Abstract
Objective
To investigate whether pre-existing diabetes modifies racial disparities in colorectal cancer (CRC) survival.
Research Design and Methods
We analyzed prospective data from 16,977 patients (age≥67 years) with CRC from the Surveillance Epidemiology and End Results (SEER)-Medicare database. SEER registries included data on demographics, tumor characteristics, and treatment. Medicare claims were used to define pre-existing diabetes and comorbid conditions. Mortality was confirmed in both sources.
Results
At baseline, 1,332 (8%) were African-Americans and 26% had diabetes (39% in blacks; 25% in whites). From 2000 to 2005, more than half of the participants died (N=8,782, 52%). This included 820 (62%) deaths (23.8 per 100 per-years) among blacks, and 7,962 (51%) deaths (16.6 per 100 person-years) among whites. Among older adults with diabetes, blacks had significantly higher risk of all-cause and CRC mortality after adjustments for demographic characteristics, [hazard ratio (HR), 95% confidence interval (CI): 1.21 (1.08–1.37) and 1.21 (1.03–1.42)], respectively, but these associations attenuated to null after additional adjustments for cancer stage and grade. Among adults without diabetes, the risk of all-cause mortality [HR (95% CI): 1.14 (1.04–1.25)] and CRC mortality [HR (95% CI): 1.21 (1.08–1.36)] remained higher in blacks than whites in fully-adjusted models that included demographic variables, cancer stage, grade, treatments, and co-morbidities.
Conclusions
Among older adults with CRC, diabetes is an effect modifier on the relationship between race and mortality. Racial disparities in survival were explained by demographics, cancer stage and grade in patients with diabetes.
Keywords: Outcome, colorectal cancer, diabetes
BACKGROUND
Racial disparities in survival from colorectal cancer have been reported in the literature with evidence suggesting that African-Americans have a higher mortality compared to Whites1–3. Although reasons for disparities in survival remain unclear, previous studies have suggested several factors, including differences in socioeconomic status4, cancer screening5, stage at diagnosis2, 6 and differences in cancer treatment7, potentially contributing to disparities.
Diabetes, a well-established risk factor for mortality, cardiovascular disease as well as other complications, disproportionately affects African-Americans compared to whites in the general population8. Several large cohort studies and meta-analyses have demonstrated that pre-existing diabetes is associated with increased risk of colorectal cancer as well as decreased survival after cancer diagnosis9–13. However, to our knowledge, no study has investigated the role of pre-existing diabetes in racial disparities of colorectal cancer survival. Therefore, we analyzed data from the Surveillance Epidemiology and End Results (SEER)-Medicare program to investigate whether pre-existing diabetes modifies racial disparities in colorectal cancer survival.
RESEARCH DESIGN AND METHODS
SEER database is an authoritative source of information on cancer incidence and survival in the United States, which currently collects cancer incidence and survival data from 17 population-based cancer registries covering approximately 26 percent of the US population14–16. The registries routinely collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment, and follow-up for vital status. Medicare claims data include inpatient hospitalizations, outpatient hospital or office visits and data from skilled nursing facility hospitalizations. It also includes data on ICD diagnostic codes, procedure codes, Healthcare Common Procedure Coding System (HCPCS codes), and dates of services. The SEER-Medicare linkage results in a unique population-based source of information that can be used for an array of epidemiological and health services research.
Study population
We identified 21,693 individuals age 67 years or older who were diagnosed with incident colorectal cancer in years 2000 and 2001 from SEER-Medicare database, and with continuous enrollment in the fee-for-service Medicare program two years prior to cancer diagnosis and through the study period up to 2005. We excluded individuals with race other than African-Americans or Whites (N=1,255), with other cancers (N=1,888), with stage 0 cancer according to the American Joint Committee on Cancers (AJCC) staging (N=308) or with missing data on any of the key variables (N=1,265). The final analysis included prospective data on 16,977 adults with colorectal cancer.
Assessment of Pre-existing Diabetes
We used Medicare claims data to identify pre-existing diabetes mellitus using the ICD-9 code 250.xx based on previously published reports17–21; anyone with one of these codes from inpatient hospitalization, outpatient or physician office visits within two years prior to cancer diagnosis was considered to have diabetes at baseline20. Although we did not attempt to differentiate type 1 from type 2 diabetes, we assumed that greater than 90% of cases were type 2 diabetes as in the general U.S. population22, 23.
