths and Limitations
Thus far, we have conducted a series of studies to demonstrate our ability to use TAC data to estimate clinically relevant parameters, including peak BrAC and total number of standard drink units consumed. Estimations were accurate under conditions where men and women drank: (a) 1 – 5 beers at rates that produced similar BrAC levels10, and (b) when men and women drank at the same pace, yielding sex-related differences in BrAC levels11.
The studies have limitations due to the controlled laboratory conditions under which they were done. Participants were limited to healthy individuals based on psychological history, pregnancy, medical conditions, drug use, history of substance dependence, and alcohol dependence. Thus, we cannot say whether these models will generalize to other populations. Moreover, participants only drank 5 drinks at the highest dose, so it is not known whether these estimations are still valid when larger amounts of alcohol are consumed. Finally, participants in these studies were required to drink alcohol at a specified pace, which is not representative of all cases of real-world drinking behavior. Varying paces of alcohol consumption could potentially affect TAC data variables (e.g., time-to-peak TAC, peak TAC, and area under the peak TAC curve). However, a recent study indicates that wide variations in the pace of drinking do not significantly affect key TAC parameters11. Despite these potential limitations, these studies clearly indicate that transdermal alcohol monitoring has the potential for utility in clinical studies of alcohol use.
Conclusion
Research utilizing transdermal alcohol monitors, heretofore primarily used in forensic settings, is becoming increasingly common in clinical research. Transdermal alcohol monitoring offers advantages in settings where self-reported drinking may be inaccurate or where drinkers may consume more than what is commonly reported15. Using the mathematical models described, TAC data can be used to accurately and objectively estimate standard units of alcohol consumed and peak BrAC10,11. The continued development of transdermal alcohol monitoring to better characterize drinking behavior will aid alcohol researchers and clinicians needing objective measurements of drinking in the "real world."
Acknowledgments
Source of Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (R01AA14988). Drs. Karns and Lake were supported by a postdoctoral training grant from the National Institute of Drug Abuse (T32DA031115). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Dougherty acknowledges support from a research endowment, the William and Marguerite Wurzbach Distinguished Professorship.
Footnotes
Conflicts of Interest
None of the authors has conflicting interests concerning this manuscript. All authors significantly contributed to this manuscript and have read and approved the final manuscript.
References
- 1.McKnight AS, Fell JC, Auld-Owens A. Transdermal alcohol monitoring: Case studies. (Report No. DOT HS 811 603) Washington DC: National Highway Traffic Safety Administration; 2012. [Google Scholar]
- 2.Robertson R, Vanlaar W, Simpson H. Continuous transdermal alcohol monitoring: A primer for criminal justice professionals. Ottawa, Ontario: Traffic Injury Research Foundation; 2007. [Google Scholar]
- 3.Alcohol Monitoring Systems, Inc. [Accessed May 1, 2014];Secure Continuous Remote Alcohol Monitor: SCRAM. [AMS Web site]. Available at: http://home.trafficresourcecenter.org/Impaired-Driving/~/media/Microsites/Files/traffic-safety/SCRAM%20II%20-%20General.ashx.
- 4.Marques PR, McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res. 2009;3:703–711. doi: 10.1111/j.1530-0277.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- 5.Barnett NP, Tidey J, Murphy JG, et al. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougherty DM, Hill-Kapturczak N, Liang Y, et al. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend. 2014 doi: 10.1016/j.drugalcdep.2014.06.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett NP, Meade EB, Glynn TR. Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Exp Clin Psychopharmacol. 2014;22:86–96. doi: 10.1037/a0034821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIAAA. [Accessed May 1, 2014];Moderate and binge drinking. 2014 [NIAAA Web site.] Available at: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 9.Weschler H, Nelson TF. Binge drinking and the American college student: What’s five drinks? Psychol Addict Behav. 2001;15:287–291. doi: 10.1037//0893-164x.15.4.287. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty DM, Charles NE, Acheson A, et al. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharmacol. 2012;20:373–381. doi: 10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill-Kapturczak N, Roache JD, Liang Y, et al. Accounting for sex-related differences in the estimation of breath alcohol levels using transdermal alcohol monitoring. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3644-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Givvon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 13.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected Papers of Hirotugu Akaike. New York, NY: Springer; 1988. pp. 199–213. [Google Scholar]
- 14.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 15.Dawson DA. Methodological issues in measuring alcohol use. Alcohol Res Health. 2003;27:18–29. [PMC free article] [PubMed] [Google Scholar]