Abstract
Background
Intestinal injury is a consequence of hemorrhagic shock and resuscitation. The intestinal mucosa has been shown to respond to ischemia/reperfusion injury with production of inflammatory mediators. Previous work in our laboratory indicates that intestinal epithelial cells secrete proinflammatory cytokines in the direction of both the lamina propria and intestinal lumen. The ability of the intestinal mucosa to transmit inflammatory signals into the gut lumen after hemorrhagic shock is unknown. We hypothesized that hemorrhagic shock results in secretion of proinflammatory cytokines into the gut lumen.
Methods
Male C57/Bl6 mice underwent femoral artery cannulation and hemorrhage to a systolic blood pressure of 20 mmHg for 1 h, then resuscitation with lactated Ringer’s (LR) solution. Sham animals were cannulated only. Mice were decannulated and sacrificed at intervals. Stool and succus were removed from intestinal segments, weighed, and placed into buffer solution. Specimens were analyzed via enzyme-linked immunosorbent assay (ELISA).
Results
Compared with sham-injured mice, hemorrhagic shock resulted in increased intestinal luminal cytokines. At 3 h after injury, elevated levels of IL-6 were found in the cecal stool. At 6 h after injury, TNFα, IL-6, and MIP-2 were significantly elevated in the cecal stool, and IL-6 and MIP-2 were significantly elevated in the distal colonic stool.
Conclusions
Hemorrhagic shock results in secretion of proinflammatory cytokines into the intestinal lumen. These findings suggest that the intestinal mucosa may transmit and receive signals in a paracrine fashion via the gut lumen.
Keywords: hemorrhagic shock, hemorrhage, luminal cytokines, stool cytokines, intestinal inflammation
INTRODUCTION
Hemorrhagic shock continues to be a leading cause of mortality, accounting for 30% to 40% of trauma related deaths [1]. Even after initial survival, patients suffering from hypotension due to hemorrhagic shock are at risk for acidosis, sepsis, and multi-organ failure later in their hospital course [2]. The intestinal mucosa is affected during hemorrhagic shock, resulting in significant local inflammation, enterocyte damage [3], breech of the gut barrier [4], and translocation of harmful mediators and pathogens from the lumen into the interstitial space [5]. It is now increasingly recognized that factors derived in the intestine serve to propagate systemic injury during shock and critical illness [6, 7].
In nontraumatic inflammatory conditions of the intestine, such as Crohn’s disease or ulcerative colitis, proinflammatory mediators have been found in the intestinal lumen. These cytokines and chemokines in the stool have been shown to correlate with disease activity, predicting relapse and disease severity. Initially, TNF-α was detected in stool of pediatric patients with active Crohn’s and ulcerative colitis [8]. Subsequently, multiple chemokines have been found in the intestinal epithelium and mucin vacuoles of goblet cells [9, 10]. Currently, other luminal markers such as fecal calprotectin are used in clinical practice to monitor patient disease [11–13].
In previous work using an in vitro model of gut inflammation, we have shown that differentiated, polarized intestinal epithelial cells are capable of secreting inflammatory mediators in the apical/luminal direction [14]. Specifically, when a monolayer of intestinal epithelial cells was treated on the apical surface with TNF-α, apical cytokine secretion resulted. When the cells were stimulated basolaterally, cytokines were produced in both the apical and basolateral directions. These data indicated that intestinal epithelial cells have the capability to produce and respond to proinflammatory stimuli from the luminal direction [14].
In the setting of trauma and hemorrhagic shock, the ability of the intestinal mucosa to secrete inflammatory cytokines into the intestinal lumen is currently unknown. We hypothesized that hemorrhagic shock induces secretion of cytokines into the intestinal lumen.
METHODS
Animals
Male C57/BL6 mice weighing 21–29 g were purchased from Charles River Laboratories, fed a standard laboratory diet and water ad libitum. Experiments were performed after acclimation for 2–3 wk in a climate-controlled room with a 12 h light-dark cycle, and were approved by the Institutional Animal Care and Use committees of the University of Cincinnati.
Hemorrhage Model
Hemorrhagic shock was induced in a similar fashion as described previously [15]. Briefly, mice were injected intraperitoneally with 0.1 mg/g body weight pentobarbital to induce anesthesia. After clipping and skin sterilization with povidone-iodine and alcohol solutions, the femoral vessels were exposed and cannulated with tapered polyethylene catheters. Pressure transducers were used to continuously monitor and record hemodynamic parameters (Harvard Apparatus, Holliston, MA). Hypothermia was avoided by placing the mice on a circulating water blanket maintained at 41°C. An equilibration period of 10 min was allowed, and then blood was withdrawn over 3 min until a systolic blood pressure (SBP) of 20 mmHg was achieved. Mice were maintained at a SBP of 20 mmHg ± 5 mmHg for 60 min by drawing or administering shed blood volume. For resuscitation, lactated Ringer’s (LR) solution was infused to a goal SBP of 80 mmHg over 5 min. Animals were monitored for 15 min to ensure adequate resuscitation, then decannulated and sacrificed at intervals. Sham animals were similarly cannulated and monitored for 90 min, but were not hemorrhaged or resuscitated. Five animals were used in the hemorrhagic shock and sham groups at each time point.