Mortality Outcomes
Cause of death was recorded in SEER, while date of death was determined based on the Medicare database. The outcomes of interest were all-cause mortality (long-term), all-cause mortality within 90 days of cancer diagnosis (short-term), cardiovascular mortality and colorectal cancer-related mortality. Cardiovascular mortality included mortality due hypertensive disease (ICD-10: I10–I13), ischemic heart disease (ICD-10: I20–I25), arrhythmia (ICD-10: I44–I49), heart failure (ICD-10: I50), cerebrovascular disease (ICD-10: I60–I69), or atherosclerosis or other diseases of the arteries (ICD-10: I70–I78). Colorectal cancer mortality was defined using ICD-10 codes C18 to C21.In SEER-Medicare dataset, only month and year of colorectal cancer diagnosis were available while the day of diagnosis was not provided. Therefore, we assigned 15th of the month to every case.
Race and Covariates
Demographic characteristics including age at cancer diagnosis, race, gender, marital status, and median income in census track were available in SEER registries. Charlson comorbidity index was used as a composite measure of comorbidity based on prior published reports24. These comorbidities were based on ICD-9 discharge diagnostic codes from inpatient hospitalizations, outpatient hospital visits or physician office visits. Baseline co-morbidities were defined based on conditions recorded within two years prior to colorectal cancer diagnosis. For cancer-directed surgery and radiation, we used data from the SEER registries, while for chemotherapy therapy we used Medicare data with HCPCS and ICD-9 codes as used in a previous study25.
Statistical analysis
We first compared baseline characteristics of the study population by race (African-Americans vs. Whites) in both diabetes and non-diabetes groups. T-test and Chi square test were used for continuous and categorical variables respectively. We presented incidence rates per 100-person years. Follow up time was calculated using date of colorectal cancer diagnosis as the baseline time with end of follow up being date of death or end of follow up (December 31st, 2005), whichever occurred first. We then conducted survival analysis using Kaplan-Meier method with log rank tests to determine the significance of racial differences for each outcome. To test if there is a statistical interaction between race and diabetes for colorectal cancer survival, we first included the interaction term, race X diabetes, in the fully adjusted models. Given that we observed statistically significant interaction, we subsequently conducted series of multivariable Cox proportional hazards models comparing the associations in blacks with whites (reference category), stratified by diabetes status. Finally, we performed subsidiary analyses stratified by cancer stages to ensure our findings were robust.
Tests of significance were two-tailed, with alpha-level of 0.05. All statistical analyses were performed using Stata version 1227.
This study was reviewed by the Johns Hopkins Medicine Institutional Review Board, and was determined that the study does not involve human subjects research under the DHHS or FDA regulations. The National Cancer Institute approved the use of SEER-Medicare data for this study. The dataset was fully de-identified before its release to this study. The SEER-Medicare data are not public use data files. Investigators are required to obtain approval from National Cancer Institute in order to obtain the data28.
RESULTS
Baseline Characteristics
Of the 16977 colorectal cancer patients, 1,332 (8%) were African-Americans, 9,883 (58%) were females, and 26% had diabetes (39% in blacks compared to 25% in whites).Table 1 compares baseline characteristics of colorectal cancer cases in blacks vs. whites by diabetes status. Mean age at diagnosis of colorectal cancer was 79 years across all groups. In both patients with and without diabetes, blacks were less likely to be married and had a significantly lower census track median income compared to whites. Several comorbidities including heart failure and dementia were more common in diabetic-blacks as compared to their white counterparts; renal disease and hemiplegia were more common in blacks than whites for both diabetic and non-diabetic individuals. In those with diabetes, blacks had a higher comorbidity burden as indicated by a higher median [IQR] Charlson comorbidity Index (3.0 [2.0–4.0]) compared to whites (2.0 [2.0–3.0]), while there was no difference in the non-diabetes group.
Table 1.
