Abstract
The papillary tumor of the pineal region (PTPR) is a distinct entity that is particularly rare in the pediatric population. The authors document the youngest reported patient with this clinicopathological entity to date. A case of PTPR in a 15-month-old boy is described. Initially thought to be a tectal glioma, the tumor was later identified as a pineal region tumor after demonstrating growth on routine imaging. Diagnosis of PTPR was established by histopathological evaluation of biopsy samples, which revealed papillary, cystic, and solid tumor components. The patient’s postoperative course was complicated by tumor growth despite several debulking procedures and chemotherapy, as well as persistent hydrocephalus requiring 2 endoscopic third ventriculostomies and eventual ventriculoperitoneal shunt placement. After a 15-month follow-up period, the patient has received proton-beam therapy and has a stable tumor size. The PTPR is a recently described tumor of the CNS that must be included in the differential diagnosis of pineal region masses. The biological behavior, prognosis, and appropriate treatment of PTPR have yet to be fully defined.
Keywords: hydrocephalus, papillary tumor, pineal tumor, pediatric brain tumor, papillary tumor of the pineal region, tectum
The PTPR is a rare entity, which was first described by Jouvet et al.12 in 2003, and was introduced into the WHO classification of CNS tumors in 2007.14,15 The PTPR is an entity derived from specialized ependymal cells of the subcommissural organ, with a poorly understood biological behavior.3,16 Clinically, these tumors have a high risk of local recurrence and an unclear prognosis.3 More than 50 cases of PTPR have been documented thus far,16 with the vast majority occurring in adults. Six cases have been described in children younger than 16 years of age, with the youngest patient in whom a case of PTPR has been reported being a 5-year-old girl.7 We report a case of PTPR in a 15-month-old boy, the youngest documented patient with this clinicopathological entity.
