Abstract
BACKGROUND
Resuscitation with blood products instead of crystalloid in the treatment of hemorrhagic shock has been associated with improved outcomes in trauma patients requiring massive transfusions and transfusion of fresh products results in reduced morbidity and mortality compared with aged blood. Processes to eliminate harmful components of aged blood are under investigation. We hypothesized that washing blood would reduce levels of proinflammatory mediators in stored units, and resuscitation with washed units would attenuate the proinflammatory response in mice after hemorrhagic shock.
METHODS
Mice underwent pressure-controlled hemorrhage and resuscitation with fresh packed red blood cells (pRBCs) or 15-day-old washed or unwashed pRBCs. Cytokine concentrations in donor samples and recipient serum were measured. In addition, cytokine concentrations were measured in 15-day-old units that underwent three interval washes versus one poststorage wash.
RESULTS
Blood stored for 15 days demonstrated increased levels of interleukin lα, keratinocyte chemoattractant, macrophage inflammatory protein 1α, and macrophage inflammatory protein 2 compared with fresh units. Washing 15-day-old pRBCs reduced concentrations of these cytokines. Cytokine levels in stored units that underwent multiple washes versus a single wash were not different. Mice resuscitated with 15-day-old unwashed pRBCs had increased levels of serum cytokines compared with mice resuscitated with fresh and 15-day-old washed pRBCs.
CONCLUSION
Aged pRBC units have elevated levels of proinflammatory cytokines compared with fresh units, and washing aged units after storage reduces cytokine concentrations. Resuscitation with washed units blunts the proinflammatory response in mice after hemorrhage. Washing aged pRBCs may improve the safety profile of aged units and may result in improved outcomes in subjects after hemorrhagic shock and resuscitation.
Keywords: Hemorrhage, shock, resuscitation, blood products, inflammation
Hemorrhagic shock is the leading cause of preventable death after trauma in both civilian and military settings.1,2 As a result, there is increased interest in examining the effects of various resuscitation methods on patient outcomes. Recent data have suggested that the immediate use of blood products for resuscitation after hemorrhagic shock, called damage control resuscitation (DCR), has been associated with improved patient outcomes when compared with patients resuscitated by the traditional Advanced Trauma Life Support guidelines of crystalloid resuscitation followed by transfusion of blood products as needed based on hematologic parameters.3,4 Approximately 25% of activated trauma patients demonstrate evidence of severe coagulopathy at the time of presentation, which is an independent predictor of mortality.5,6 DCR strategies address the “lethal triad” of hypothermia, acidosis, and coagulopathy by immediately instituting the use of blood products for hemostatic resuscitation.7,8
Increased emphasis on the use of blood products for resuscitation has led to a significant amount of investigation into the effect of storage on blood products, specifically packed red blood cells (pRBCs). The US Food and Drug Administration standards define pRBC units with less than 75% RBC viability 24 hours after transfusion as unsuitable for transfusion.9 This change occurs at 42 days of storage. In addition to posttransfusion RBC viability, alterations in ATP, 2,3-diphosphoglycerate, nitric oxide, pH, and RBC morphology diminish the ability of RBCs to deliver oxygen.9–11 Collectively, the changes seen in stored pRBCs as they age are known as the “RBC storage lesion. ”
The red blood cell storage lesion may play a role in post transfusion complications.12 Recent cardiac surgical data suggest that the transfusion of pRBCs stored for greater than 14 days results in poorer outcomes, including prolonged intubation, renal failure, sepsis, and death.13 Retrospective studies of trauma patients transfused with blood stored for greater than 14 days have suggested that there are increased rates of nosocomial infections and multisystem organ failure.13,14 Additional data suggest that the age of blood transfused is an independent risk factor for the rate of morbidity and mortality in trauma patients.14–17 Investigations into the effect of aged RBC transfusions in other patient populations are ongoing and controversial; however, the relationship between transfusion of aged blood and poor outcomes in trauma patients is well established.18
Previous studies from our laboratory have examined the RBC storage lesion in mice compared with that in humans.19 Like human pRBCs, aged murine pRBCs prepared in the same storage solution as human pRBCs demonstrate an increase in lactate and potassium, a decrease in pH, morphologic changes, and an increased rate of hemolysis. Other biochemical and morphologic parameters not assessed in our previous studies may also contribute to the RBC’s ability to deliver oxygen at a cellular level. Changes in murine blood occur at an accelerated rate such that mouse blood aged to 15 days is approximately equivalent to 45-day-old human blood based on the parameters measured.19 In the present study, we used a murine model of blood banking and hemorrhagic shock to further evaluate the RBC storage lesion with regard to immunologic parameters. Further, we implemented a washing protocol to pRBCs to determine if washing before transfusion affected the acute proinflammatory response of mice after hemorrhagic shock and resuscitation.
