Abstract
Though RecQL4 was shown to be essential for the initiation of DNA replication in mammalian cells, its role in initiation is poorly understood. Here, we show that RecQL4 is required for the origin binding of Mcm10 and Ctf4, and their physical interactions and association with replication origins are controlled by the concerted action of both CDK and DDK activities. Although RecQL4-dependent binding of Mcm10 and Ctf4 to chromatin can occur in the absence of pre-replicative complex, their association with replication origins requires the presence of the pre-replicative complex and CDK and DDK activities. Their association with replication origins and physical interactions are also targets of the DNA damage checkpoint pathways which prevent initiation of DNA replication at replication origins. Taken together, the RecQL4-dependent association of Mcm10 and Ctf4 with replication origins appears to be the first important step controlled by S phase promoting kinases and checkpoint pathways for the initiation of DNA replication in human cells.
Keywords: CDK, DDK, cell cycle, checkpoint, Ctf4/And-1, DNA replication, Mcm10, RecQL4
Abbreviations
- CDK
cyclin dependent kinase
- DDK
Dbf4 dependent kinase
- pre-RC
pre-replicative complex
- BiFC
bimolecular fluorescence complementation
- ChIP
chromatin immunoprecipitation
Introduction
RecQL4 is a member of RecQ family of helicases that play important roles in maintaining genome integrity. In humans, 5 RecQ related helicases, BLM, WRN, RecQL1, RecQL4, and RecQL5 were identified, and 3 of these, BLM, WRN, and RecQL4 have been associated with genetic disorders characterized by genome instability, cancer predisposition, and premature aging phenotypes.1,2 RecQL4 is a conserved protein in higher eukaryotes showing 3′ to 5′ DNA helicase activity,3 and mutations in this gene are associated with Rothmund-Thomson Syndrome, which leads to developmental abnormalities, signs of premature aging, and high incidences of osteosarcoma.4 While RecQL4 was shown to be involved in the cellular response to DNA damage or oxidative stress5 and telomere maintenance,6 RecQL4 also plays essential roles in DNA replication. Unlike other RecQ family proteins, RecQL4 has a distinct N-terminus showing limited homology to Sld2 which is an essential initiation factor in yeast,7 and this N-terminal domain of RecQL4 was shown to be essential for the initiation of DNA replication in vertebrates.8,9
The initiation of DNA replication in eukaryotes is critical for the onset and progression of S phase, as well as the target of cell cycle checkpoint machineries that control S phase. To ensure faithful and complete duplication of the whole genome of cells, pre-replicative complexes (pre-RCs) required for the initiation of DNA replication are assembled on replication origins before S phase, and then sequentially activated during S phase.10
Pre-RCs are assembled in G1 by the stepwise recruitment of the origin recognition complex, Cdc6, Cdt1 and the Mcm2-7 complex. The Mcm2-7 complex is the core of the replicative DNA helicase activity required for the unwinding of and formation of template DNAs, but remains inactive until S phase.11,12 For the activation of the pre-RC and formation of the replicative helicase, Cdc45 and GINS are recruited onto pre-RCs, and interact with the Mcm2-7 complex to form the active replicative helicase complex containing Cdc45, Mcm2-7, and GINS, collectively called the CMG complex.13 In yeast systems, this activation minimally requires additional factors including Dpb11/Cut5, Sld2, Sld3, Mcm10, and is governed by 2 S phase promoting kinases, the cyclin-dependent