Case Report
History and Examination
This previously healthy 15-month-old white male infant initially presented with a 1-month history of gait disturbance and behavioral changes. One month prior to presentation, his parents noticed a new gait abnormality, whereby he would lean to the left and run into objects as if he was unable to avoid them. In addition, he experienced decreased appetite, increased sleepiness, fussiness, and irritability. Approximately 2 weeks later, the patient experienced several days of nonbloody, nonbilious emesis, which was accompanied by a fever of up to 102.7° lasting for 5 days. During this period, he also experienced some intermittent, watery, nonbloody diarrhea. One week prior to presentation, he welts, with subsequent progression to bull’s-eye lesions, and finally a macular rash.
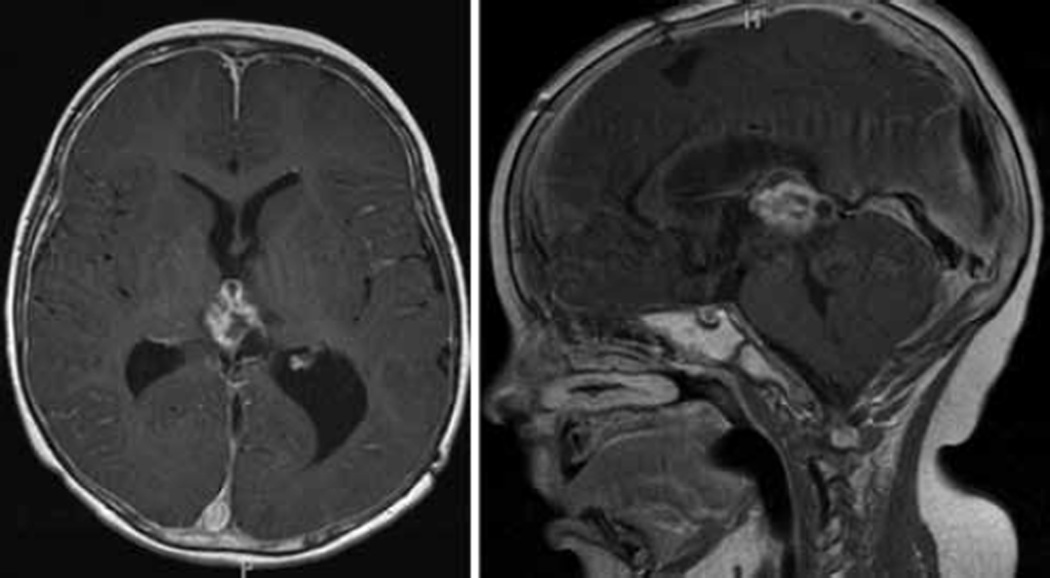
The patient was admitted to a local hospital, where a diagnosis of Lyme disease was made based on positive enzyme-linked immunosorbent assay serological findings and Western Blot results, and treatment with antibiotics was started. Because he had continued gait difficulty, a CT scan of the head was performed prior to lumbar puncture. The CT imaging demonstrated obstructive hydrocephalus secondary to a presumed tectal mass, with associated enlargement of the third and lateral ventricles (Fig. 1). The MR imaging studies demonstrated a 1 ×1–cm, well-circumscribed mass, which was thought to arise either from the tectum or the ependyma of the cerebral aqueduct (Fig. 2). At this time, the patient was transferred to the Johns Hopkins Hospital for further evaluation and tumor management.
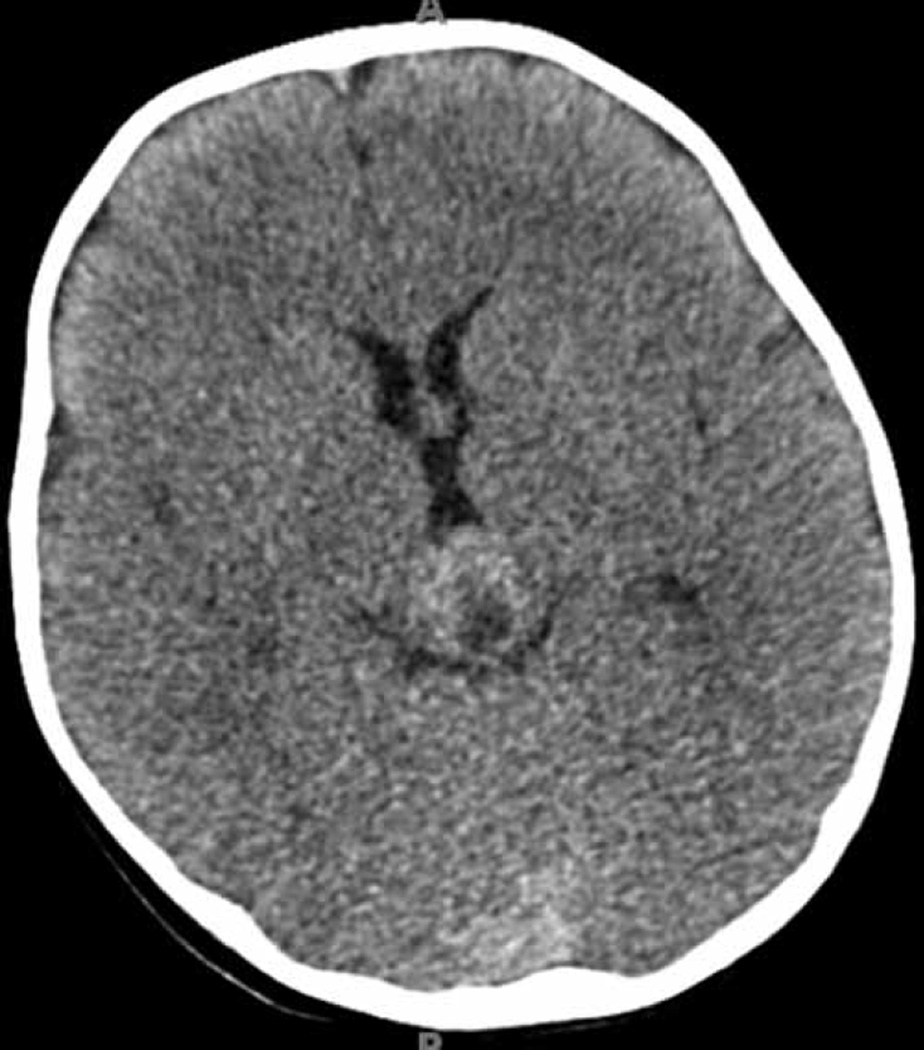
Fig. 1.
