Abstract
Purpose of review
IDH1/2 mutations occur in up to 70% of low-grade gliomas and secondary glioblastomas. Mutation of these enzymes reduces the wildtype function of the enzyme (conversion of isocitrate to α-ketoglutarate) while conferring a new enzymatic function, the production of d-2-hydroxyglutarate (d-2-HG) from α-ketoglutarate (α-KG). However, it is unclear how these enzymatic changes contribute to tumorigenesis. Here, we discuss the recent studies that demonstrate how IDH1/2 mutation may alter the metabolism and epigenome of gliomas, how these changes may contribute to tumor formation, and opportunities they might provide for molecular targeting.
Recent findings
Metabolomic studies of IDH1/2 mutant cells have revealed alterations in glutamine, fatty acid, and citrate synthesis pathways. Additionally, d-2-HG produced by IDH1/2 mutant cells can competitively inhibit α-KG-dependent enzymes, including histone demethylases and DNA hydroxylases, potentially leading to a distinct epigenetic phenotype. Alterations in metabolism and DNA methylation present possible mechanisms of tumorigenesis.
Summary
Recent attempts to improve outcomes for glioma patients have resulted in incremental gains. Studies of IDH1/2 mutations have provided mechanistic insights into tumorigenesis and potential avenues for therapeutic intervention. Further study of IDH1/2 mutations might allow for improved therapeutic strategies.
Keywords: astrocytoma, epigenetics, glioblastoma, glioma, IDH1, IDH2, metabolism, oligodendroglioma
INTRODUCTION
Gliomas are a collection of nervous system tumors arising from glial cells such as astrocytes and oligodendroglia. The most deadly and aggressive of these gliomas is the grade IV astrocytomas or glioblastoma multiforme (GBM). To better understand the genetic basis of GBM, 22 GBM genomes were sequenced. This study quantified the frequency of previously known mutations (e.g. TP53, EGFRvIII, and PI3KCA) and led to the discovery of acquired mutations in IDH1, which were not previously known or associated with cancer [1]. Subsequent studies discovered mutations in IDH2, the mitochondrial homolog of IDH1 [2]. The role of IDH1/2 mutation in tumorigenesis and tumor maintenance is unclear, but recent findings have implicated altered metabolism, modified epigenetic states, and possible connections between the two as potential tumorigenic mechanisms.
METABOLISM AND CANCER
Associations between metabolism and cancer were first documented in the 1920s by Otto von Warburg, who demonstrated that cancer cells exhibited increased rates of glycolysis and decreased dependence on oxidative phosphorylation, despite the presence of sufficient oxygen [3,4]. Recently, understanding and therapeutically exploiting altered metabolism in cancer cells has received renewed interest. Oncogenes and tumor suppressors (e.g. MYC, p53, PI3KCA, etc.) contribute to the regulation of both tumor and normal cell metabolism [5–7]. However, relatively few mutations in genes encoding metabolic enzymes are known to contribute to oncogenesis. These known mutations include succinate dehydrogenase (SDH), fumarate dehydrogenase, and IDH1/2 [1,6,7]. SDH and fumarate dehydrogenase mutations are associated with familial cancer syndromes and lead to enzymatic inactivation with subsequent accumulation of their substrates, succinate and fumarate. Accumulation of these metabolites contributes to tumorigenesis by stabilizing HIF-1α in the presence of sufficient oxygen through inhibition of prolyl hydroxylase domain 2 (PHD2). PHD2 utilizes O2 and α-ketoglutarate (α-KG) to hydroxylate HIF-1α, leading to its degradation. Inhibition of PHD2 in the presence of sufficient oxygen leads to increased HIF-1α and promotes tumorigenesis through increased glycolysis and angiogenesis [6–10].