Baseline Characteristics by race and baseline diabetes status
| Diabetes | No Diabetes | |||||
|---|---|---|---|---|---|---|
| Black | White | P-value | Black | White | P-value | |
| Demographics | ||||||
| Number | 518 | 3,927 | NA | 814 | 11,718 | NA |
| Mean age at diagnosis in years | 77.9 (6.9) | 78.2 (6.5) | 0.30 | 78.6 (7.1) | 79 (7.1) | 0.11 |
| Female % | 64.9 | 55.2 | <0.001 | 63.3 | 58.6 | 0.008 |
| Census tract median income (in thousands) | 29.7 [22.9–41.7] | 45.1 [34.4–60.1] | <0.001 | 30.5 [22.9–42.0] | 45.5 [34.8–61.5] | <0.001 |
| Married (%) | 31.9 | 45.9 | <0.001 | 30.0 | 45.7 | <0.001 |
| Metropolitan (%) | 88.8 | 82.2 | <0.001 | 88.9 | 79.6 | <0.001 |
| Comorbidity No. (%) | ||||||
| Acute myocardial infarction | 62 (12.0) | 526 (13.4) | 0.40 | 45 (5.5) | 842 (7.2) | 0.08 |
| Congestive heart failure | 180 (34.8) | 1184 (30.2) | 0.03 | 147 (18.1) | 1975 (16.9) | 0.38 |
| Peripheral vascular disease | 117 (22.6) | 754 (19.2) | 0.07 | 99 (12.2) | 1217 (10.4) | 0.11 |
| Cerebrovascular disease | 153 (29.5) | 1171 (29.8) | 0.90 | 155 (19.0) | 2155 (18.4) | 0.64 |
| Dementia | 52 (10.0) | 197 (5.0) | <0.001 | 44 (5.4) | 493 (4.2) | 0.10 |
| Chronic obstructive pulmonary disease | 169 (32.6) | 1328 (33.8) | 0.59 | 209 (25.7) | 3263 (27.9) | 0.18 |
| Rheumatoid (Connective tissue disease) | 37 (7.1) | 243 (6.2) | 0.40 | 33 (4.1) | 523 (4.5) | 0.58 |
| Peptic ulcer disease | 36 (7.0) | 272 (6.9) | 0.98 | 40 (4.9) | 566 (4.8) | 0.91 |
| Mild liver disease | 4 (0.8) | 39 (1.0) | 0.63 | 3 (0.4) | 73 (0.6) | 0.37 |
| Hemiplegia | 26 (5.0) | 96 (2.4) | 0.001 | 19 (2.3) | 152 (1.3) | 0.014 |
| Renal disease | 57 (11.0) | 224 (5.7) | <0.001 | 33 (4.1) | 295 (2.5) | 0.008 |
| Severe liver disease | 8 (1.54) | 15 (0.4) | 0.001 | 3 (0.4) | 24 (0.2) | 0.33 |
| Acquired immune deficiency syndrome | 2 (0.4) | 2 (0.05) | 0.017 | 1 (0.1) | 5 (0.04) | 0.31 |
| Median Charlson comorbidity index, [IQR] | 3.0 [2.0–4.0] | 2.0 [2.0–4.0] | 0.015 | 1.0 [0.0–2.0] | 1.0 [0.0–2.0] | 0.20 |
| AJCC Cancer Stage (%) | ||||||
| Stage 1 | 17.8 | 22.9 | <0.001 | 16.8 | 21.5 | <0.001 |
| Stage 2 | 26.3 | 27.9 | 25.7 | 28.8 | ||
| Stage 3 | 16.8 | 22.4 | 18.7 | 21.7 | ||
| Stage 4 | 18.9 | 13.3 | 19.9 | 14.0 | ||
| Un-staged | 20.3 | 13.6 | 18.9 | 14.0 | ||
| Cancer Grade (%) | ||||||
| Grade 1 | 6.4 | 8.8 | <0.001 | 6.8 | 8.3 | <0.001 |
| Grade 2 | 57.7 | 60.7 | 61.3 | 60.5 | ||
| Grade 3 | 15.1 | 18.1 | 13.0 | 18.8 | ||
| Grade 4 | 0.0 | 1.0 | 0.7 | 0.7 | ||
| Unknown | 20.9 | 11.4 | 18.2 | 11.7 | ||
| Cancer Treatment (%) | ||||||
| Surgery performed | 72.4 | 83.4 | <0.001 | 74.9 | 83.0 | <0.001 |
| Received chemotherapy | 24.4 | 29.4 | 0.01 | 27.0 | 32.2 | 0.001 |
| Received radiation | 6.8 | 6.7 | 0.82 | 7.6 | 7.9 | 0.14 |
All values expressed as mean (SD) or number (%) except if otherwise specified.
For tumor related variables, blacks were more likely to have stage 4 colorectal cancer or being un-staged compared to whites. We also observed a significant difference in cancer treatment between blacks and whites – whites were more likely to receive cancer-directed surgery or chemotherapy compared to blacks.