MATERIALS AND METHODS
Animal Model
Male C57BL/6 mice weighing 22 g to 30 g were purchased from Harlan Laboratories (Indianapolis, Ind), fed standard laboratory diet and water ad libitum, and acclimated for I week in a climate-controlled room with a 12-hour light–12-hour dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Preparation of Storage Solutions
Citrate phosphate double dextrose (CP2D; 257.6 mmol/L of glucose, 105.0 mmol/L of citrate-citric acid, and 18.5 mmol/L of monosodium phosphate, pH 5.7) was prepared daily and used for anticoagulation. AS-3 (55.5 mmol/L of glucose, 70.1 mmol/L of sodium chloride, 20 mmol/L of sodium phosphate, 12 mmol/L of citric acid, and 2.2 mmol/L of adenine, pH 5.8) was used as medium for the storage of pRBCs.19
Blood Collection, Separation of Components, and Storage
Blood was collected and stored as described previously.19 Briefly, donor mice were anesthetized with intraperitoneal pentobarbital (0.1 mg/g body weight), and blood was collected via cardiac puncture into syringes pretreated with CP2D anticoagulant. CP2D was immediately added to whole blood in a ratio of 1:7, and blood was gently mixed to avoid cell lysis. Next, whole blood was centrifuged at 3000 rpm for 15 minutes at 4°C and the platelet poor plasma was discarded. AS-3 was added to the pRBC pellet and huffy coat in a 2:9 ratio using the original volume of whole blood to yield one unit of non-leukoreduced pRBCs. Samples were stored at 4°C and mixed daily for 15 days.
Washing pRBC Units
After 15 days of storage, half of the pRBC units underwent a washing protocol and the other half remained unwashed. To wash the pRBCs, each stored unit was diluted I:I (vol/vol) with AS-3. Next, samples were centrifuged at 3000 rpm for 15 minutes at 4°C. Supernatant was discarded and AS-3 was added to the pRBC pellet in 2:9 ratio using the original volume of whole blood. Fresh or unwashed pRBC units remained unwashed.
Hemorrhage and Resuscitation
Anesthetized mice underwent femoral cannulation and pressure-controlled hemorrhage as previously described.20 Briefly, after sterile preparation, the left femoral vessels were exposed and the artery was cannulated using a polyethylene catheter connected to a pressure transducer for continuous hemodynamic monitoring (Harvard Apparatus, Holliston, Mass). Body temperature was maintained by placing the mice on a 41°C circulating water blanket. After cannulation and a 10-minute period of equilibration, mice were hemorrhaged to a mean (SEM) systolic blood pressure (SBP) of 25 (5) mm Hg by withdrawing blood from the femoral catheter. After 60 minutes of hemorrhage, mice were resuscitated with fresh pRBCs, 15-day-old washed pRBCs, or 15-day-old unwashed pRBCs to a mean (SEM) SBP of 80 (5) mm Hg. Sham mice were cannulated but neither hemorrhaged nor resuscitated. After 20 minutes of resuscitation, mice were decannulated, allowed to recover, and killed after an additional 30 minutes.