kinase (CDK) and Dbf4-dependent Cdc7 kinase (DDK).14 In budding yeast, CDK phosphorylates Sld2 and Sld3, and these modifications are required to support their interaction with the BRCT domains of Dpb11, which are critical for the initiation of DNA replication.15,16 DDK phosphorylation of the Mcm2-7 complex, including the N-terminus of Mcm4, appears to cause conformational changes required for the activation of pre-RCs.17 Although the detailed mechanisms are not completely understood, the combined action of these 2 kinases facilitates the CMG complex formation and the initiation of DNA replication.14
While the role of these factors and their control by CDK and DDK are relatively well characterized in yeast systems, their effects in mammalian cells are poorly understood. In mammalian cells, some homologues of the yeast proteins appear to play similar roles during the initiation process. RecQL4 was shown to be essential for the initiation of DNA replication and assembly of the CMG complex.8,18 Treslin/Ticrr, initially identified as a TopBP1 interacting protein in Xenopus, was shown to be essential for the initiation of DNA replication, and appears to be the mammalian ortholog of Sld3.19-21 Mcm10 was also shown to be required for the initiation of DNA replication, the recruitment of DNA polymerase α, and the association of RecQL4 with Mcm2-7.18,22,23 On the other hand, TopBP1, the mammalian homolog of Dpb11/Cut5, appears to play roles in both DNA replication and S phase checkpoint control, but its role in the initiation process is still controversial.18,24 Ctf4/And-1/Wdhd1, which supports sister chromatid cohesion in yeast cells,25 was also shown to be required for the binding of DNA polymerase α to chromatin and the initiation of DNA replication in mammalian cells.18,22 Recent studies showed that Treslin is phosphorylated by S-CDK, a modification essential for its interaction with TopBP1 and DNA replication.20,21 While these results suggest that initiation of DNA replication is conserved from yeast to humans, the detailed mechanism controlling the initiation of DNA replication in mammalian cells is still poorly understood.
In this study, we examined the interactions between RecQL4, Mcm10 and Ctf4 and their association with replication origins in human cells. We find that RecQL4 is required for chromatin and origin binding of Mcm10 and Ctf4, and their interactions and binding to replication origins are controlled by S-phase promoting kinases and DNA damage checkpoint pathways. The results described here provide novel events that influence the initiation of DNA replication in mammalian cells.
Results
RecQL4 is required for the interaction of Mcm10 with Ctf4 on chromatin in human cell
In order to understand the role of replication proteins required for the assembly of CMG complex in human cells, we examined their physical interactions. When RecQL4, Mcm10 and Ctf4 were over-expressed in Sf 9 cells using the baculovirus expression system, immunoprecipitation of RecQL4 or Ctf4 resulted in the co-precipitation of other 2 proteins (Fig. 1A, lanes 1-6), suggesting that these 3 proteins form a complex in the absence of CMG or pre-RC components. Examination of the interactions between 2 of the 3 proteins revealed that Mcm10 interacted stably with RecQL4 or Ctf4 in the absence of Ctf4 or RecQL4, respectively (Fig. 1A, lanes 7-18). RecQL4 and Ctf4 however, did not interact with each other in the absence of Mcm10 (Fig. 1A, lanes 19-24). Therefore, Mcm10 appears to play an essential role in the formation of the 3 protein complex by mediating interactions between RecQL4 and Ctf4.
Figure 1.