Specimen Preparation
Blood was collected via direct cardiac puncture at intervals after resuscitation, allowed to clot, centrifuged at 6800 g to separate the serum and cellular components, and stored at −80°C until analysis.
Intestinal tissue was separated into jejunum, ileum, cecum, and distal colon segments. Jejunum was defined as a 15 cm segment beginning at the ligament of Treitz, and ileum was defined as a 15 cm segment proximal to the ileocecal valve. Solid and liquid stool was removed from the cecum and distal colon and placed into 1 mL of extraction buffer (0.1M Tris-buffered saline with 0.3% BSA, 0.01% sodium azide, and 0.002% Tween). The jejunum and ileum were opened longitudinally and intestinal contents were absorbed with filter paper. Two pieces of 100 mm × 5 mm Whatman 42 (Whatman International Ltd., Maidstone, England) filter paper were used on each the jejunum and ileum. The filter paper was placed into 1 mL of extraction buffer. All stool specimens were gently agitated overnight at 4°C then centrifuged at 18,000 g for 15 min. Supernatants were transferred to clean containers and stored at −80°C until analysis.
After removing the luminal contents, intestinal tissue was snapfrozen in liquid nitrogen. Specimens were homogenized, and then sonicated for 10 s in 1 mL phosphate-buffered saline (PBS) containing complete protease inhibitor cocktail tablets (Roche, Indianapolis, IN) and 2 mM PMSF (Sigma, St. Louis, MO). Samples were centrifuged at 12,000 g at 4°C for 45 min. Supernatant density was determined using BCA Protein Assay Kit (Pierce Protein Research Products, Rockford, IL, USA). Samples were stored at −80°C until analysis.
Cytokine Analysis
Tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and macrophage inflammatory protein 2 (MIP-2) levels were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R and D Systems, Minneapolis, MN) per the manufacturer’s instructions. Serum values are presented as pg/mL. Stool values are presented as pg/mL in the jejunum and ileum specimens, and as pg/g stool in cecum and distal colon specimens, adjusting for the stool weight. Tissue values are presented as ng/g protein, as determined by BCA assay. Statistical analysis was performed by t-test between hemorrhage and sham groups using SigmaPlot 11 software (Systat, Chicago, IL) with P values <0.05 considered significant. Data are reported as mean ± standard error of the mean (SEM).
RESULTS
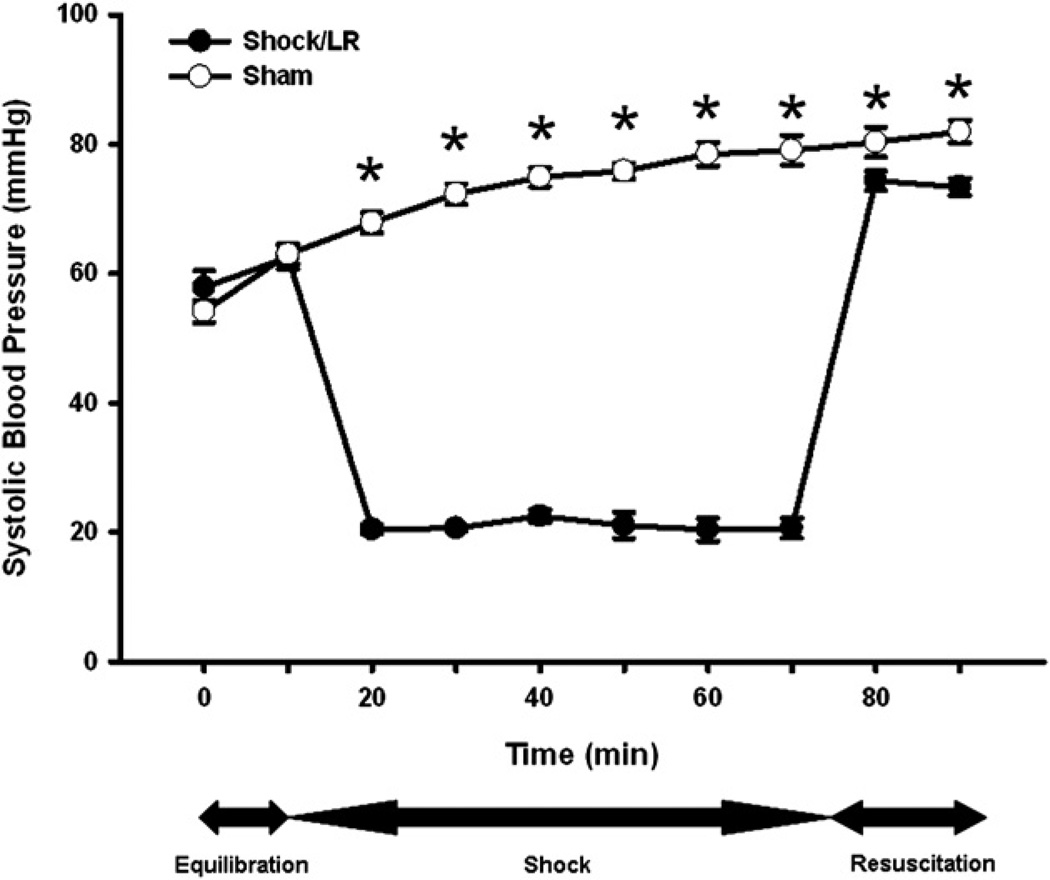
Blood Pressure and Fluid Volumes During Hemorrhagic Shock and Resuscitation
To test our hypothesis, we used a previously described model of murine pressure-clamp hemorrhagic shock [15]. During the equilibration phase, SBP was mildly decreased in all animals due to induction of anesthesia, but there was no difference in SBP between groups. The SBP of hemorrhaged mice was significantly lower than in sham injured animals during the 60 min period of hemorrhagic shock (Fig. 1). Hemorrhaged animals were resuscitated with LR to a SBP of 73.37 ± 1.33 mmHg. At the end of 90 minutes of monitoring, the SBP of sham injured animals increased to 81.93 ± 1.75 mm Hg. (Fig. 1)
FIG. 1.