IDH1/2 MUTATION FREQUENCY
IDH1 mutations occur in up to 12% of all GBMs [1]. GBMs develop de novo (primary) or from lower grade gliomas (secondary). Mutations in IDH1/2 occur in up to 70% of low-grade gliomas and secondary GBMs [2]. IDH1 and IDH2 mutations occur in 16% of acute myelogenous leukemias (AMLs) and up to 33% in AMLs with normal karyotypes [11], 56% of central and periosteal cartilaginous tumors [12], and 40% of gliomatosis cerebri cases [13–15].
The role of IDH1 or IDH2 mutation as an indicator of progression and survival is not well established. IDH1/2 mutation may result in decreased prognosis for AML [16–18] and increased prognosis for both low-grade gliomas (65 months for mutant IDH1/2 versus 38 months for wildtype) and secondary GBMs (31 months for mutant IDH1/2 versus 15 months for wildtype) [2]. However, IDH1/2 mutant tumors may be recognized earlier than IDH1/2 wildtype tumors, resulting in an apparent, rather than real, association with increased survival.
IDH1 MUTATION AND ENZYMATIC FUNCTION
IDH1 mutations occur at a single amino acid residue, R132 [1,2], whereas IDH2 mutations occur at two residues, R140 or R172 (the functional equivalent of R132 in IDH1) [11]. The most common mutation is the R132H IDH1 mutation [2]. IDH1/2 mutations result in two enzymatic changes: decreased wildtype IDH function (oxidative decarboxylation of isocitrate to α-KG with simultaneous reduction of NADP+) [19▪,20,21] and gain of a new enzymatic capability [reduction of α-KG to d-2-hydroxyglutarate (d-2-HG)] with simultaneous oxidation of NADPH [19▪,20] (Fig. 1). Changes in the active site of mutant IDH1/2 enzymes prime the enzyme to produce d-2-HG by increasing its affinity for α-KG [19▪,20] and shifting the conformation of the enzyme to favor an intermediate transition state [22].
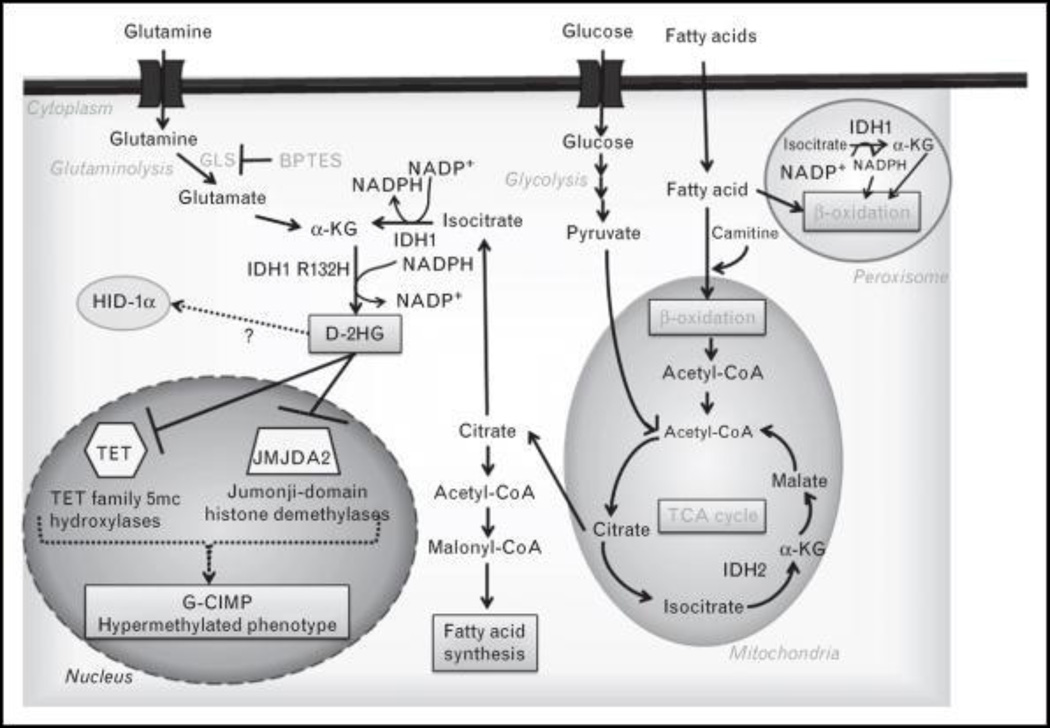
FIGURE 1.