Axial CT scan demonstrating an iso- to slightly hyperdense mass with a cystic portion. This mass was initially thought to be arising from the tectum; however, it was later found to be a papillary tumor of the pineal region.
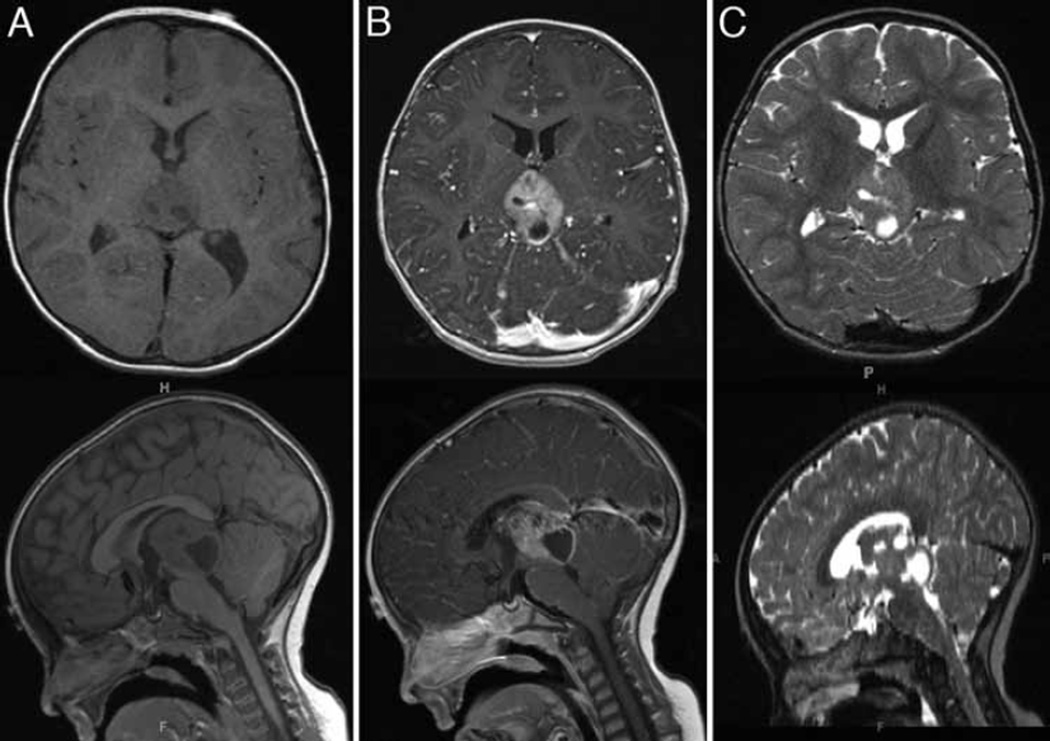
Fig. 2.
Axial and sagittal MR imaging views of a mixed solid and cystic component mass arising from the pineal region. It is isointense on the T1-weighted sequences (A), with strong contrast enhancement (B). On T2-weighted images, the solid portion of the mass is isointense, with the hyperintense cystic components (C). This mass was originally thought to be arising from the tectum or ependyma of the fourth ventricle.