Systolic blood pressure during hemorrhagic shock and resuscitation. Animals were subjected to hemorrhagic shock (closed symbols) or sham shock (open symbols). *P < 0.05 shock versus sham by t-test; n = 15 animals in each group.
In hemorrhaged mice, shed blood volumes and resuscitation volumes of LR were similar in all animals. There were no differences in shed blood volumes or resuscitation volumes between animals sacrificed at 1, 3, and 6 h after shock (Table 1). All animals survived to time of sacrifice.
TABLE 1.
Shed Blood and Resuscitation Volume in Hemorrhaged Mice
| 1 h | 3 h | 6 h | |
|---|---|---|---|
| Volume of shed blood (mL) | 0.500 ± 0.051 | 0.490 ± 0.037 | 0.510 ± 0.066 |
| Volume of lactated Ringers (mL) | 1.020 ± 0.037 | 1.040 ± 0.025 | 1.110 ± 0.056 |
Hemorrhaged mice were sacrificed at 1, 3, and 6 h after shock. Shed blood volumes and LR resuscitation volumes were equal in all groups; n = 5 for each group at each time point.
Stool Weights in Hemorrhaged and Sham Mice
Stool specimens from cecum and distal colon in all mice were weighed. Specimens from the lumen of the jejunum and ileum were liquid absorbed by the filter paper, and were not weighed. Animals sacrificed 1 h after hemorrhagic shock had significantly more stool in the distal colon than in sham injured animal (Table 2), but there was no difference in weight of cecal stool. There were no differences in stool weight in the cecum or distal colon in animals sacrificed 3 h after injury. Compared with sham injured animals, animals sacrificed 6 h after undergoing hemorrhagic shock had significantly higher stool weight in the cecum and distal colon (Table 2).
TABLE 2.
Stool Weights in Hemorrhaged Versus Sham Animals
| 1 h | 3 h | 6 h | ||||
|---|---|---|---|---|---|---|
| Sham | Shock | Sham | Shock | Sham | Shock | |
| Cecum | 0.734 ± 0.063 | 0.771 ± 0.025 | 0.739 ± 0.051 | 0.793 ± 0.039 | 0.641 ± 0.033 | 0.789 ± 0.045* |
| Distal colon | 0.486 ± 0.025 | 0.581 ± 0.027* | 0.478 ± 0.017 | 0.518 ± 0.019 | 0.454 ± 0.021 | 0.559 ± 0.026* |
Stool samples from each mouse were weighed prior to processing.
P < 0.05 by t-test comparing shock to sham at each time point in each organ; n = 5 for each group at each time point.
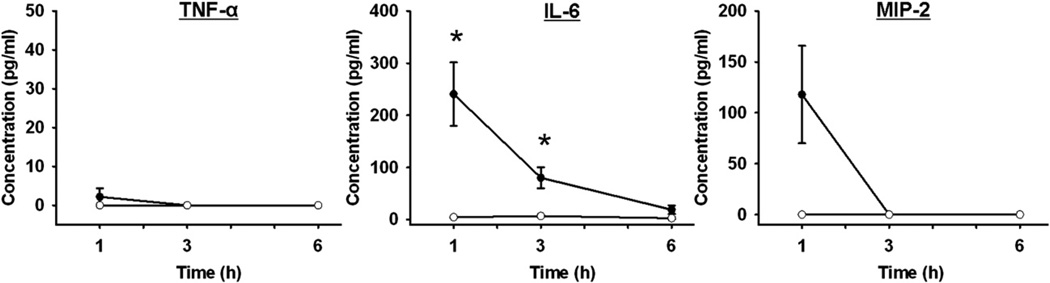
Serum Cytokine Levels After Hemorrhagic Shock
Serum samples taken at intervals after hemorrhagic shock (HS) were analyzed for TNF-α, IL-6, and MIP-2 levels (Fig. 2). There were no significant differences in serum TNF-α or MIP-2 levels after hemorrhagic shock. After 1 h of shock, MIP-2 levels were increased compared with sham animals, but the difference was not statistically different due to the variability in the hemorrhaged animals. At 1 and 3 h after shock, serum IL-6 levels were significantly increased compared with sham injured animals (Fig. 2).