Epigenetic and metabolic alterations in IDH1/2 mutant tumor cells. IDH1/2 mutant enzyme activity results in high intracellular d-2-hydroxyglutarate (d-2-HG) levels and alterations in glutamine metabolism, citrate and acetyl-coA levels, and fatty acid metabolism. Simultaneously, d-2-HG is able to compete with α-ketoglutarate for binding to histone demethylases and DNA hydroxylases likely contributing to a hypermethylated phenotype.
IDH1/2 MUTATIONS, D-2-HYDROXYGLUTARATE, AND METABOLIC DISORDERS
d-2-HG accumulation is a biochemical hallmark of IDH1/2 mutant tumors. Cells expressing wildtype IDH1/2 have low levels of d-2HG, a byproduct of l-hydroxylysine breakdown, which has no known function within the cell. d-2-HG is removed from the cell through conversion to α-KG by d-2-hydroxyglutarate dehydrogenase. Interestingly, d-2-HG and its stereoisomer l-2-hydroxyglutarate (l-2-HG) accumulate in certain metabolic disorders, d-2 or l-2 hydroxyglutaric aciduria (d-2 or l-2-HGA), respectively [23–26].
l-2-HGA results from inactivation of l-2-hydroxyglutaric dehydrogenase [25], whereas d-2-HGA results from inactivation of d-2-hydroxyglutarate dehydrogenase or IDH2 mutations [23,25,27,28]. IDH2 mutations found in d-2-HGA are identical to those documented in gliomas or AML. l-2-HGA patients have been documented to develop gliomas [28–32]; however, no cases of glioma have been documented in d-2-HGA patients, despite d-2-HG accumulation [23,25–27]. This may suggest that increased d-2-HG levels alone are not sufficient to cause tumorigenesis.
IDH1/2 MUTATIONS AND TUMORIGENESIS
IDH1/2 mutations are significant driver mutations in glioma development as evidenced by computational analyses using Cancer-Specific High-Throughput Analysis of Somatic Mutations (CHASM) [33]. Additionally, IDH1/2 mutations occur first in grade II gliomas and their frequency does not increase with tumor grade, suggesting that these mutations are critical for initial tumor development [2,34]. How IDH1/2 mutation contributes to the development of cancer is currently under investigation.
Initially, studies suggested that IDH1/2 mutations contribute to tumorigenesis through stabilization of HIF-1α, similar to SDH and fumarate dehydrogenase mutations. The authors suggested α-KG levels were significantly decreased in IDH1/2 mutant cells, leading to inactivation of PHD2 and stabilization of HIF-1α [21]. However, subsequent studies have found that α-KG levels are not significantly altered in IDH1/2 mutant cells, despite increased cellular demand for d-2-HG production [19▪,20,35,36▪,37▪▪]. Additionally, studies in AML and glioma with IDH1/2 mutations did not detect increased stabilization of HIF-1α [38,39]. Therefore, although HIF-1α stabilization could contribute to tumorigenesis, other pathways may play a larger role (Fig. 1).