Cytokine Analysis
Fresh and 15-day-old unwashed pRBC units were centrifuged at 5000 rpm for 10 minutes at 4°C, and the supernatant was analyzed using a multiplex enzyme-linked immunosorbent assay (ELISA; Quansys Biosciences, Logan, Utah) according to the manufacturer’s instructions. The multiplex ELISA contained a panel of 13 cytokines, including granulocyte macrophage colony-stimulating factor, interferon γ, interleukin 1α (IL-1α), IL-1β, IL-6, IL-10, keratinocyte chemoattractant (KC), macrophage-derived chemokine, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α (MIP-lα), MIP-2, RANTES, and tumor necrosis factor α. Included in the results are cytokine concentrations with differences that reached statistical significance. Washed blood samples underwent the washing protocol followed by centrifugation at 5,000 rpm for 10 minutes at 4°C, and the supernatant was collected for cytokine analysis.
Thirty minutes after hemorrhagic shock, resuscitation, and decannulation, blood from hemorrhaged mice was collected via cardiac puncture. Plasma samples were allowed to clot and were centrifuged at 5000 rpm for 10 minutes at 4°C to separate the serum from cellular components. A panel of proinflammatory cytokines was measured using multiplex ELISA.
Statistics
Results are reported as mean (SEM). Data were analyzed using analysis of variance with subsequent Student-Newman-Kuels test where appropriate to determine significance (p ≤ 0.05). Statistical analysis was performed using SigmaPlot 10 software (Systat Software, Chicago, Ill).
RESULTS
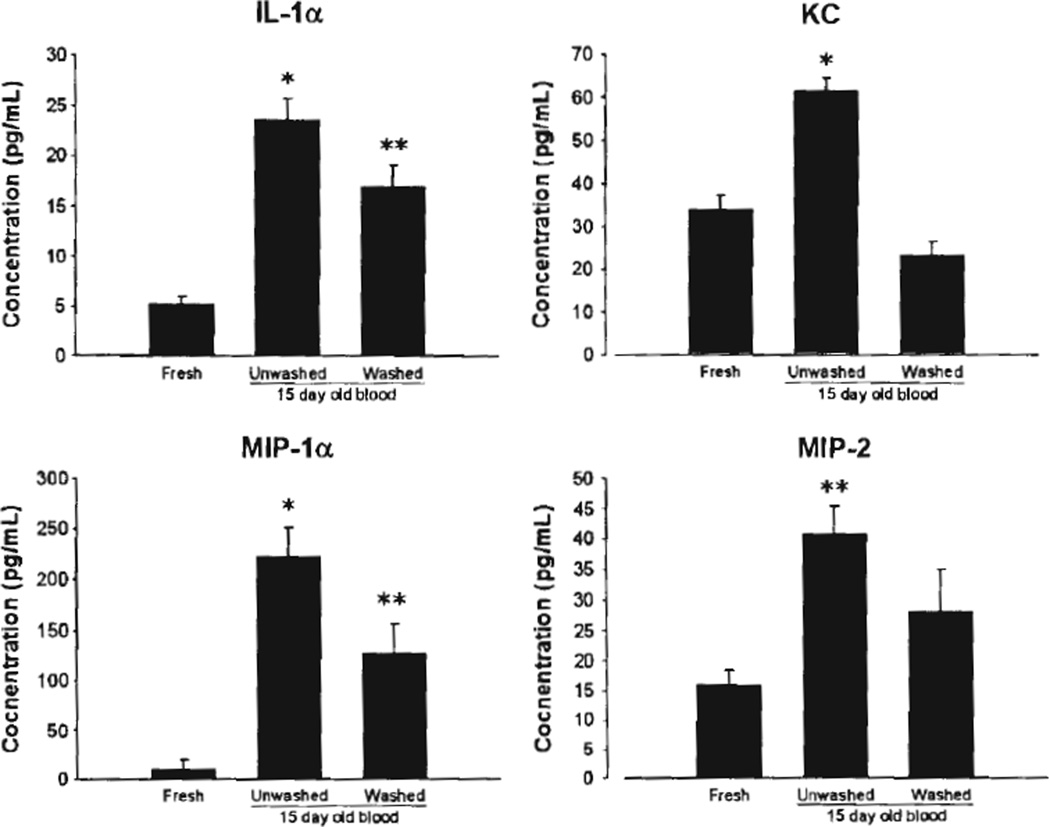
To determine the effect of storage on pRBC cytokine levels, we analyzed aliquots of stored blood by ELISA. The storage of mouse pRBCs for 15 days resulted in increased pRBC supernatant levels of IL-1α, KC, MIP-lα, and MIP-2 in aged as compared with freshly prepared pRBC units (Fig. 1). Washing pRBCs before analysis resulted in decreased proinflammatory mediator concentrations in the pRBC supernatant (Fig. 1).