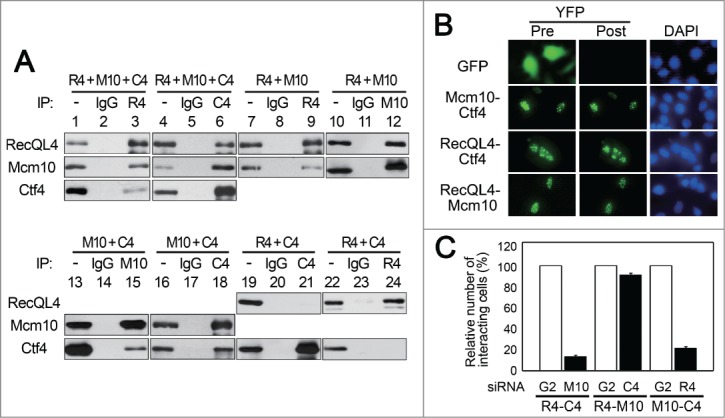
RecQL4, Mcm10 and Ctf4 interact with each other on chromatin and RecQL4 is required for the interaction between Mcm10 and Ctf4 on chromatin. (A) SF9 cells were infected with combinations of baculoviruses expressing RecQL4 (R4), Mcm10 (M10), and Ctf4 (C4), and incubated for 50 hr. Whole cell extracts were used for immunoprecipitations with antibodies against the indicated proteins or control IgG. Lanes 1, 4, 7, 10, 13, 16, 19, and 22 represent input material (10%) for each immunoprecipitation. (B) HeLa cells were transfected with GFP expressing vector or BiFC plasmids expressing RecQL4, Mcm10, and Ctf4 as indicated. After incubation for 24 hr, cells were washed with PBS containing 0.1 % Triton X-100, and cell fluorescence was examined. Pre- and post-treated images were obtained with live cells using a fluorescent microscope. (C) HeLa cells were transfected with siRNAs as indicated and incubated for 24 hr. Cells were then co-transfected with siRNAs and BiFC expression vectors as indicated. After incubation for 24 hr, the percent of cells showing positive interactions were counted. Each bar represents the mean value of 3 independent experiments; the error bar indicates the standard error observed between experiments.
We further examined interactions between RecQL4, Mcm10 and Ctf4 in HeLa cells using bimolecular fluorescence complementation (BiFC) assays. In BiFC assays, the generation of a functional fluorescence protein from 2 fragments and the emission of a fluorescence signal depend on the interaction between 2 fused proteins.26 In this assay, N-terminal or C-terminal fragments of the highly fluorescent mutated YFP (called Venus) were fused to the C-terminus of RecQL4, Mcm10 or Ctf4 proteins. When two of those proteins were expressed in HeLa cells, strong fluorescence signals were observed in the nucleus in any combination, suggesting that these 3 proteins are associated stably with, or are at least in close proximity to each other in cells. Those fluorescence signals were still observed after treating cells with a buffer containing non-ionic detergent (Fig. 1B), suggesting that these proteins interacted with each other on chromatin (Fig. 1B). This notion was further confirmed by the co-immunoprecipitation experiments with separated soluble and chromatin fractions of 293T cells. Immunoprecipitation of RecQL4 resulted in the co-precipitation of Mcm10 and Ctf4 only from the chromatin fraction (Fig. S1).
Consistent with the in vitro interaction patterns shown in Fig. 1A, interactions between RecQL4 and Ctf4 depended on the presence of Mcm10, and RecQL4 interacted with Mcm10 in the absence of Ctf4 in HeLa cells (Fig. 1C). On the other hand, interaction of Mcm10 with Ctf4 was significantly reduced following depletion of RecQL4 in HeLa cells (Fig. 1C), which appeared to contradict the protein interaction results shown in Fig. 1A (lanes 13-18). These results suggest that additional mechanisms might contribute to the control of the association of these proteins in HeLa cells. Loss of those interactions observed in Mcm10 or RecQL4 depleted cells could be recovered by the expression of siRNA resistant form of Mcm10 or RecQL4, respectively (Fig. S2), suggesting that Mcm10 or RecQL4 is indeed required for those interactions. Since both RecQL4 and Mcm10 are required for the initiation of DNA replication, their depletion might lead to the activation of checkpoint pathways, which affect the interaction of other proteins. To test this possibility, we examined the effects of caffeine, an inhibitor of the ATM/ATR-dependent checkpoint pathway. Addition of caffeine after RecQL4 or Mcm10 depletion did not rescue interactions between Mcm10 and Ctf4 or RecQL4 and Ctf4 in HeLa cells (Fig. S3), suggesting that RecQL4 or Mcm10 is involved directly in the interaction of Ctf4 with Mcm10 or RecQL4.