In cells harboring IDH1/2 mutations, intracellular d-2-HG levels can reach up to 10mM [19▪,20,40]. d-2-HG and α-KG are similar in structure, and recent studies have shown that d-2-HG serves as competitive inhibitor of enzymes that utilize α-KG as a co-factor [38,41▪]. More than 60 enzymes utilize α-KG as a cofactor [42], and d-2-HG outcompetes α-KG for binding to several classes of histone demethylases, PHD2 and TET 5-methylcytosine hydroxylases [38,41▪]. Crystallographic studies confirm that d-2-HG fits into binding sites for α-KG in dual-specificity histone demethylase KIAA1718 (KDM7A), factor inhibiting hypoxia-inducible factor (FIH), and jumonji domain containing protein 2A (JMJD2A) [38,41▪]. High intracellular levels of d-2-HG in IDH1/2 mutant tumors are likely sufficient for potent enzymatic inhibition [38] and suggest a possible mechanism by which IDH1/2 mutations contribute to tumorigenesis.
Interestingly, mutant IDH1/2 cases in AML have a characteristic hypermethylated phenotype which may result from inhibition of TET2 by d-2-HG [43▪]. TET2 catalyzes the conversion of methylcytosine to 5-hydroxymethylcytosine and is mutated in approximately 24% of secondary AMLs. TET2 mutations result in a hypermethylated phenotype similar to that seen in mutant IDH1/2 AMLs, although the two mutations are mutually exclusive [43▪]. Differentially methylated genes in IDH1 mutant gliomas include processes known to contribute to tumor progression as well as several metabolic pathways (Table 1). In gliomas, a hypermethylated phenotype has also been identified, the glioma-CpG island methylator phenotype (G-CIMP) which correlates tightly with IDH1 mutations. Although 78% of G-CIMP positive tumors possess an IDH1 mutation, no causative link between IDH1/2 mutations and this phenotype has been directly studied [35,44▪].
Table 1.
Pathways potentially altered by IDH1/2 mutations in glioma
| Tissue studied | Alteration | ||
|---|---|---|---|
| Methylation | IDH1 mutant gliomas | Hypermethylated pathways | Hypomethylated pathways |
| Methane metabolism | Protein kinase A signaling | ||
| Pregnane X receptor/retinoid X receptor activation | Angiopoietin signaling | ||
| Retinol metabolism | Ras-related nuclear protein signaling | ||
| Phenylalanine metabolism | Retinol transport | ||
| Starch and sucrose metabolism | Cell cycle regulation | ||
| Pentose and glucuronate interconversion | Methylated-DNA-protein-cysteine methyltransferase (MGMT) | ||
| Androgen and estrogen metabolism | Fatty acid binding | ||
| Transcription | G-CIMP-positive gliomas | Increased | Decreased |
| Transcriptional regulation | Polysaccharide binding | ||
| Nucleic acid synthesis | Heparin binding | ||
| Metabolic processes | Glucosaminoglycan binding | ||
| Cadherin based cell adhesion | Collagen | ||
| Zinc finger transcription factors | Thrombospondin | ||
| Cell morphogenesis | |||
| Retinoic acid signaling | |||
| Fatty acid binding | |||
| Metabolism | IDH1/2 mutant cells or cells treated with d-2-HG | Increased | Decreased |
| d-2-HG | NAAG | ||
| Glycerol-3-phosphate | NAA | ||
| Glycerol-2-phosphate | 2-Methyl butyryl carnitine | ||
| Glycine | Isobutyl carnitine | ||
| Asparagine | α-Aminoadipate | ||
| Glutamine | Phosphocholine | ||
| Serine | Propionylcarnitine | ||
| Threonine | Malate | ||
| Phenylalanine | Fumarate | ||
| Tyrosine | Citrate | ||
| Tryptophan | |||
| Methionine | |||
IDH1/2 MUTATIONS AND METABOLIC ALTERATIONS
Transcriptional analysis of aberrantly methylated genes in G-CIMP tumors by Noushmehr et al. [44▪] found significant upregulation in genes involved in metabolic processes including carbohydrate metabolism, oxidative stress response, and nucleic acid synthesis (Table 1). Pathways transcriptionally repressed in G-CIMP tumors include those involved in tumor invasion, extracellular matrix remodeling, retinoic acid signaling, and mesenchymal markers (Table 1) [44▪]. Although the mechanisms for these changes are not yet known, d-2-HG produced by IDH1/2 mutations may alter the epigenetic profile and thereby alter the expression of metabolic and other genes that promote tumor formation and growth.