Operation
The patient initially underwent a third ventriculostomy to treat the obstructive hydrocephalus. However, he continued to experience intermittent vomiting and abnormal eye movements. Serial MR imaging demonstrated increasing tumor size and enhancement over the course of 1 month, and the mass was better visualized as a pineal region tumor, extending into the posterior third ventricle. A neuraxis survey performed at this time was negative for drop metastases. Given the rapid tumor progression, the patient underwent a right frontal craniotomy via a transchoroidal approach for biopsy sampling and tumor debulking. A transfrontal, transchoroidal approach from the supplementary motor area region was selected over a posterior approach because the vein of Galen and internal cerebral veins were noted to be situated superiorly and dorsally. The tumor was very vascular, which limited the amount of resection, and required a significant amount of careful bipolar cauterization. Unfortunately, the tissue sent for specimen analysis did not yield a diagnosis.
The patient returned for a repeat biopsy procedure and further debulking via the same transcortical approach, and pathological evaluation of the tumor specimen revealed PTPR. The postoperative clinical course was complicated by obstructive hydrocephalus secondary to a blood clot in the cerebral aqueduct. The patient returned to the operating room for endoscopic removal of the clot and revision of the third ventriculostomy. Although he initially did well following this procedure, within weeks he again experienced deterioration in his neurological status and ultimately required a ventriculoperitoneal shunt to treat the hydrocephalus.
Pathological Findings
Examination of H & E–stained sections showed a cellular neoplasm demonstrating both solid and papillary growth patterns (Fig. 3A and B). The papillary regions were composed primarily of perivascular pseudorosettes, with no true rosettes identified. The nuclei showed moderate to severe pleomorphism, with dense hyperchromatic chromatin. The cytoplasm was eosinophilic, with some areas showing vacuolization. Mitoses were identified at approximately 3 per 10 hpfs, and necrosis was evident. Immunohistochemical stains specific for S100 and cytokeratin 8 (clone CAM5.2) were strongly positive in the neoplastic cells (Fig. 3C and D). Immunohistochemical stains specific for EMA and GFAP were negative in the tumor. The MIB-1 rate was approximately 5%–8% in the tumor cells. Taken together, the morphological and immunohistochemical profiles were typical of papillary tumor of the pineal region.7,12
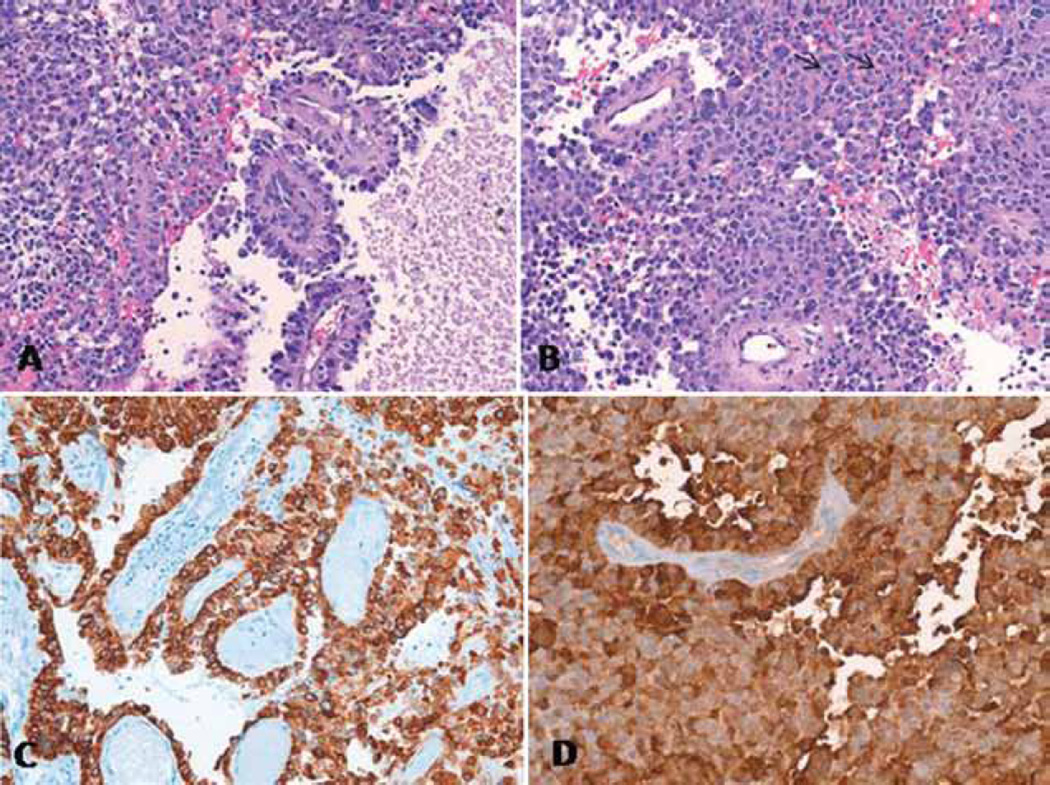
Fig. 3.