Mortality Rates
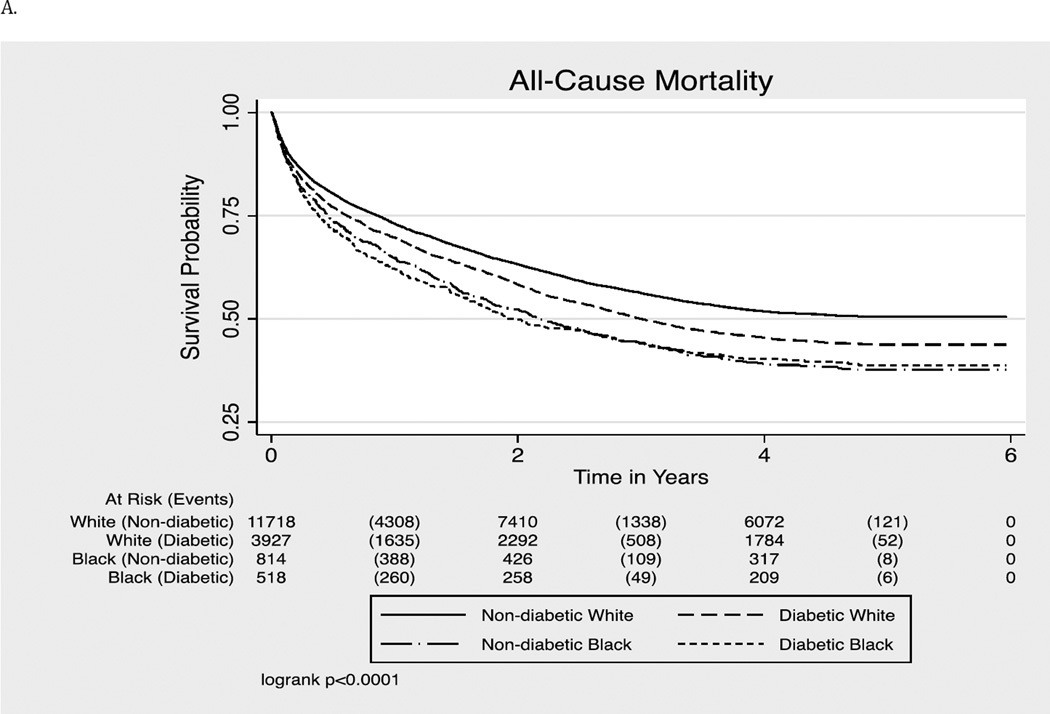
More than half of the participants died (N=8,782, 52%) during a median (highest) follow up of 3.8 (6.0) years. This included 820 (62%) deaths (23.8 per 100 per-years) among blacks, and 7,962 (51%) deaths (16.6 per 100 person-years) among whites. As shown in Table 2, in adults with and without diabetes, unadjusted incidence rates were higher in blacks than whites for all mortality outcomes.
Table 2.
Mortality rates (95% confidence intervals) by race with 95% confidence interval
| Diabetes | No Diabetes | |||
|---|---|---|---|---|
| Death (Person- Years) |
Mortality Rate (95% Confidence Interval) |
Death (Person- Years) |
Mortality Rate (95% Confidence Interval) |
|
| All-Cause Mortality | ||||
| White | 2195 (11225) | 19.55 (18.75, 20.38) | 5767 (35592) | 15.71 (15.31, 16.12) |
| Black | 315 (1314) | 23.87 (21.37, 26.66) | 505 (2136) | 23.71 (21.73, 25.87) |
| 90-day Mortality | ||||
| White | 636 (11225) | 5.67 (5.24, 6.12) | 1628 (35592) | 4.43 (4.23, 4.66) |
| Black | 99 (1314) | 7.5 (6.16, 9.13) | 149 (2136) | 6.99 (5.96, 8.21) |
| CVD-Mortality | ||||
| White | 456 (18070) | 2.53 (2.31, 2.78) | 904 (54757) | 1.63 (1.53, 1.74) |
| Black | 62 (2355) | 2.64 (2.06, 3.39) | 74 (3833) | 1.93 (1.54, 2.43) |
| Cancer-Mortality | ||||
| White | 1154 (15058) | 7.68 (7.25, 8.14) | 3552 (43806) | 7.93 (7.67, 8.19) |
| Black | 177 (1862) | 9.47 (8.17, 10.98) | 332 (2738) | 12.01 (10.79, 13.38) |
Time = Person time in years, Incidence rate = Crude incidence rates per 100-person years. CVD = Cardiovascular disease.
We conducted a series of Cox proportional hazards models by diabetes status to assess if diabetes modified the race-cancer survival associations. As shown in Table 3, demographic variables partially explained racial disparities in survival in both diabetic and non-diabetic groups. For long-term all-cause mortality, in those with pre-existing diabetes, after adjustments for cancer stage and grade, racial disparities attenuated to null. Additional adjustments for cancer treatment and comorbidities attenuated the association further. In contrast, in those without pre-existing diabetes, significant racial disparities persisted even after the adjustments for cancer stage, grade, cancer treatment and comorbidities. In the fully adjusted model, non-diabetic blacks had a 14.0% (HR 1.14, 95% CI: 1.04–1.25) higher risk of long-term mortality from all causes compared to non-diabetic whites.
Table 3.