RecQL4 is required for the recruitment of Mcm10 and Ctf4 to chromatin
Since depletion of RecQL4 abolishes the interaction of Ctf4 with Mcm10 on chromatin in HeLa cells (Fig. 1C), we reasoned that RecQL4 might affect the binding of Mcm10 and Ctf4 to chromatin. To test this possibility, we examined the binding of Mcm10 and Ctf4 to chromatin using immunofluorescence after depletion of RecQL4. When HeLa cells pre-treated with non-ionic detergents were stained with antibodies against Mcm10 or Ctf4, nuclear fluorescence was observed in cells containing Cyclin E, suggesting that these proteins binds to chromatin at late G1 and S phase during the cell cycle (Fig. 2A). When RecQL4 was depleted with siRNA, the nuclear staining of Mcm10 or Ctf4 in Cyclin E-positive cell was significantly reduced in cells pre-treated with a buffer containing non-ionic detergents (Figs. 2B, D), while the staining of Mcm10 or Ctf4 was not affected in cells that were not pre-treated with non-ionic detergent by the depletion of RecQL4 (Fig. 2C). These results suggested that RecQL4 did not significantly affect the cellular level of Mcm10 and Ctf4 proteins but was required for the association of Mcm10 and Ctf4 with chromatin.
Figure 2.

RecQL4 is required for chromatin binding of Mcm10 and Ctf4. (A) HeLa cells were pre-treated with PBS containing 0.1% Triton X-100 for 30 sec, fixed with 3.7% paraformaldehyde, and stained with antibodies against indicated proteins. (B–D) HeLa cells transfected with siRNAs against GL2 or RecQL4” were pre-treated, fixed, and stained with antibodies against indicated proteins (B, D), or fixed without pre-treatment and stained with indicated antibodies (C). Representative examples are shown in (B) and (C), and the percentage of cells showing positive staining of Mcm10 or Ctf4 from the pre-treated cells stained with Cyclin E is presented as bar-graph at (D). Error bars represent the standard deviation from 3 independent experiments. Scale bars represent 10 μm.
Physical interactions between RecQL4, Mcm10, and Ctf4 are controlled by S phase promoting kinases, CDK and DDK
We examined whether the interactions between RecQL4, Mcm10 and Ctf4 were affected by the S phase promoting kinases, CDK and DDK. As shown in Figs. 3A and B, treatment of cells with the CDK2 inhibitor, NU6140 or siRNA against Cdc7 significantly reduced the co-immunoprecipitation of Mcm10 and Ctf4, or Mcm10 and RecQL4 by antibodies against RecQL4 or Ctf4, respectively. The requirements of CDK and DDK for those interactions were further confirmed by BiFC assay (Fig. S4). Since the effects of a CDK inhibitor or Cdc7 depletion could be indirect, we examined whether the phosphorylation of those proteins was required for their physical interactions. For this purpose, these proteins were overexpressed in Sf9 cells using the baculovirus expression system and their interactions were examined using co-immunoprecipitation assays before and after λ phosphatase treatment. While RecQL4, Mcm10, and Ctf4 proteins interacted and formed a complex in crude Sf9 cell extracts, λ phosphatase treatment of these extracts almost completely abolished complex formation as well as interactions between Mcm10 and RecQL4 or Mcm10 and Ctf4 (Fig. 3C). Although the targets of these kinases required for the interactions between RecQL4, Mcm10, and Ctf4 proteins are unknown, these findings suggest that phosphorylation is required for their physical interactions and CDK and DDK play essential roles in their interactions.
Figure 3.