Figure 1.
Cytokine concentrations in donor pRBC samples. *p < 0.05 versus other groups, **p < 0.05 versus fresh blood, n = 4 samples for each group.
We then examined whether additional interval washing would lead to further decreases in proinflammatory mediators in aged pRBC units. Units of pRBCs were stored and washed either every 5 days (three total washes) or once, at day 15. ELISA for cytokines in the supernatant was then performed. However, there were no significant differences in cytokine concentrations between aged units that underwent multiple washes versus one wash (Table 1). These data suggested that the addition of multiple washes to the protocol did not significantly reduce proflammatory mediators in the aged pRBC units.
TABLE 1.
Cytokine Concentrations in pRBC Samples After Zero, One, or Three Washes
| Group | IL-1α | KC | MIP-1α | MIP-2 |
|---|---|---|---|---|
| Unwashed | 45.94 (20.36) | 221.9 (135.98) | 80.05 (26.23) | 35.2 (11.82) |
| One wash | 10.97 (0) | 94.22 (76.23) | 49.14 (24.82) | 38.75 (10.15) |
| Three washes | 16.73 (5.76) | 13.55 (3.84)* | 27.33 (1.175) | 22.14 (6.47) |
Values are presented as mean (SEM).
p < 0.03 versus 1 wash, n = 5 samples for each group.
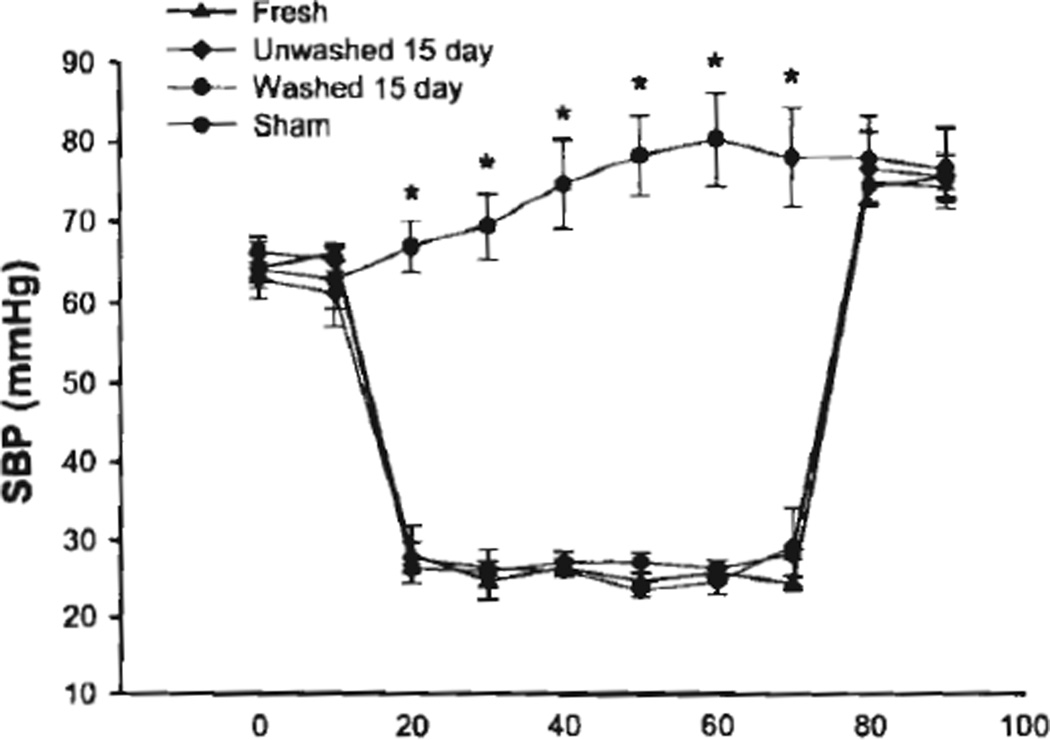
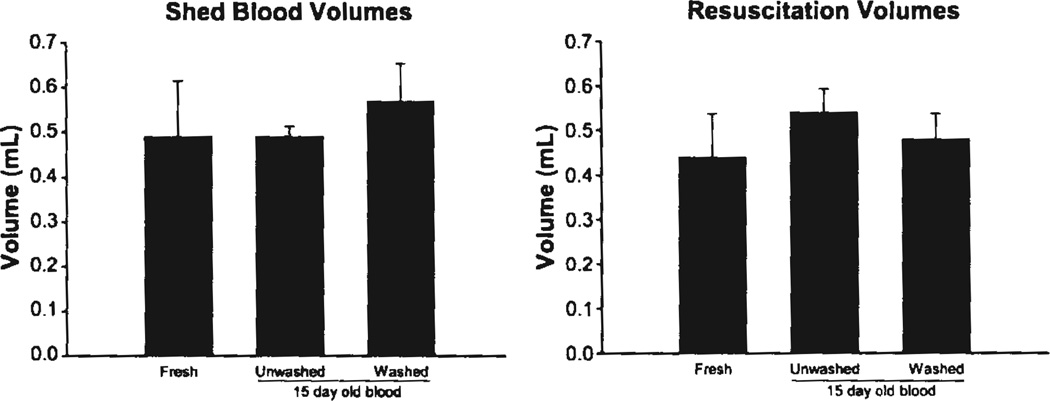
To investigate the effect of using aged pRBC units in resuscitation from hemorrhage, we subjected mice to hemorrhagic shock, then resuscitated them with freshly prepared pRBC units, or washed or unwashed aged pRBC units. SBP during the hemorrhagic shock and resuscitation periods did not differ between resuscitation groups. (Fig. 2) In addition, shed blood volumes and volume of transfusion required for resuscitation were not statistically different between resuscitation groups (Fig. 3).
Figure 2.
SBP during of mice undergoing hemorrhagic shock and resuscitation versus sham mice. *p < 0.001 versus hemorrhage group, n = 5 mice for each group.
Figure 3.
Shed blood and resuscitation volumes for mice undergoing hemorrhage and resuscitation, n = 5 mice for each group.