Adjusted Hazard ratios (95% confidence interval) of mortality outcomes in blacks versus whites by diabetes status
| Diabetes | No Diabetes | |||||
|---|---|---|---|---|---|---|
| Model a | Model b | Model c | Model a | Model b | Model c | |
| All-Cause Mortality | 1.21 (1.08, 1.37) |
1.06 (0.94, 1.19) |
0.94 (0.83, 1.06) |
1.38 (1.26, 1.52) |
1.22 (1.11, 1.33) |
1.14 (1.04, 1.25) |
| 90-day Mortality | 1.22 (0.99, 1.52) |
0.94 (0.76, 1.17) |
0.80 (0.64, 0.99) |
1.34 (1.14, 1.59) |
1.15 (0.97, 1.37) |
1.01 (0.86, 1.20) |
| CVD Mortality | 1.04 (0.80, 1.37) |
1.08 (0.82, 1.41) |
0.98 (0.75, 1.29) |
1.16 (0.92, 1.48) |
1.18 (0.93, 1.5) |
1.14 (0.90, 1.45) |
| Colorectal Cancer Mortality | 1.21 (1.03, 1.42) |
0.97 (0.82, 1.14) |
0.96 (0.82, 1.14) |
1.42 (1.26, 1.59) |
1.23 (1.09, 1.37) |
1.21 (1.08, 1.36) |
Referent category is whites. All hazard ratios are for blacks compared to whites.
Model a: Adjusted for demographic characteristics [age at diagnosis (continuous), sex (male or female), marital status (married, unmarried or unknown), census track median income (log transformed), living in metropolitan city (yes or no)].
Model b: Model a + cancer stage (stage 1 (reference), 2, 3 and 4) and grade (grade 1 (reference), 2, 3 and 4)
Model c: Model b + surgery, chemotherapy, radiation and Charlson comorbidity index (used as a continuous variable with score ranging from 0–13)
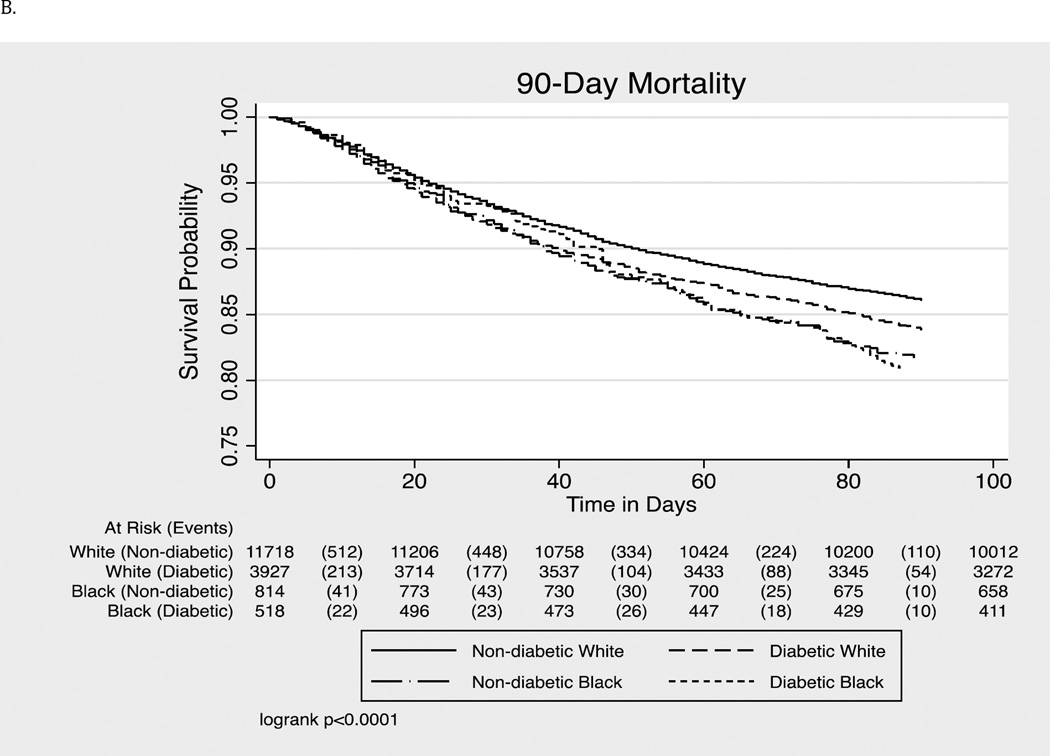
For mortality within 90 days of cancer diagnosis, among those with pre-existing diabetes, there was no significant racial difference after adjustment for the demographic variables. Among those without diabetes, racial disparities disappeared after adjustments for demographic characteristics, cancer stage, grade, and cancer treatment.