A: Photomicrograph of an H & E–stained section, original magnification ×100, showing a combination of solid and papillary architectures, with perivascular rosette formation. An area of necrosis is present. B: Photomicrograph of an H & E–stained section, original magnification ×100, showing a solid tumor area with slightly vacuolated cytoplasm and multiple mitoses (black arrows). C: Photomicrograph of an immunoperoxidase-stained section, original magnification × 64, showing strong immunoreactivity for cytokeratin 8 (clone CAM5.2). D: Photomicrograph of an immunoperoxidase-stained section, original magnification × 100, showing strong immunoreactivity for S100.
Postoperative Course
In light of the diagnosis of PTPR and continued tumor growth on follow-up imaging, the patient began an individualized chemotherapy regimen consisting of vincristine, methotrexate, etoposide, cyclophosphamide, and cisplatin. After the second round of chemotherapy, his mother noted that he had increased fullness surrounding his craniotomy site, prompting readmission for evaluation. A head CT scan performed at that time demonstrated an increase in the cystic portion of the tumor. Brain MR imaging confirmed an increase of the cystic component, with associated brainstem compression, and noted a decrease in the solid component of the tumor (Fig. 4). The patient underwent a third debulking surgery that involved fenestration of the recurrent cystic tumor components. Despite treatment with chemotherapy, the tumor was noted to be very vascular during each surgical intervention. At the time of this report, 15 months after his initial presentation, the patient’s MR imaging continues to show a residual PTPR, albeit with a decrease in the size of nodular and cystic components (Fig. 5). He has completed a total of 4 cycles of chemotherapy and 6 weeks of proton-beam radiation to the residual tumor. Neurologically, he has a residual partial left third-nerve palsy, and has otherwise been clinically stable.
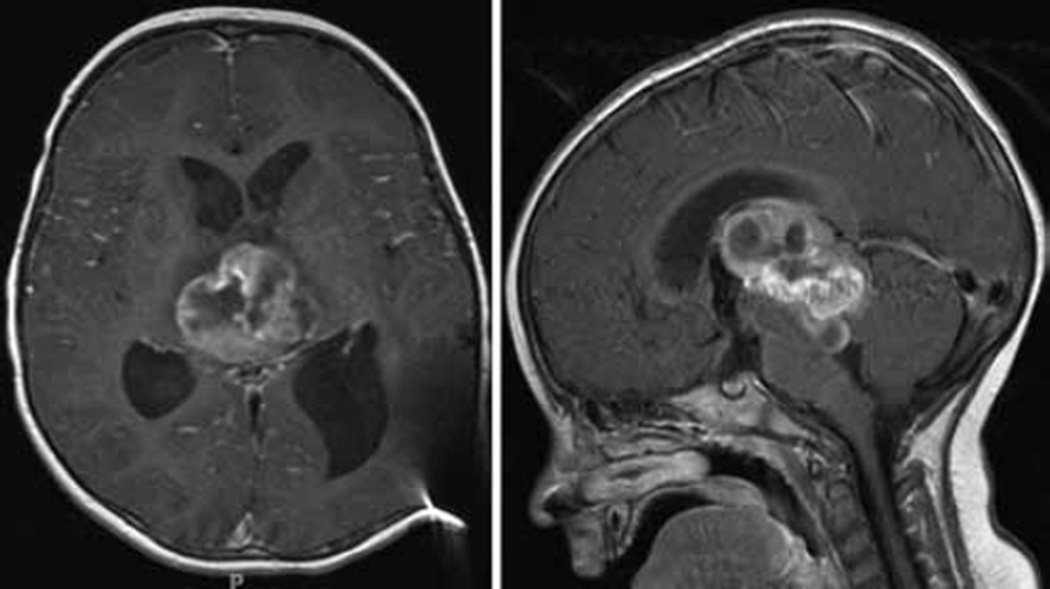
Fig. 4.