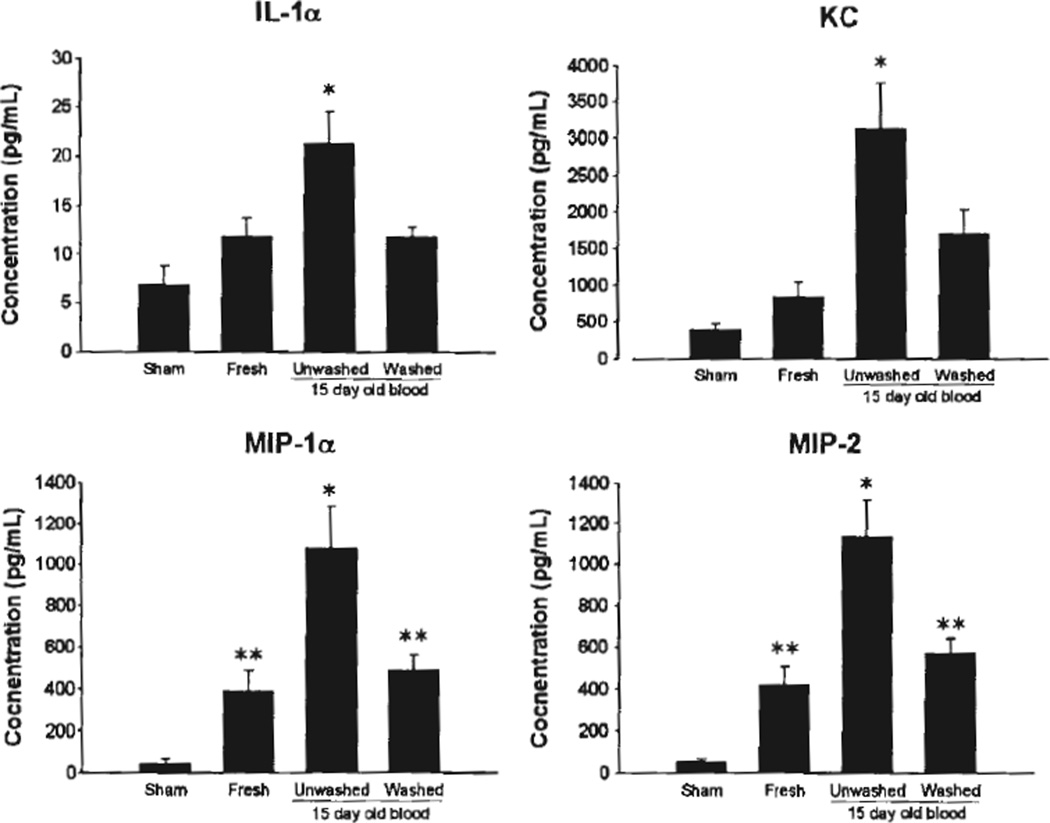
After hemorrhage and resuscitation with fresh pRBCs, 15-day-old washed pRBCs, or 15-day-old unwashed pRBCs, mice were killed and their serum was harvested for cytokine analysis. Mice resuscitated with 15-day-old unwashed pRBCs demonstrated a significant increase in serum IL-1α, KC, MIP-1α, and MIP-2 when compared with sham-injured animals and those resuscitated with fresh blood and 15-day-old washed pRBCs (Fig. 4). There was no significant difference in serum cytokines between mice resuscitated with fresh blood compared with those resuscitated with 15-day-old washed blood. There was a statistically significant difference in serum concentrations of MIP-1α and MIP-2 between sham-injured mice and hemorrhaged mice regardless of resuscitation strategy. Serum concentrations of KC and IL-1α were not different between sham-injured mice and mice resuscitated with fresh pRBCs or 15-day-old washed pRBCs (Fig. 4).
Figure 4.
Cytokine concentrations in recipient serum after hemorrhage and resuscitation. *p < 0.05 versus other groups, **p < 0.05 versus sham, n = 5 for each group.
DISCUSSION
In the present study, we used a murine model of blood banking to evaluate the effect of washing on the presence of inflammatory mediators in aged pRBCs. In addition, we used a pressure-controlled model of hemorrhagic shock and resuscitation to determine if washing aged pRBCs attenuated the proinflammatory effects of hemorrhagic shock and resuscitation with aged blood.