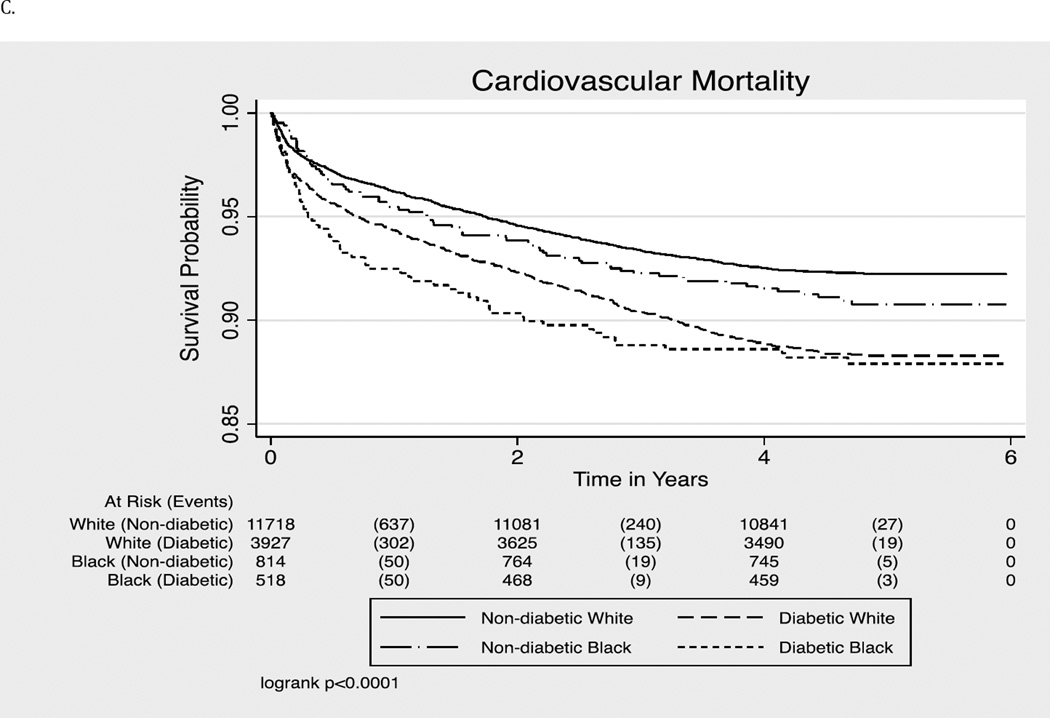
For cardiovascular mortality, we did not observe significant racial difference in adults with or without diabetes, although blacks had a higher mortality than whites in all models (Table 3).
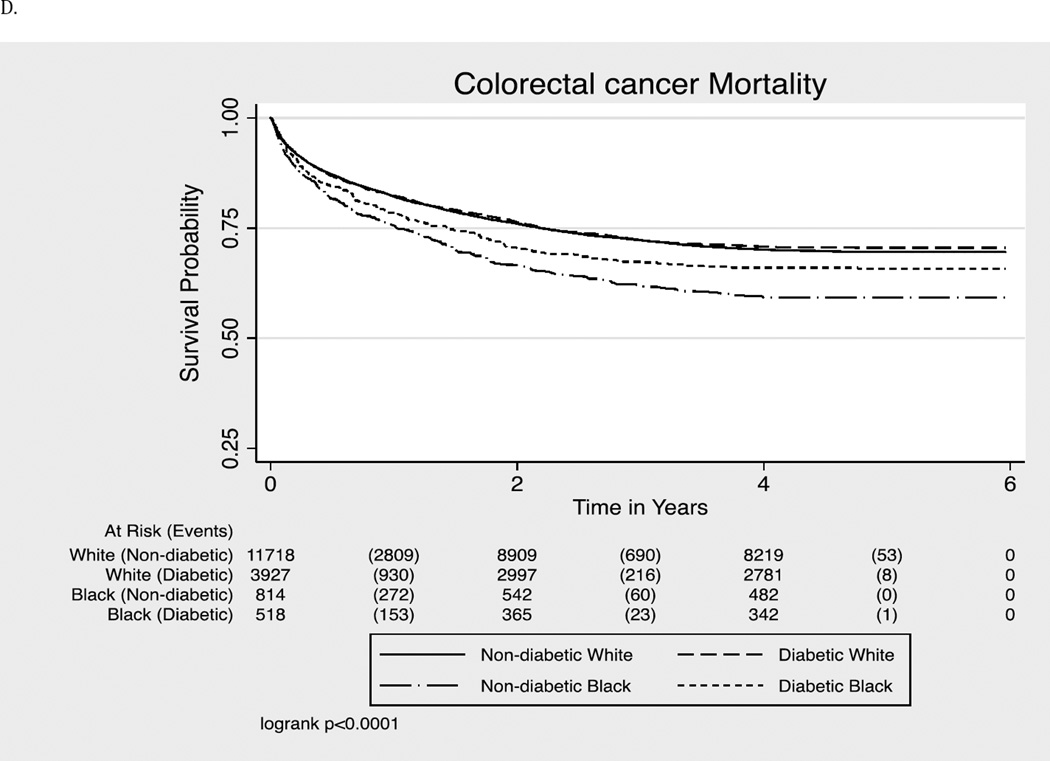
For colorectal cancer mortality, while no racial disparities were observed in individuals with diabetes, non-diabetic blacks had a higher risk compared to non-diabetic whites even after adjustment for all the covariates [HR (95% CI): 1.21 (1.08–1.36)]
For all mortality outcomes, advanced cancer stage and grade were the most significant predictors. We also confirmed that both cancer-directed surgery and chemotherapy were beneficial to survival (data not shown).