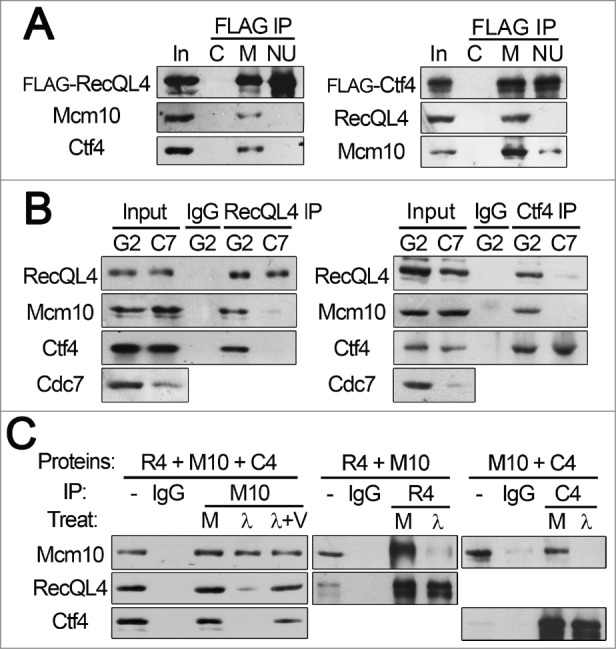
Interactions between RecQL4, Mcm10, and Ctf4 require the S phase promoting kinases, CDK and DDK. (A) The 293T cells stably expressing FLAG-RecQL4 (left panel) or FLAG-Ctf4 (right panel) were treated with 10 μM NU6140 (NU) or mock-treated (M) for 24 hr, and immunoprecipitation was carried out using anti-FLAG M2 agarose beads. For control immunoprecipitation (lane C), FLAG peptides (0.2 mg/ml) were added to cell extracts for immunoprecipitation. (B) HeLa cells transfected with GL2 (G2) or Cdc7 (C7) siRNAs were incubated for 24 hr, and immunoprecipitations were carried out using antibodies against RecQL4 or Ctf4. (C) Sf9 cells infected with baculoviruses expressing indicated proteins were incubated for 48 hr. Whole cell extracts were prepared and incubated in the absence (lane M), the presence of λ phosphatase (lane λ) or λ phosphatase and sodium vanadate (lane λ + V). Immunoprecipitations were carried out using antibodies against indicated proteins. R4, M10, and C4 denote RecQL4, Mcm10, and Ctf4, respectively.
RecQL4 dependent association of Mcm10 and Ctf4 with chromatin and their interaction with replication origins are controlled by CDK and DDK
Since the physical interactions between RecQL4, Mcm10, and Ctf4 require CDK and DDK activities, we examined whether their binding to chromatin is controlled by CDK and DDK. As shown in Fig. 4A, the binding of RecQL4 to chromatin was unaffected by CDK inhibition or depletion of Cdc7 kinase, while Ctf4 binding to chromatin was significantly reduced by CDK inhibition or Cdc7 depletion. Mcm10 binding to chromatin was unaffected by CDK inhibition, but was reduced significantly by Cdc7 depletion. Taken together, these results suggest that RecQL4 binds to chromatin first in the absence of CDK and DDK activities, and then Mcm10 and Ctf4 proteins are recruited to RecQL4 on chromatin in a DDK and DDK/CDK-dependent reaction, respectively.
Figure 4.

The influence of CDK, DDK and pre-RC proteins on the binding of RecQL4, Mcm10 and Ctf4 to chromatin. (A) HeLa cells were treated with 10 μM roscovitine (ROS) or transfected with siRNA against Cdc7. After incubation for 24 hr, soluble (SF) and chromatin (CF) fractions were prepared, and subjected to Western blotting. GL2 siRNA was used as the negative control. (B) HeLa cells transfected with siRNAs against pre-RC proteins or GL2 were incubated for 24 hr, and the level of indicated proteins in soluble (SF) and chromatin fractions (CF) determined by Western blotting.
Since RecQL4, Mcm10, and Ctf4 were shown to be required for the assembly of CMG complex on pre-RCs,18 we examined whether their recruitment to chromatin and ability to form a complex required pre-RCs. Surprisingly, their chromatin binding (Fig. 4B) and interactions (Fig. S5) were not affected significantly following depletion of pre-RC components such as Orc2, Cdc6 or Mcm7. These results suggest that the initial binding of RecQL4 may occur at non-origin regions on chromatin, and that Mcm10 and Ctf4 can be recruited onto RecQL4 loaded on chromatin in the absence of pre-RCs.