Using cytokine concentrations as an immunologic parameter, we determined that aged pRBCs have a significantly higher concentration of proinflammatory cytokines when compared with fresh pRBCs. Upon poststorage washing of 15-day-old pRBC units, cytokine concentrations were significantly reduced when compared with 15-day-old unwashed units. The institution of a more aggressive washing protocol involving multiple washes demonstrated no difference between three washes at various storage intervals versus a single poststorage wash. Serum cytokine concentrations of mice after hemorrhagic shock and resuscitation with unwashed 15-day-old pRBCs were significantly increased compared with mice resuscitated with fresh pRBCs. Resuscitation with washed 15-day-old pRBCs attenuated the proinflammatory response in recipients. The host inflammatory response may be a result of direct transfer of cytokines and subsequent activation of the inflammatory response or a result of direct transfer alone. Host cytokine concentrations were significantly elevated over donor pRBC cytokine levels, suggesting that there is activation of the host inflammatory response in addition to direct transfer of cytokines from donor to recipient. Serum cytokine concentrations in mice resuscitated with 15-day-old washed pRBCs were equivalent to those of mice resuscitated with fresh pRBCs.
The red blood storage lesion is complex and poorly understood.11 Changes that occur with storage include decreased RBC viability, RBC aggregation, RBC adhesion, and procoagulant effects as well as altered ATP levels, 2,3-diphosphoglycerate, nitric oxide, pH, and RBC morphology. Our data suggest that effects of the RBC storage lesion on the recipient are, at least in part, mediated by the supernatant of stored RBC units. Importantly, it appears that the altered RBC morphology seen during storage does not mediate the inflammatory response in recipients.
Our murine model of blood banking, pressure-controlled hemorrhagic shock, and resuscitation provides a useful method of evaluating aged blood products and their effects on recipients after hemorrhage. Resuscitation with aged blood results in an acute proinflammatory response, which may be part of the mechanism behind the poorer outcomes seen in trauma patients transfused with aged blood compared with those resuscitated with fresh blood products. Our results suggest that washing pRBC units alters the inflammatory response to transfusion of aged blood. Thus, washing aged pRBCs could be useful to improve the safety profile of blood products being used in the treatment of hemorrhagic shock.
In addition to data linking aged blood transfusions with worse outcomes as well as the limited availability of fresh blood products, scientists and clinicians have become interested in methods of improving the shelf life of stored blood products as well as eliminating harmful components in stored blood. Although we did not examine the leukoreduction in the current study, it represents a method used by many blood banks to decrease potentially harmful components of stored blood products. Nineteen countries including Canada, France, and the United Kingdom require leukoreduction of all blood products either before or after storage. The United States and Japan do not practice universal leukoreduction.21, 22 The evidence to support leukoreduction is mixed and suggests that leukoreduction has no effect on clinical outcomes in certain patient populations, including trauma patients.22–25 Furthermore, leukoreduction is expensive.26 Because of conflicting data supporting its efficacy concerning patient outcomes and significant difference in cost, further studies are required before leukoreduction will be required worldwide.
In addition to leukoreduction, other methods of improving the safety profile of blood products have been attempted, including washing blood products before transfusion. Blumberg et al27 evaluated the effect of washing leukoreduced pRBCs and platelets before transfusion into adult patients with acute leukemia. Patients receiving unwashed, leukoreduced blood products had a higher rate of transfusion reactions, and survival was inferior to the group receiving washed blood products. In addition, pediatric critical care data suggest that the transfusion of washed pRBCs and platelets in cardiac surgical patients is associated with decreased systemic inflammation and a trend toward reduced mortality postoperatively.28 The present study extends these findings. Our data suggest that washing decreased proinflammatory mediator levels in stored pRBCs, which, in turn, led to decreased systemic inflammation when used to resuscitate hemorrhaged mice.
Currently, RBC transfusion remains an important and critical therapy used in the setting of hemorrhagic shock. Given recent data demonstrating improved patient outcomes using DCR strategies after hemorrhage, much interest has been directed toward modulating the RBC storage lesion and optimizing the safety and efficacy profiles of blood products. The washing protocol described in this study is a technically simple and rapid method of reducing the levels of proinflammatory cytokines in aged pRBCs that appear to be responsible, in part, for the inflammatory response seen in mice after hemorrhagic shock and resuscitation. Future studies should include the effect of the inflammatory response after resuscitation with washed and unwashed units on organ systems including pulmonary function and lung injury.