Subsidiary Analysis
In our subsidiary analyses stratified by cancer stage, our findings remained similar among patients diagnosed with stage 1, 2, or 3 colorectal cancers (n=14,549). In contrast, for patients diagnosed to have stage 4 cancer, no significant racial disparities were observed between blacks and whites for all mortality outcomes in older adults with or without pre-existing diabetes.
DISCUSSION
We confirmed that racial disparities exist in survival from colorectal cancer between blacks and whites1–3. In this study, we found pre-existing diabetes is an effect modifier on the relationship between race and colorectal cancer survival. In those with pre-existing diabetes, disparities were primarily due to differences in cancer stage and grade at diagnosis; disparities attenuated after we adjusted for these variables. In contrast, among those without history of diabetes at baseline, although cancer stage and grade explained major proportion of black-white disparities, differences persisted even after adjustments for these variables. Additional adjustments for cancer treatment and co-morbidities attenuated the associations further, but disparities remained except for short-term mortality for which the disparity no longer existed after adjustment for cancer stage and grade. In our subsidiary analysis where we stratified by cancer stage, for colorectal cancer stage I, II and III, our findings remained similar to our main analyses; while for colorectal cancer stage IV, we observed no difference between the two races for any of the mortality outcome for both diabetic and non-diabetic adults.
Our findings are consistent with previous studies that blacks were more likely to have advanced stage disease at diagnosis than whites, were less likely to receive cancer directed surgery or chemotherapy6, 29, 30, and had higher mortality rates3. In the analysis using earlier SEER-Medicare data, Gomez et al concluded disease characteristics (cancer stage, grade and cancer location) were major contributors for black-white disparities31. However, none of the studies have investigated the role of pre-existing diabetes specifically.
It was intriguing to observe that racial disparities in all-cause mortality and cancer-specific mortality were present only in those without pre-existing diabetes, while for those with diabetes, there was no difference in cancer survival between the two races after adjustment for cancer stage and grade. Although it remains unclear what could be the reason for this effect modification by pre-existing diabetes, we speculated survival bias might be a potential reason. Since mean age of our study population at diagnosis of colorectal cancer was over 75 years, those who survived to this age were likely to be relatively healthier, particularly among those with diabetes. Therefore, racial disparities were not apparent in this survivor population.
Our finding that short-term mortality was significantly lower in diabetic blacks compared to diabetic whites in the fully adjusted model was also unanticipated. One possible explanation was whites were more likely to receive cancer-directed surgery than blacks, and surgery related short-term mortality might drive this finding.
Major strengths of our study include a large sample with well-characterized cancer data and prospective diagnosis data on outcomes and comorbid conditions. However, it is also important to mention some of the potential limitations. First, we lacked data on fasting glucose level or HbA1c, and diabetes was defined based on diagnosis codes in administrative claims. In SEER-Medicare, comorbidities may not be reliably captured, as this database is not designed for research. However, we believe that misclassification was likely to be non-differential, which would result in conservative estimates. Second, we lacked data on duration of diabetes, glycemic control, and adiposity, which could be potential confounders or mediators in the racial disparity and survival relationship. Third, although both blacks and whites in our sample have equal access to health care under the Medicare coverage, there was no prior data on whether diagnosis of diabetes differs by race in cancer patients in the SEER population. We cannot rule out the possibility of residual confounding in the association between race and cancer survival in non-diabetic patients.
Our study has at least two implications. First, better cancer screening in blacks with pre-existing diabetes would minimize racial disparity in cancer survival. Our study supports the recommendation from the American Cancer Society-American Diabetes Association Consensus panel that “patients with diabetes should be strongly encouraged by their health care professionals to undergo appropriate cancer screenings as recommended for all people in their age and sex”32. Second, for older adults without pre-existing diabetes, in addition to better cancer screening, improvement in cancer treatment and care of comorbid conditions might substantially increase survival in blacks. Although Medicare coverage provides equal health care access to blacks and whites, disparity of care quality is equally important.
In conclusion, our study showed that in older adults with colorectal cancer, in those with pre-existing diabetes, racial disparities in cancer survival were explained mainly by cancer stage and grade; while among those without diabetes, racial disparities remained even after adjustments for demographic variables, tumor characteristics, cancer treatment and co-morbid conditions.