Axial and sagittal T1-weighted MR imaging studies obtained after addition of contrast material, prior to the third debulking surgery. A significant increase in the cystic component of the tumor, resulting in brainstem compression, prompted repeat surgical intervention. Despite previous treatment with chemotherapy, the tumor was still highly vascular, as was noted during the first 2 debulking procedures.
Fig. 5.
Axial and sagittal T1-weighted MR images obtained after addition of contrast material at follow-up more than 15 months after the patient’s original presentation. A residual tumor portion is seen, with an overall decrease in the size of the nodular and cystic components.
Discussion
Tumors of the pineal region account for approximately 1% of all intracranial tumors9,16 and 3%–4% of pediatric intracranial tumors.3,6 The differential diagnosis of pineal region tumors is quite diverse, and includes pineal parenchymal tumors, brainstem gliomas, meningiomas, arachnoid cysts, germ cell tumors, ependymomas, and metastases.3,10 A relatively new entity to be included in this differential list is the PTPR, which was first reported in 2003 by Jouvet et al.12 in a series of 6 cases. Since the initial description there have been more than 50 reported cases of PTPR. A review that summarizes the literature on PTPR has recently been published by Cardenas et al.4 The age at diagnosis has ranged from 5 years to 66 years, with most tumors arising in young adulthood (mean patient age 32 years), with no predilection for either sex.14,16 Five cases of PTPR have thus far been reported in children younger than 16 years of age.7 Our report describes the youngest patient to present with PTPR, and is only the second case in a patient younger than 10 years of age.7
The PTPRs are thought to arise from the specialized ependymal cells of the subcommissural organ, which is supported by the tumor’s location, immunohistochemical staining profile, and ultrastructural features.1 Histologically, PTPRs are characterized by certain shared features, including loose papillary and dense cellular areas exhibiting pseudorosettes, true rosettes, variable necrosis, and sometimes vacuolated cytoplasm.2,7,8,13 Mitoses vary from sparse to frequent.2 In their series of 31 cases, Fèvre-Montange et al.7 reported that all tumors were characterized by an epithelial growth–like pattern in which the vessels were covered by layers of columnar or cuboidal tumor cells, forming perivascular pseudorosettes. In terms of immunohistochemical profile, PTPRs are strongly positive for cytokeratins (particularly CK8 and CK18), neuron-specific enolase, S100 protein, and vimentin, as well as MAP-2. Expression of synaptophysin, EMA, transthyretin, neural cell adhesion molecule, and nestin was encountered in some tumors. The PTPR should be differentiated from other tumor types that are frequently found in the pineal region or that demonstrate papillary architecture, including pineal parenchymal tumors, choroid plexus tumors, papillary meningioma, medulloepithelioma, metastatic carcinoma, or germ cell neoplasm.12,16 Differentiating PTPR from papillary ependymoma can be particularly challenging, because both contain perivascular pseudorosettes and can show dotlike EMA positivity, which is indicative of ependymal differentiation, on immunohistochemical studies. In general, the two tumor types can be differentiated based on GFAP immunoreactivity, because GFAP is variably positive or weakly positive in PTPR, in contrast to ependymomas, which often show strong and diffuse positivity. Furthermore, MAP2 and CK18 immunoreactivity has been reported in PTPR, whereas these stains are not generally positive in ependymoma.8,15
Expansion of PTPRs typically results in clinical symptoms associated with obstructive hydrocephalus. Neurological examination may reveal symptoms including headache, diplopia, vomiting, and asthenia,2,5,16 as well as neurological signs including Argyll Robertson pupils, Parinaud sign, horizontal nystagmus, and essential tremor.2,3,11 In the current report, the patient presented first with ataxia and irritability, followed by emesis, fever, and diarrhea. The latter two symptoms were attributed to his Lyme disease and/or a viral illness, whereas the remaining symptoms were consistent with progressive obstructive hydrocephalus, as seen on neuroimaging.