Acknowledgments
DISCLOSURE
This work was supported by the US Air Force (grant no. FA8650-10-2-6801) and the National Institutes of Health (grants no. GM08478 to RMB and GM088589 to TAP).
Footnotes
This study was presented as a poster at the Advanced Technology Applications for Combat Casualty Care Conference in Fort Lauderdale, Florida, August 15–18, 2011.
AUTHORSHIP
R.M.B., A.T.M., E.C.M., D.I.S., M.D.G., W.C.D., A.B.L., and T.A.P. designed this study. R.M.B., A.T.M., M.D.G., A.B.L., and T.A.P. conducted the literature search. R.M.B., A.T.M.,. E.M.C., D.I.S., M.D.G., and L.A.F. collected the data. R.M.B., A.T.M., E.M.C., D.I.S., M.D.G., W.C.D., L.A.F., A.B.L., and T.A.P. analyzed the data. R.M.B., A.T.M., W.C.D., A.B.L., and T.A.P. interpreted the data. R.M.B., A.T.M., A.B.L., and T.A.P. handled the statistical analysis. R.M.B., A.T.M., A.B.L., and T.A.P. were responsible for manuscript preparation and revision.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66:S69–S76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 4.Duchesne JC, Barbeau JM, Islam TM, Wahl G, Greiffenstein P, McSwain NE., Jr Damage control resuscitation: from emergency department to the operating room. Am Surg. 2011;77:201–206. doi: 10.1177/000313481107700222. [DOI] [PubMed] [Google Scholar]
- 5.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 6.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 7.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 8.Sihler KC, Napolitano LM. Massive transfusion: new insights. Chest. 2009;136:1654–1667. doi: 10.1378/chest.09-0251. [DOI] [PubMed] [Google Scholar]
- 9.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 10.Kor DJ, Van Buskirk CM, Gajic O. Red blood cell storage lesion. Bosn J Basic Med Sci. 2009;9:21–27. doi: 10.17305/bjbms.2009.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’ Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–88. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. 8:s26–s30. doi: 10.2450/2010.005S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- 14.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriell J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., 3rd Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavenski K, Saidenberg E, Lavoie M, Tokessy M, Branch DR. Red blood cell storage lesions and related transfusion issues: a Canadian blood services research and development symposium. Transfus Med Rev. 2012;26:68–84. doi: 10.1016/j.tmrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–45. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makley AT, Goodman MD, Friend LA, Deters JS, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. 2010;68:305–311. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371–379. [PubMed] [Google Scholar]
- 22.Fergusson D, Hebert PC, Barrington KJ, Shapiro SH. Effectiveness of WBC reduction in neonates: what is the evidence of benefit? Transfusion. 2002;42:159–165. doi: 10.1046/j.1537-2995.2002.00022.x. [DOI] [PubMed] [Google Scholar]
- 23.Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oneal Stem Cell Ther. 2008;1:106–123. doi: 10.1016/s1658-3876(08)50042-2. [DOI] [PubMed] [Google Scholar]
- 24.Englehart MS, Morris MS, Gee AC, Riha G, Underwood SJ, Differding JA, Luem ND, Wiesberg TT, Boshkov LK, Schreiber MA. Use of leukoreduced blood does not reduce infection, organ failure, or mortality following trauma. World J Surg. 2009;33:1626–1632. doi: 10.1007/s00268-009-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelan HA, Sperry JL, Friese RS. Leukoreduction before red blood cell transfusion has no impact on mortality in trauma patients. J Surg Res. 2007;138:32–36. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro MJ. To filter blood or universal leukoreduction: what is the answer? Crit Care. 2004;8:S27–S30. doi: 10.1186/cc2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, Swartz M, Gensini F, Daugherty LE, Nazarian E, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2012;13:290–299. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]