Although the binding of these proteins to chromatin and their assembly into a complex occur in the absence of pre-RCs, they should interact with the pre-RC components present at replication origins for the assembly of CMG complex and the initiation of DNA replication. Therefore, we examined the origin binding properties of these proteins using in vivo crosslinking followed by chromatin immunoprecipitation (ChIP). As shown in Fig. 5A, RecQL4, Mcm10, and Ctf4 all interacted with replication origins (lanes 7, 17, 19) and, in contrast to their chromatin binding or interactions, their binding to origin DNA required pre-RC proteins, such as Cdc6 and Mcm7 (lanes 7-9, 17-20). RecQL4, which binds to chromatin in the absence of pre-RCs, did not interact with replication origins in the absence of pre-RCs (lanes 8, 9), suggesting that RecQL4 can bind to non-origin regions on chromatin, and then interacts with replication origins via pre-RCs. At present, the initial binding sites of RecQL4 on chromatin are unknown.
Figure 5.

The association of RecQL4, Mcm10 and Ctf4 with replication origins requires CDK, DDK and pre-RC proteins. (A and B) HeLa cells were transfected with siRNAs against GL2 (G2), Cdc6 (C6), Mcm7 (M7), RecQL4 (R4), Mcm10 (M10) or Ctf4 (C4). After incubation for 24 hr, the binding of RecQL4, Mcm10 or Ctf4 to the replication origin located in the Mcm4 upstream promoter region (M4 Ori) was determined by ChIP analyses. The primer set for the 4 kb distal region (Distal) from the replication origin was used as the internal control in PCR analyses. (C) HeLa cells were transfected with siRNA against Cdc7 (C7) or treated with NU6140 (NU), and ChIP analyses carried out as above.
The binding of Mcm10 as well as Ctf4 at replication origins required RecQL4 (Fig. 5B, lanes 18 and 20), while Ctf4 was not required for the origin binding of RecQL4 (Fig. 5B, lane 8). Interestingly, Mcm10 was also required for the origin binding of RecQL4 (Fig. 5B, lane 9). Since RecQL4 is required for the chromatin binding of Mcm10, this result suggests that Mcm10 interacts with RecQL4 and contributes to the interaction of RecQL4-Mcm10 complex with replication origins presumably by the interaction of Mcm10 with pre-RC components. Consistent with this notion, Mcm10 was shown to directly interact with pre-RC component proteins such as the Mcm2-7 complex in other studies.22,27
We also found that the origin binding of RecQL4 depended upon CDK and DDK activities (Fig. 5C). Collectively, these results suggest that RecQL4 binds to chromatin in the absence of CDK and DDK, and then Mcm10 and Ctf4 proteins are sequentially recruited to chromatin in DDK- and DDK/CDK-dependent manner, which leads to the association of those protein complexes with replication origins.
Association of RecQL4, Mcm10, and Ctf4 with replication origins is inhibited by DNA damage checkpoint pathways
Activation of DNA damage checkpoint pathways prevents the initiation of DNA replication by actively blocking origin firing. Since most checkpoint pathways target CDK activities and the binding of RecQL4, Mcm10 and Ctf4 to replication origins is controlled by CDK, the association of those proteins with replication origins could be targets of the checkpoint pathways. We first examined whether the binding of RecQL4, Mcm10, and Ctf4 to chromatin was affected by DNA damaging agents. As shown in Fig. 6A, UV irradiation, treatment of cells with an alkylating agent, 4-nitroquinoline 1-oxide (4NQO), or zeocin which intercalates into DNA and induces strand breaks, significantly reduced Ctf4 binding to chromatin, but not the binding of RecQL4 and Mcm10 to chromatin. Treatment of cells with UV also significantly decreased the co-precipitation Ctf4 following immunoprecipitation of RecQL4, and this decrease was reversed by the treatment of cells with caffeine, an inhibitor of the major checkpoint kinases, ATM/ATR (Fig. 6B). These results suggest that the binding of Ctf4 to the RecQL4 and Mcm10 complex on chromatin is prevented by DNA damage, presumably by the activation of ATM/ATR-dependent checkpoint pathways.