FIG. 2.
Serum cytokine levels after hemorrhagic shock. TNF-α, IL-6, and MIP-2 levels in the serum at intervals after hemorrhage (closed symbols) or sham injury (open symbols). *P < 0.05 shock versus sham at each time point; n = 5 for each group at each time point.
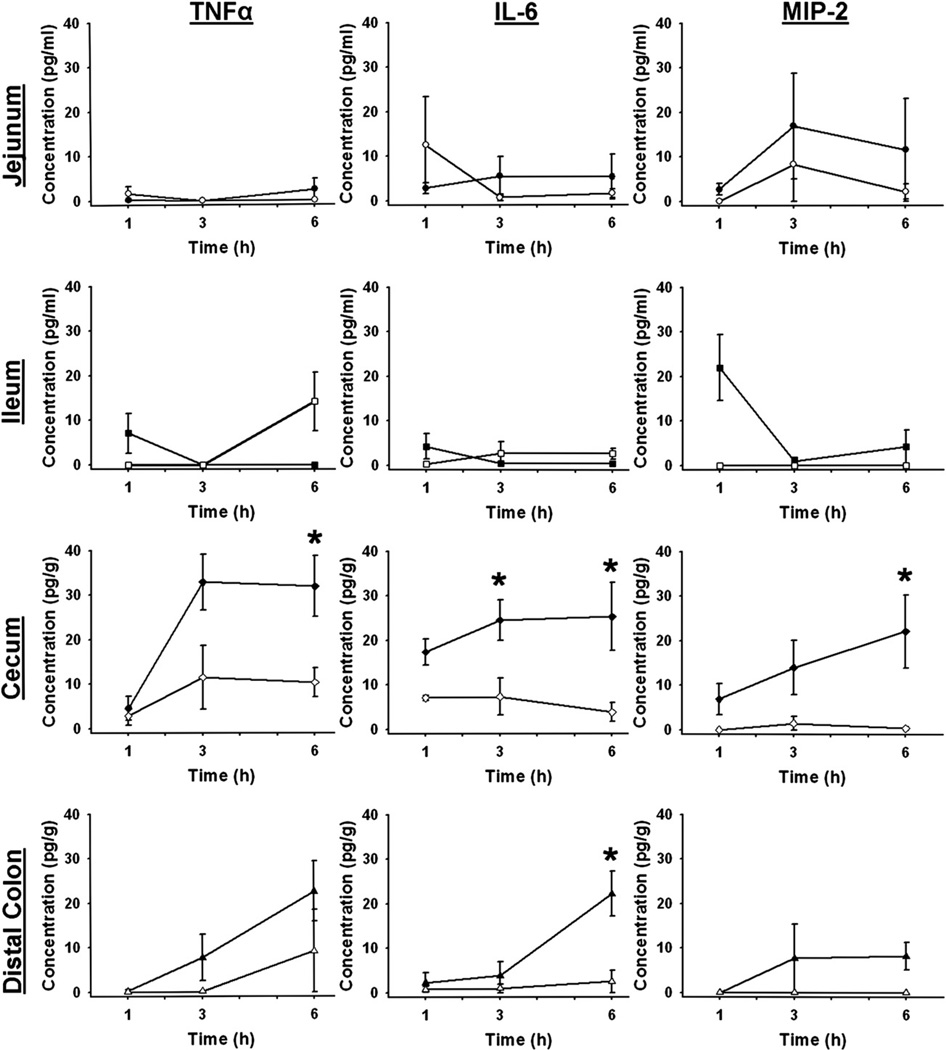
Stool Cytokine Levels after Hemorrhagic Shock
Luminal contents of the four intestinal segments were sampled at intervals and analyzed for TNF-α, IL-6, and MIP-2 levels. In the jejunum and ileum, there were no significant differences in luminal cytokines between sham and hemorrhaged animals at any time point (Fig. 3). Although luminal MIP-2 levels appeared to increase initially in the ileum, this did not reach statistical significance due to variability in the hemorrhaged animals. In stool recovered from the cecum, no differences were found at the 1 h time point in any cytokine. Three hours after resuscitation, stool IL-6 was significantly increased (Fig. 3). Six hours after hemorrhagic shock, significant increases were seen in all three cytokines (Fig. 3).
FIG. 3.
Stool cytokine levels after hemorrhagic shock. TNF-α, IL-6, and MIP-2 levels in luminal contents from each intestinal segment at intervals after hemorrhage (closed symbols) or sham injury (open symbols). *P < 0.05 shock versus sham at each time point; n = 5 for each group at each time point.