Radiologically, PTPRs are mildly lobulated, partially cystic, heterogeneously enhancing masses, and they are usually associated with obstructive hydrocephalus.1,12 They are difficult to distinguish from other clinical entities, including pineocytoma.1 The T2-weighted MR imaging modality often reveals heterogeneous, hyperintense regions, whereas T1-weighted imaging often reveals low-to-intermediate intensities.1,11,17 In the current case, the tumor was originally thought to be a tectal glioma with secondary hydrocephalus, because the contrast-enhancing lesion appeared to arise directly from the tectum or the ependyma of the cerebral aqueduct. Only after the tumor demonstrated growth on routine imaging was it thought to be a pineal region tumor, which was later confirmed pathologically to be a PTPR.
The expected course for a PTPR is uncertain, and data on the prognosis of patients with this disease are scarce. In the 31 cases reported by Fèvre-Montange et al.,7 gross-total resection was achieved in 21, and 15 patients received radiotherapy after resection of the primary tumor. These authors reported estimates for 5-year overall survival and progression-free survival as 73% and 27%, respectively. In addition to a paucity of information regarding clinical course, appropriate treatment for PTPRs remains unclear. Several studies have reported differing combinations of surgery, chemotherapy, and radiation; radiotherapy is often necessary due to the high risk of local recurrence.2,5,7,12 In this case, similar to previous reports, our patient had a slow-growing tumor complicated by local recurrence following initial therapy. To date, our patient has undergone a total of 3 debulking surgeries, 4 rounds of chemotherapy, and proton-beam radiation therapy for recurrent PTPR.
Conclusions
In this study we present a case of PTPR, which is a rare tumor of the CNS that had previously been reported in patients ranging from 5 to 66 years of age. Our description of PTPR in a 15-month-old boy represents the youngest patient in whom a case has been reported to date. As a relatively novel clinicopathological entity, PTPR must be included in the differential diagnosis of pineal region masses and considered in all age groups of patients with pineal region tumors.
Abbreviations used in this paper
- EMA
epithelial membrane antigen
- GFAP
glial fibrillary acidic protein
- PTPR
papillary tumor of the pineal region.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: VR Recinos, PF Recinos, Jallo. Acquisition of data: Li, PF Recinos, Orr. Analysis and interpretation of data: PF Recinos, Burger, Orr. Drafting the article: Li, PF Recinos, Orr. Critically revising the article: all authors. Reviewed final version of the manuscript and approved it for submission: all authors. Administrative/technical/material support: Burger, Orr. Study supervision: VR Recinos, Jallo.