Metabolic changes have been confirmed by metabolomics studies on IDH1/2 mutant cells [36▪,37▪▪]. Reitman et al. [36▪] indentified changes resulting from expression of mutant IDH1/2 or treatment with d-2-HG (1). Some metabolic alterations were found under both conditions; however, approximately half of the observed changes in IDH1/2 mutant expressing cells could not be replicated by treatment with exogenous d-2-HG. Most notably, decreases in glutamate levels and metabolites whose synthesis involves glutamate were not replicated by d-2-HG treatment. Therefore, these changes are a direct result of the enzymatic activity of IDH1/2 mutant enzymes.
One such metabolic alteration is the reduction of the neuropeptide N-acetyl-aspartyl glutamate (NAAG) and its precursor N-acetylated aspartic acid (NAA) in IDH1/2 mutant cells and tumors by up to 50-fold [36▪]. NAAG is synthesized from NAA and glutamate by NAAG synthase and functions in glutamatergic pathways of the brain [45]. Reitman et al. found that even when NAA levels are restored, NAAG levels are not fully restored indicating that the glutamate necessary for generation of NAAG may be shuttled for production of α-KG and subsequently d-2-HG. In either case, the consequences of lowered intracellular NAAG levels on tumor formation and maintenance are unknown and warrant further study.
Mutant IDH1/2 produce d-2-HG from glutamine-derived α-KG, resulting in increased flux through this pathway [20]. Therefore, cells expressing mutant IDH1/2 may be more dependent upon this pathway for growth and survival. Interestingly, inhibition of α-KG synthesis from glutamine led to a 15–20% decrease in growth for cells expressing mutant IDH1 [37▪▪]. To further characterize this growth inhibition, metabolite levels were evaluated following treatment with BPTES, a small molecule inhibitor of glutamine, the first enzyme in the synthesis of α-KG from glutamine. Glutaminase inhibition lowered glutamate and α-KG levels in IDH1 wildtype and mutant cells, but d-2-HG levels were not reduced, indicating the presence of a compensatory mechanism [37▪▪].
Changes in the other metabolites following BPTES treatment supported this hypothesis including increases in glycolytic intermediates and decreased levels of TCA cycle intermediates which were seen in both wildtype and mutant IDH1-expressing cells [37▪▪]. Consequently, α-KG might be produced or diverted away from the TCA cycle to compensate for inhibition of α-KG produced from glutaminase. Therefore, metabolic inhibition has been shown as a potential approach for selective targeting of IDH1/2 mutant tumors. However, glutaminase inhibition alone is not sufficient as a therapeutic strategy and will require additional components for an effective therapeutic response.
Changes in fatty acid metabolism may also result from IDH1/2 mutant expression. Mutant IDH1-expressing cells exhibit decreased levels of citrate compared with wildtype-expressing IDH1 cells, coupled with increases in acetyl-CoA and triglyceride and phospholipid precursors [36▪,37▪▪]. The combination of decreased citrate and increased lipid precursors may indicate that IDH1 mutant expressing cells shuttle citrate out of the TCA cycle to produce lipids required for cell growth. Increases in fatty acid/lipid synthesis are a common characteristic of many cancers, including gliomas [46], and it is possible IDH1/2 mutation may contribute to this phenotype. In addition, mutant IDH1 may lead to decreased fatty acid oxidation in two ways: reducing available cofactors for peroxisomal β-oxidation and by decreasing carnitine biosynthesis for mitochondrial fatty acid transport.