After hemorrhage and resuscitation, IL-6 was significantly increased in the stool recovered from the distal colon compared to specimens from sham animals (Fig. 3). There were no differences seen in TNF-α or MIP-2 levels (Fig. 3).
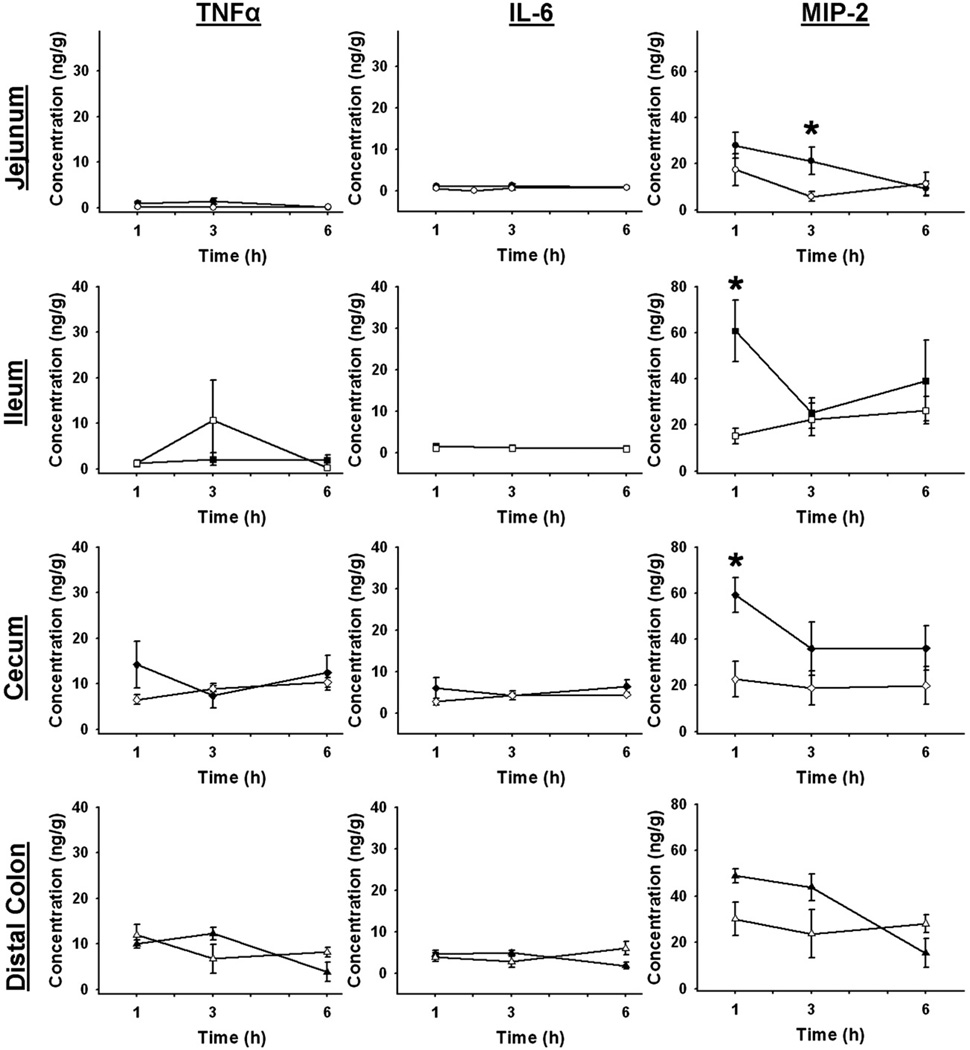
Intestinal Tissue Cytokine Levels after Hemorrhagic Shock
Segments of whole gut tissue were analyzed for TNF-α, IL-6, and MIP-2 by ELISA. In jejunal tissue, MIP-2 was significantly increased in injured mice compared witih sham (Fig. 4). No differences were found in jejunal tissue levels of TNF-α or IL-6 between sham and hemorrhaged animals at any time point. In the ileum, tissue levels of MIP-2 were increased 1 h after hemorrhagic shock (HS 60.724 ± 13.338 ng/g versus sham 15.262 ± 3.409 ng/g, P = 0.021), while no differences were seen in TNF-α or IL-6 (Fig. 4). In the cecum, tissue levels of MIP-2 were elevated at 1 h after shock (HS 59.180 ± 7.616 ng/g versus sham 22.603 ± 7.758 ng/g, P = 0.013). No differences were found between sham and hemorrhaged animals in the cecum levels of TNF-α and IL-6. No cytokine differences were found in the distal colonic tissue at any time point (Fig. 4).
FIG. 4.
Gut tissue cytokine levels after hemorrhagic shock. TNF-α, IL-6, and MIP-2 levels in the tissue of each intestinal segment at intervals after hemorrhage (closed symbols) or sham injury (open symbols). *P < 0.05 shock versus sham at each time point; n = 5 for each group at each time point.