Figure 1.
- All-Cause Mortality (long-term)
- All-Cause Mortality (within 90-days of cancer diagnosis)
- Cardiovascular Mortality
- Colorectal Cancer-related Mortality
Acknowledgments
Salman Waheed was supported by National Heart, Lung, and Blood Institute grant 5T32HL007024. H-C.Y. has received support from the Maryland Cigarette Restitution Fund (FH B33 CRF), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diabetes Research Center (P30DK07963) (), and National Institute of Lung, Heart, and Blood (P50HL105187). Funding for the SEER-Medicare was provided by grants from the National Cancer Institute (U01-CA-086308).
Footnotes
Competing Interests
No potential conflicts of interest relevant to this article were reported.
References
- 1.Du XL, Meyer TE, Franzini L. Meta-analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109:2161–2170. doi: 10.1002/cncr.22664. [DOI] [PubMed] [Google Scholar]
- 2.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401–405. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 3.White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: To what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116:4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: Findings from the national longitudinal mortality study, 1979–2003. Cancer. 2011;117:3242–3251. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall MJ, Ruth K, Giri VN. Rates and predictors of colorectal cancer screening by race among motivated men participating in a prostate cancer risk assessment program. Cancer. 2012;118:478–484. doi: 10.1002/cncr.26315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doubeni CA, Field TS, Buist DS, Korner EJ, Bigelow C, Lamerato L, Herrinton L, Quinn VP, Hart G, Hornbrook MC, Gurwitz JH, Wagner EH. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109:612–620. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 7.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in african american and white adults: The atherosclerosis risk in communities study. Jama. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: An updated systematic review and meta-analysis. Digestive diseases and sciences. 2012 doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 10.Luo W, Cao Y, Liao C, Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: A meta-analysis of twenty four cohort studies. Colorectal Dis. 2011 doi: 10.1111/j.1463-1318.2012.02875.x. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: A systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Yu S. Diabetes mellitus is an independent risk factor for colorectal cancer. Digestive diseases and sciences. 2012 doi: 10.1007/s10620-012-2059-x. [DOI] [PubMed] [Google Scholar]
- 13.Kramer HU, Schottker B, Raum E, Brenner H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur J Cancer. 2012;48:1269–1282. doi: 10.1016/j.ejca.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Ries LKC, Hankey B, et al. SEER Cancer Statistics Review. Bethesda: MNCI; 1999. [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the seer-medicare data: Content, research applications, and generalizability to the united states elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB. Overview of the seer--medicare health outcomes survey linked dataset. Health Care Financ Rev. 2008;29:5–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Twombly JG, Long Q, Zhu M, Fraser LA, Olson DE, Wilson PW, Narayan KM, Phillips LS. Validity of the primary care diagnosis of diabetes in veterans in the southeastern united states. Diabetes Res Clin Pract. 2011;91:395–400. doi: 10.1016/j.diabres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Young TK, Roos NP, Hammerstrand KM. Estimated burden of diabetes mellitus in manitoba according to health insurance claims: A pilot study. Cmaj. 1991;144:318–324. [PMC free article] [PubMed] [Google Scholar]
- 19.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using medicare claims data. Am J Med Qual. 1999;14:270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths RI, Danese MD, Gleeson ML, Valderas JM. Epidemiology and outcomes of previously undiagnosed diabetes in older women with breast cancer: An observational cohort study based on seer-medicare. BMC Cancer. 2012;12:613. doi: 10.1186/1471-2407-12-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. http://diabetes.niddk.nih.gov/dm/pubs/statistics/-Types.
- 23. http://www.who.int/mediacentre/factsheets/fs312/en/.
- 24.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Lang K, Korn JR, Lee DW, Lines LM, Earle CC, Menzin J. Factors associated with improved survival among older colorectal cancer patients in the us: A population-based analysis. BMC Cancer. 2009;9:227. doi: 10.1186/1471-2407-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslow NE DNSMiCRL. France: International Agency for Research on Cancer, IARC Publications; 1990. pp. 76–78. Publication 32. [Google Scholar]
- 27.StataCorp LP. Statistics/Data Analysis. TX: 4905 Lakeway Drive CS; [Google Scholar]
- 28. http://healthservices.cancer.gov/seermedicare.
- 29.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629–639. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 30.Haas JS, Brawarsky P, Iyer A, Fitzmaurice GM, Neville BA, Earle C. Association of area sociodemographic characteristics and capacity for treatment with disparities in colorectal cancer care and mortality. Cancer. 2011;117:4267–4276. doi: 10.1002/cncr.26034. [DOI] [PubMed] [Google Scholar]
- 31.Gomez SL, O'Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: Impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]