Oxidation of α-phytanic acids and β-oxidation of certain fatty acids within peroxisomes requires both NADPH and α-KG, products of wildtype IDH1 activity. IDH1 has been shown to be the sole source of these molecules within the peroxisomes [47]. Mutant IDH1 has decreased the ability to produce NADPH and α-KG, suggesting that mutation of IDH1 may lead to decreased levels of these substrates within peroxisomes and potentially decreased rates of fatty acid oxidation. Chowdhury et al. [38] demonstrated that high levels of d-2-HG can inhibit γ-butyrobetaine hydroxylase 1, the last enzymatic step in carnitine biosynthesis. Carnitine is required for activation and transport of fatty acids into the mitochondria to undergo β-oxidation. Consistent with this finding, levels of propionylcarnitine (a carnitine ester which sustains endogenous carnitine pools) were decreased in IDH1/2 mutant and d-2-HG-treated cells [36▪]. Therefore, IDH1/2 mutations may contribute to tumorigenesis by priming cells for growth by increasing fatty acid synthesis and reducing oxidation of certain fatty acids.
IDH1/2 MUTATIONS AND ANTICANCER METABOLISM BASED THERAPY
The discovery of the IDH1/2 mutation has profound implications for the understanding and treatment of cancer. Elevated d-2-HG levels are characteristic of IDH1/2 mutant tumors and could serve as a biomarker to identify IDH1/2 mutational status or monitor tumor growth or treatment efficacy. Studies in AML have shown that d-2-HG can be detected in serum, and increased d-2-HG levels correlate with IDH1/2 mutational status [19▪,40]. The use of d-2-HG as a biomarker in gliomas is currently under investigation; however, detection may be technically challenging as the degree of d-2-HG diffusion from solid glial tumors into serum, cerebrospinal fluid, or urine is unknown. Alternatively, d-2-HG can be detected by magnetic resonance spectroscopy (MRS), allowing for noninvasive monitoring of tumor progression or classification of IDH1/2 mutational status [48].
As it is unclear whether mutant IDH1/2 protein is important for tumor growth or maintenance, targeting the mutant enzymes may not be therapeutically beneficial. Additionally, it is unknown whether changes in methylation status in IDH1/2 mutant cells are reversible by disrupting mutant protein function. The distinct metabolic alterations resulting from IDH1/2 mutations reveal a potential opportunity for therapeutic intervention. Therefore, targeting unique metabolic pathways with IDH1/2 mutant tumors may be an alternative strategy. One potential strategy for the treatment of IDH1/2 mutant tumors is inhibition of α-KG synthesis from glutamine. Studies from our group have demonstrated that inhibition of this pathway can specifically slow the growth of mutant IDH1-expressing cells [37▪▪]. However, compensatory mechanisms and the mutational background of glioma cells suggest that multiple metabolic components may need to be targeted for the development of a successful therapeutic strategy. Further investigation of the role of IDH1/2 and d-2-HG on the epigenome and the metabolome will likely reveal additional targets.
CONCLUSION
IDH1/2 mutations are central to the development of a particular subset of gliomas, and further studies could identify targeted treatments for individuals with these mutations. Epigenetic and metabolic alterations in IDH1/2 mutant cells provide clues to the mechanism by which these mutations lead to tumor development and/or maintenance; however, the relative contribution of these changes to each process is currently unknown.
To our knowledge, cell lines and animal models derived from a tumor with a naturally occurring IDH1/2 mutation have not been established. The literature and our experience suggest that IDH1/2 mutant tumors are resistant to cell culture establishment using standard protocols [49]. Therefore, studies of IDH1/2 mutations have been conducted in genetic and metabolic backgrounds which have not developed in concert with an IDH1/2 mutation. Although these efforts have yielded useful insights, cell culture systems and animal tumor models derived from tumors with naturally occurring IDH1/2 mutations need to be developed to better understand the role of mutant IDH1/2 in tumorigenesis and tumor maintenance.
KEY POINTS.
IDH1/2 mutations occur frequently and early in the development of low-grade gliomas and secondary GBM.
IDH1/2 mutations produce high levels of d-2-HG, which can inhibit α-KG-dependent enzymes potentially leading to genome-wide epigenetic alterations and altered cellular metabolism.