DISCUSSION
In the present study we demonstrate the presence of inflammatory cytokines in the lumen of the intestine after hemorrhagic shock. Our data suggest that hemorrhagic shock and resuscitation results in an increase in intestinal luminal cytokine levels that is not easily attributed to serum or mucosal increases in these cytokines. This is consistent with previous work from our laboratory where we found evidence of directed vectorial secretion of IL-8 in response to TNF-α treatment in vitro in cultured intestinal epithelial cells [14]. The present experiment extends these findings into an in vivo model of hemorrhage and resuscitation. Taken together, these studies suggest that cytokine levels in the intestinal lumen may change during acute inflammation and that intestinal epithelial cells are the likely source of luminal cytokines.
In these experiments, analysis of fecal samples showed elevations in TNF-α, IL-6, and MIP-2 in mice undergoing hemorrhage and resuscitation mice. Increases in these three inflammatory mediators were found in the cecum at 6 h after shock. IL-6 was also elevated in the cecum at the 3 h interval and in the distal colon at 6 h after injury. Interestingly, no overlap was found between elevations in stool cytokines in these locations and time points and the corresponding intestinal tissue cytokine levels. In the intestinal tissue, only MIP-2 was elevated above control levels, at 1 h in the ileum and cecum and 3 h in the jejunum. In order to compare with stool and intestinal tissue levels, we measured serum levels of TNF-α, IL-6, and MIP-2. Consistent with this model of hemorrhagic shock, no significant elevation in TNF-α or MIP-2 was seen. Serum IL-6 was elevated at early time points and decreased to sham levels by 6 h after shock.
These findings suggest an overall increase in the inflammatory status of the intestinal luminal milieu. While the current study does not investigate the effect of intraluminal cytokines on the intestinal epithelium, mucosal leukocytes, or intestinal flora under these conditions, the systemic and intestinal functions of TNF-α, IL-6, and MIP-2 are well described, each having important relationships to the inflammatory cascade in intestinal tissue. TNF-α has been shown to induce intestinal mucosal damage via epithelial apoptosis after thermal injury [16]. Anti-TNF therapy is an important component of the treatment of inflammatory bowel diseases. In ischemia and reperfusion, TNF-α has been demonstrated to be responsible for impaired barrier function [17, 18]. IL-6 is a pleiotropic cytokine, exhibiting multiple effects, but in hemorrhagic shock, has been found to contribute to NF-κB and STAT3 activation as well as PMN infiltration into liver and lung tissues [19]. Interestingly, orally administered recombinant IL-6 has been shown to protect from bacteremia after hemorrhagic shock [20] and prevent intestinal epithelial cells from hypoxia induced apoptosis in vitro [21]. Additionally, IL-6 plays a role in shock-induced intestinal dysmotility [22]. MIP-2, a murine analog of human IL-8, plays an important role in neutrophil and lymphocyte recruitment to the intestine [23, 24] and is important mediator in neutrophil recruitment to the lung and acute lung injury [25]. This scenario of intraluminal cytokines, produced by the intestinal epithelium and to which the intestinal epithelium respond, may represent a novel autocrine or paracrine mechanism of regulation.
During stool and organ harvest, stool weights were measured. We demonstrated a difference in distal colonic stool weight at 1 h after shock and in cecal and distal colonic stool weight at 6 h after shock. These differences in stool weight are potentially the result of ileus induced by shock and reperfusion. This is consistent with other work demonstrating smooth muscle dysfunction due to elevated ICAM and IL-6 expression and leukocyte infiltration [26, 27]. Increased stool weight or volume in mice treated with hemorrhagic shock and resuscitation does not explain increased luminal cytokine levels in those animals, as these were adjusted for weight during data analysis. Indeed, the actual increase in luminal cytokine levels may be somewhat diluted by increased stool volume.
Samples from the lumen of the jejunum and ileum were taken using a filter paper blotting technique. Other experiments have demonstrated this to be an effective technique, which was superior to lavage of intestinal segments. Filter paper blotting has been used with success during endoscopy procedures for sampling of the luminal milieu [28–30]. Alternatively, a whole gut lavage model has been used in human studies, but is not practical for this murine model [31].