References
- 1.Amemiya S, Shibahara J, Aoki S, Takao H, Ohtomo K. Recently established entities of central nervous system tumors: review of radiological findings. J Comput Assist Tomogr. 2008;32:279–285. doi: 10.1097/RCT.0b013e31814ce981. [DOI] [PubMed] [Google Scholar]
- 2.Boco T, Aalaei S, Musacchio M, Byrne R, Cochran E. Papillary tumor of the pineal region. Neuropathology. 2008;28:87–92. doi: 10.1111/j.1440-1789.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 3.Buffenoir K, Rigoard P, Wager M, Ferrand S, Coulon A, Blanc JL, et al. Papillary tumor of the pineal region in a child: case report and review of the literature. Childs Nerv Syst. 2008;24:379–384. doi: 10.1007/s00381-007-0500-9. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas R, Javalkar V, Haydel J, Wadhwa R, Fowler M, Scheithauer B, et al. Papillary tumor of pineal region: prolonged control rate after gamma knife radiosurgery—a case report and review of literature. Neurol India. 2010;58:471–476. doi: 10.4103/0028-3886.66051. [DOI] [PubMed] [Google Scholar]
- 5.Coello AF, Torres A, Acebes JJ, Boluda S. Papillary tumor of the pineal region. Neurology. 2009;73:486. doi: 10.1212/WNL.0b013e3181b16637. [DOI] [PubMed] [Google Scholar]
- 6.Drummond KJ, Rosenfeld JV. Pineal region tumours in childhood. A 30-year experience. Childs Nerv Syst. 1999;15:119–127. doi: 10.1007/s003810050347. [DOI] [PubMed] [Google Scholar]
- 7.Fèvre-Montange M, Hasselblatt M, Figarella-Branger D, Chauveinc L, Champier J, Saint-Pierre G, et al. Prognosis and histopathologic features in papillary tumors of the pineal region: a retrospective multicenter study of 31 cases. J Neuropathol Exp Neurol. 2006;65:1004–1011. doi: 10.1097/01.jnen.0000240462.80263.13. [DOI] [PubMed] [Google Scholar]
- 8.Hasselblatt M, Blümcke I, Jeibmann A, Rickert CH, Jouvet A, van de Nes JA, et al. Immunohistochemical profile and chromosomal imbalances in papillary tumours of the pineal region. Neuropathol Appl Neurobiol. 2006;32:278–283. doi: 10.1111/j.1365-2990.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 9.Herrick MK, Rubinstein LJ. The cytological differentiating potential of pineal parenchymal neoplasms (true pinealomas). A clinicopathological study of 28 tumours. Brain. 1979;102:289–320. doi: 10.1093/brain/102.2.289. [DOI] [PubMed] [Google Scholar]
- 10.Hirato J, Nakazato Y. Pathology of pineal region tumors. J Neurooncol. 2001;54:239–249. doi: 10.1023/a:1012721723387. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Kumabe T, Kanamori M, Sonoda Y, Watanabe M, Tominaga T. Papillary tumor of the pineal region: a case report. Brain Tumor Pathol. 2008;25:85–90. doi: 10.1007/s10014-008-0231-y. [DOI] [PubMed] [Google Scholar]
- 12.Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A, et al. Papillary tumor of the pineal region. Am J Surg Pathol. 2003;27:505–512. doi: 10.1097/00000478-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Kuchelmeister K, Hügens-Penzel M, Jödicke A, Schachenmayr W. Papillary tumour of the pineal region: histodiagnostic considerations. Neuropathol Appl Neurobiol. 2006;32:203–208. doi: 10.1111/j.1365-2990.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakazato Y. Revised WHO classification of brain tumours. Brain Nerve. 2008;60:59–77. (Jpn) [PubMed] [Google Scholar]
- 16.Sharma MC, Jain D, Sarkar C, Suri V, Garg A, Sharma BS, et al. Papillary tumor of the pineal region—a recently described entity: a report of three cases and review of the literature. Clin Neuropathol. 2009;28:295–302. doi: 10.5414/npp28295. [DOI] [PubMed] [Google Scholar]
- 17.Shibahara J, Todo T, Morita A, Mori H, Aoki S, Fukayama M. Papillary neuroepithelial tumor of the pineal region. A case report. Acta Neuropathol. 2004;108:337–340. doi: 10.1007/s00401-004-0898-z. [DOI] [PubMed] [Google Scholar]