Mutant IDH1/2 enzymatic activity leads to metabolic alterations including changes in glutamine, N-acetylated amino acid, and fatty acid metabolism.
Targeting the altered metabolism in IDH1/2 mutant gliomas may be a useful therapeutic strategy.
Acknowledgements
None.
Support for this work was generously provided by the Virginia and D. K. Ludwig Fund for Cancer Research, the Conrad N. Hilton Foundation, the Johns Hopkins Department of Neurosurgery, and the Irving J. Sherman M. D. Neurosurgery Research Professorship to G.J.R.
Footnotes
Conflicts of interest
G.J.R. is coinventor on IDH1 related intellectual property owned and managed by Johns Hopkins University in accordance with its conflict of interest policy.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 113).
- 1.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie ED, Selak MA, Tennant DA, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 13.Desestret V, Ciccarino P, Ducray F, et al. Prognostic stratification of gliomatosis cerebri by IDH1 (R132H) and INA expression. J Neurooncol. 2011:1–6. doi: 10.1007/s11060-011-0587-4. [DOI] [PubMed] [Google Scholar]
- 14.Narasimhaiah D, Miquel C, Verhamme E, et al. IDH1 mutation, a genetic alteration associated with adult gliomatosis cerebri. Neuropathology. 2011 doi: 10.1111/j.1440-1789.2011.01216.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Seiz M, Tuettenberg J, Meyer J, et al. Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes. Acta Neuropathol. 2010;120:261–267. doi: 10.1007/s00401-010-0701-2. [DOI] [PubMed] [Google Scholar]
- 16.Patnaik MM, Lasho TL, Finke CM, et al. WHO-defined ‘myelodysplastic syndrome with isolated del(5q)’ in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24:1283–1289. doi: 10.1038/leu.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thol F, Damm F, Wagner K, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 2010;116:614–616. doi: 10.1182/blood-2010-03-272146. [DOI] [PubMed] [Google Scholar]
- 18.Thol F, Weissinger EM, Krauter J, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 2010;95:1668–1674. doi: 10.3324/haematol.2010.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. This study confirmed production of d-2-HG in AML and pointed towards accumulation of d-2-HG as a potential common mechanism for tumorigenesis.
- 20.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang B, Zhong C, Peng Y, et al. Molecular mechanisms of ‘off–on switch’ of activities of human IDH1 by tumor-associated mutation R132H. Cell Res. 2010;20:1188–1200. doi: 10.1038/cr.2010.145. [DOI] [PubMed] [Google Scholar]
- 23.Kranendijk M, Struys EA, Gibson KM, et al. Evidence for genetic heterogeneity in d-2-hydroxyglutaric aciduria. Hum Mutat. 2010;31:279–283. doi: 10.1002/humu.21186. [DOI] [PubMed] [Google Scholar]
- 24.Steenweg ME, Jakobs C, Errami A, et al. An overview of l-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype–phenotype study. Hum Mutat. 2010;31:380–390. doi: 10.1002/humu.21197. [DOI] [PubMed] [Google Scholar]
- 25.Struys EA, Korman SH, Salomons GS, et al. Mutations in phenotypically mild d-2-hydroxyglutaric aciduria. Ann Neurol. 2005;58:626–630. doi: 10.1002/ana.20559. [DOI] [PubMed] [Google Scholar]
- 26.Struys EA, Salomons GS, Achouri Y, et al. Mutations in the d-2-hydroxyglutarate dehydrogenase gene cause d-2-hydroxyglutaric aciduria. Am J Hum Genet. 2005;76:358–360. doi: 10.1086/427890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranendijk M, Struys EA, van Schaftingen E, et al. IDH2 mutations in patients with d-2-hydroxyglutaric aciduria. Science. 2010;330:336. doi: 10.1126/science.1192632. [DOI] [PubMed] [Google Scholar]