The origin of the intraluminal cytokines seen in the present study is likely derived from intestinal epithelial cells. It has previously been well described that production of TNF-α, IL-6, and MIP-2 may originate from intestinal epithelial cells in sepsis, burn, hemorrhage, or other disease processes [32–35]. Washout from the serum into the intestinal lumen is an unlikely explanation for our findings. Previous work from our laboratory in this model of hemorrhagic shock has shown significant increase in vascular permeability in the lung tissue after hemorrhagic shock, using an Evans blue infusion method [15]. More recent experiments have not shown increased vascular permeability in intestinal tissue under these conditions (Makley AT and Pritts TA, unpublished observations, 2011). Other studies have shown that sepsis- and coronary bypass surgery- induced changes in intraluminal rectal levels of lactic acid, a very small molecule compared with cytokine peptides, were not the result of lactate washout from the serum, except in cases of profound serum lactate elevation [36, 37]. In our present study, IL-6 serum levels were markedly elevated for a considerable period of time (at 1 and 3 h), and intraluminal IL-6 elevated at 6 h after shock could be a result of serum washout. Serum levels of TNF-α and MIP-2 were very low after resuscitation, making this an unlikely source of those luminal cytokines. Additionally, while the presence of biliary IL-6 has been demonstrated in a liver transplant rejection model [38], the absence of cytokines in the proximal intestine in this study makes the bile an unlikely source. Moreover, previous studies have shown that there is preferential activation of NF-κB and cytokine production in the proximal gut (jejunum and ileum), suggesting that active production of proinflammatory mediators occurs in these segments [39, 40]. These studies, combined with our current data, strongly suggest that proinflammatory cytokines are actively secreted into the intestinal lumen after resuscitation from hemorrhagic shock, where they accumulate in the fecal mass of the cecum and distal colon.
In the clinical setting, measurement of intestinal luminal factors has become an important tool in the management of inflammatory bowel diseases (IBD). TNF-α levels found in the stool of patients with active Crohn’s disease and ulcerative colitis represented the first use of this tool. Interestingly, healthy control subjects as well as control subjects with infectious diarrhea had no elevations in TNF-α [8]. A later study consisting of immunohistochemical staining of human biopsy specimens from patients with inflammatory bowel disease probed for IL-8, MIP-1α, MCP-1, and other cytokines. A heavy staining pattern was found not only in the epithelial cells, but in the apical secretory vacuoles of goblet cells and in the lumen [9]. Since cytokines are small, readily degradable peptides, other more stable proteins are currently in clinical use as luminal markers of inflammation due to IBD. The most useful markers being neutrophil elastase, lactoferrin, and fecal calprotectin, which are present in the intestinal lumen, correlate with disease activity and are stable at room temperature for 1–7 d, making them useful in the process of diagnosis and following disease progression [11, 12, 41]. Our data add to this previous experience and suggest that cytokines may be present in the intestinal lumen after hemorrhagic shock.
CONCLUSION
In conclusion, this study demonstrates the presence of inflammatory cytokines in the intestinal lumen after hemorrhagic shock. The precise source and function of these cytokines remains to be elucidated, but we feel that this may represent a form of autocrine signaling in the intestine after injury.
ACKNOWLEDGMENTS
The work was supported in part by United States Army grant W81XWH-09-1-0625.
REFERENCES
- 1.Evans JA, van Wessem KJ, McDougall D, et al. Epidemiology of traumatic deaths: Comprehensive population-based assessment. World J Surg. 2010;34:158. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.de Haan JJ, Lubbers T, Derikx JP, et al. Rapid development of intestinal cell damage following severe trauma: A prospective observational cohort study. Crit Care. 2009;13:R86. doi: 10.1186/cc7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuijls G, de Haan JJ, Derikx JP, et al. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 5.Baker JW, Deitch EA, Li M, et al. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Coopersmith CM. Intestinal crosstalk: A new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: The gut-lymph hypothesis, a review. Front Biosci. 2006;11:520. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 8.Braegger CP, Nicholls S, Murch SH, et al. Tumor necrosis factor α in stool as a marker of intestinal inflammation. Lancet. 1992;339:89. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 9.Banks C, Bateman A, Payne R, et al. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199:28. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 10.Daig R, Andus T, Aschenbrenner E, et al. Increased interleukin-8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: From occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859. doi: 10.1136/gut.2008.170019. [DOI] [PubMed] [Google Scholar]
- 12.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Peterson CG, Sangfelt P, Wagner M, et al. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest. 2007;67:810. doi: 10.1080/00365510701452838. [DOI] [PubMed] [Google Scholar]
- 14.Sonnier DI, Bailey SR, Schuster RM, et al. TNF-α induces vectorial secretion of IL-8 in Caco-2 cells. J Gastrointest Surg. 2010;14:1592. doi: 10.1007/s11605-010-1321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makley AT, Goodman MD, Friend LA, et al. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. 68:305–311. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spies M, Chappell VL, Dasu MR, et al. Role of TNF-α in gut mucosal changes after severe burn. Am J Physiol Gastrointest Liver Physiol. 2002;283:G703. doi: 10.1152/ajpgi.00149.2001. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi S, Wu R, Zhou M, et al. Gut hyperpermiability after ischemia and reperfusion: Attenuation with adrenomedullin and its binding protein treatment. Int J Clin Exp Pathol. 2008;1:409. [PMC free article] [PubMed] [Google Scholar]
- 18.Tamion F, Richard V, Lyoumi S, et al. Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol. 1997;273:G314. doi: 10.1152/ajpgi.1997.273.2.G314. [DOI] [PubMed] [Google Scholar]
- 19.Meng ZH, Dyer K, Billiar TR, et al. Essential role for IL-6 in post-resuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol. 2001;280:C343. doi: 10.1152/ajpcell.2001.280.2.C343. [DOI] [PubMed] [Google Scholar]
- 20.Rollwagen FM, Li YY, Pacheco ND, et al. Systemic bacteremia following hemorrhagic shock in mice: Alleviation with oral interleukin-6. Cytokine. 1996;8:121. doi: 10.1006/cyto.1996.0017. [DOI] [PubMed] [Google Scholar]
- 21.Rollwagen FM, Madhavan S, Singh A, et al. IL-6 protects enterocytes from hypoxia-induced apoptosis by induction of bcl-2 mRNA and reduction of fas mRNA. Biochem Biophys Res Commun. 2006;347:1094. doi: 10.1016/j.bbrc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu JT, Kan WH, Hsieh CH, et al. Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent up-regulation of HO-1. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1825. doi: 10.1152/ajpregu.00112.2008. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsuka Y, Lee J, Stamm DS, et al. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomas JL, Chung CS, Grutkoski PS, et al. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: Assessment by adoptive cells transfer in mice. Shock. 2003;19:358. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Hierholzer C, Kalff JC, Chakraborty A, et al. Impaired gut contractility following hemorrhagic shock is accompanied by IL-6 and G-CSF production and neutrophil infiltration. Dig Dis Sci. 2001;46:230. doi: 10.1023/a:1005524021552. [DOI] [PubMed] [Google Scholar]
- 27.Kalff JC, Hierholzer C, Tsukada K, et al. Hemorrhagic shock results in intestinal muscularis intercellular adhesion molecule (ICAM-1) expression, neutrophil infiltration, and smooth muscle dysfunction. Arch Orthop Trauma Surg. 1999;119:89. doi: 10.1007/s004020050363. [DOI] [PubMed] [Google Scholar]
- 28.Carty E, De Brabander M, Feakins RM, et al. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 2000;46:487. doi: 10.1136/gut.46.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendel J, Nielsen OH, Madsen S, et al. A simple filter-paper technique allows detection of mucosal cytokine levels in vivo in ulcerative colitis. Interleukin-1 and interleukin-1-receptor antagonist. Dig Dis Sci. 1996;41:1775. doi: 10.1007/BF02088744. [DOI] [PubMed] [Google Scholar]
- 30.Kristjansson G, Venge P, Wanders A, et al. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: Studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnott ID, Drummond HE, Ghosh S. Gut mucosal secretion of interleukin 1beta and interleukin-8 predicts relapse in clinically inactive Crohn’s disease. Dig Dis Sci. 2001;46:402. doi: 10.1023/a:1005617302718. [DOI] [PubMed] [Google Scholar]
- 32.Lammers KM, Innocenti G, Venturi A, et al. The effect of transient intestinal ischemia on inflammatory parameters. Int J Colorectal Dis. 2003;18:78. doi: 10.1007/s00384-002-0413-8. [DOI] [PubMed] [Google Scholar]
- 33.Ogle CK, Guo X, Hasselgren PO, et al. The gut as a source of inflammatory cytokines after stimulation with endotoxin. Eur J Surg. 1997;163:45. [PubMed] [Google Scholar]
- 34.Ogle CK, Mao JX, Wu JZ, et al. The 1994 Lindberg Award. The production of tumor necrosis factor, interleukin-1, interleukin-6, and prostaglandin E2 by isolated enterocytes and gut macrophages: Effect of lipopolysaccharide and thermal injury. J Burn Care Rehabil. 1994;15:470. [PubMed] [Google Scholar]
- 35.Stadnyk AW. Cytokine production by epithelial cells. FASEB J. 1994;8:1041. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 36.Solligard E, Wahba A, Skogvoll E, et al. Rectal lactate levels in endoluminal microdialysate during routine coronary surgery. Anaesthesia. 2007;62:250. doi: 10.1111/j.1365-2044.2006.04937.x. [DOI] [PubMed] [Google Scholar]
- 37.Tenhunen JJ, Kosunen H, Alhava E, et al. Intestinal luminal microdialysis: A new approach to assess gut mucosal ischemia. Anesthesiology. 1999;91:1807. doi: 10.1097/00000542-199912000-00035. [DOI] [PubMed] [Google Scholar]
- 38.Tono T, Monden M, Yoshizaki K, et al. Biliary interleukin 6 levels as indicators of hepatic allograft rejection in rats. Transplantation. 1992;53:1195. doi: 10.1097/00007890-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Pritts TA, Moon MR, Wang Q, et al. Activation of NF-κB varies in different regions of the gastrointestinal tract during endotoxemia. Shock. 2000;14:118. doi: 10.1097/00024382-200014020-00007. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Wang JJ, Boyce S, et al. Endotoxemia and IL-1β stimulate mucosal IL-6 production in different parts of the gastrointestinal tract. J Surg Res. 1998;76:27. doi: 10.1006/jsre.1998.5288. [DOI] [PubMed] [Google Scholar]
- 41.Sipponen T, Savilahti E, Karkkainen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-α therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]