- 28.Yazici N, Sarialioglu F, Alkan O, et al. Glutaric aciduria type II [corrected] and brain tumors: a case report and review of the literature. J Pediatr Hematol Oncol. 2009;31:865–869. doi: 10.1097/MPH.0b013e3181b258c6. [DOI] [PubMed] [Google Scholar]
- 29.Aghili M, Zahedi F, Rafiee E. Hydroxyglutaric aciduria and malignant brain tumor: a case report and literature review. J Neurooncol. 2009;91:233–236. doi: 10.1007/s11060-008-9706-2. [DOI] [PubMed] [Google Scholar]
- 30.Coskun T. l-2-Hydroxyglutaric aciduria and brain tumors. J Pediatr Hematol Oncol. 2010;32:339–340. doi: 10.1097/MPH.0b013e3181d74596. [DOI] [PubMed] [Google Scholar]
- 31.Haliloglu G, Jobard F, Oguz KK, et al. l-2-Hydroxyglutaric aciduria and brain tumors in children with mutations in the L2HGDH gene: neuroimaging findings. Neuropediatrics. 2008;39:119–122. doi: 10.1055/s-2008-1081217. [DOI] [PubMed] [Google Scholar]
- 32.Moroni I, Bugiani M, D’Incerti L, et al. l-2-Hydroxyglutaric aciduria and brain malignant tumors: a predisposing condition? Neurology. 2004;62:1882–1884. doi: 10.1212/01.wnl.0000125335.21381.87. [DOI] [PubMed] [Google Scholar]
- 33.Carter H, Chen S, Isik L, et al. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res. 2009;69:6660–6667. doi: 10.1158/0008-5472.CAN-09-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69:9157–9159. doi: 10.1158/0008-5472.CAN-09-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ▪. Reitman ZJ, Jin G, Karoly ED, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA. 2011;108:3270–3275. doi: 10.1073/pnas.1019393108. Comprehensive metabolomic analysis was performed to assess metabolic alterations resulting from IDH1/2 mutations and 2-HG exposure.
- 37. Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. This study was the first to show that metabolic targeting of IDH1 mutant glioma is a viable therapeutic strategy and reveals possible compensatory mechanisms of the cell following glutaminase inhibition.
- 38.Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams SC, Karajannis MA, Chiriboga L, et al. R132H-mutation of isocitrate dehydrogenase-1 is not sufficient for HIF-1alpha upregulation in adult glioma. Acta Neuropathol. 2011;121:279–281. doi: 10.1007/s00401-010-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. The mechanism of inhibition of several enzymes by 2-HG is demonstrated in this study, demonstrating the potential oncogenic mechanism of IDH1/2 mutations.
- 42.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 43. Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. This study illustrated that TET2 mutations and IDH1/2 mutations are mutually exclusive. Additionally, it demonstrated that mutations in these genes results in similar hypermethylation phenotypes in AML.
- 44. Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. This study identifies a distinct hypermethylated profile of certain gliomas, named G-CIMP which was found to correlate closely with IDH1/2 mutations.
- 45.Slusher BS, Vornov JJ, Thomas AG, et al. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 46.Wolf A, Agnihotri S, Guha A. Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget. 2010;1:552–562. doi: 10.18632/oncotarget.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh HJ, Lee SM, Son BG, et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J Biol Chem. 2004;279:39968–39974. doi: 10.1074/jbc.M402260200. [DOI] [PubMed] [Google Scholar]
- 48.Aydin K, Ozmen M, Tatli B, Sencer S. Single-voxel MR spectroscopy and diffusion-weighted MRI in two patients with l-2-hydroxyglutaric aciduria. Pediatr Radiol. 2003;33:872–876. doi: 10.1007/s00247-003-1029-z. [DOI] [PubMed] [Google Scholar]
- 49.Piaskowski S, Bienkowski M, Stoczynska-Fidelus E, et al. Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer. 2011;104:968–970. doi: 10.1038/bjc.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]