Figure 6.

Activation of DNA damage checkpoint pathways prevents the binding of RecQL4, Mcm10, and Ctf4 to replication origins. (A) HeLa cells were irradiated with UV (10 or 20 J/m2), treated with 2 μg/ml 4NQO (4N) or 200 μg/ml zeocin (Ze) for 3 hr. The level of indicated protein in the soluble (SF) or chromatin (CF) fraction was examined by Western blotting. Lane M represented the mock-treated control. (B) The 293T cells stably expressing FLAG-RecQL4 proteins were irradiated with 20 J/m2 UV, and incubated either in the absence or presence of 5 mM caffeine for 3 hr. After preparation of cell extracts, immunoprecipitations were carried out using anti-FLAG M2 agarose beads. FLAG peptide (0.2 mg/ml) was added to whole cell extracts for control immunoprecipitations (Con). (C) HeLa cells irradiated with 20 J/m2 UV were incubated either in the absence or presence of 5 mM caffeine for 3 hr, and the binding of RecQL4, Mcm10, and Ctf4 to replication origins in the Mcm4 upstream promoter region (M4 Ori) or the β-globin locus (β-globin Ori) was examined by ChIP analysis. The primer sets for the 4 kb distal region of M4 Ori and the 9 kb distal region of β-globin Ori were used as internal controls in PCR analyses.
Treatment of cells with DNA damaging agents significantly reduced the origin binding of RecQL4, Mcm10 and Ctf4, and caffeine treatment reversed these effects (Fig. 6C). These binding patterns are similar to those observed in CDK inhibited cells (Figs. 4A and 5C). Thus, the inhibition of CDK activity following activation of the ATM/ATR-dependent checkpoint pathway may contribute to the decrease in the origin binding of RecQL4, Mcm10, and Ctf4 and prevents damaged cells from entering into S phase.
Discussion
In this study, it was shown that RecQL4 is required for the association of Mcm10 and Ctf4 with replication origins by processes controlled by CDK, DDK, and DNA damage checkpoint pathways. As shown, RecQL4 binds to chromatin in the absence of the CDK and DDK activities, and this binding did not depend on the pre-RCs. Therefore, the initial binding of RecQL4 to chromatin appears to occur at non-origin regions. Concomitant with the activation of DDK activity in late G1 or the G1/S transition, Mcm10 interacts with RecQL4 on chromatin. This complex then interacts with Ctf4 and pre-RCs at replication origins following CDK activation by interaction of Mcm10 with Ctf4 and Mcm2-7 complex in pre-RCs. Interaction of the Mcm10-RecQL4 with replication origins was observed even in the absence of Ctf4 (Fig. 5B), and Ctf4 interacted with Mcm10 and RecQL4 in the absence of the pre-RC components (Fig. S5). Therefore, those 2 interactions appear to be independent events, but can occur at the same time when CDK is activated.
Interaction of Mcm10 with RecQL4 has been reported previously.23 This study showed that RecQL4 interacted with replication proteins including Mcm10, Mcm2-7, Cdc45, and GINS, and their interaction and association with replication origin were cell-cycle regulated. Also, Mcm10 was shown to interact directly with RecQL4 and to mediate the association of RecQL4 with Mcm2-7 complex. Our results of protein interactions and origin binding properties as shown in Fig. 1 and 5 are consistent with those observations, and we further explored their molecular interactions and found several novel findings including an essential role of Mcm10 in the interaction of RecQL4 with Ctf4 (Fig. 1), an essential role of RecQL4 in the association of Mcm10 and Ctf4 with chromatin and replication origins (Figs. 2 and 5), and control of interactions and origin association of RecQL4, Mcm10, and Ctf4 by CDK, DDK, and DNA damage checkpoint pathways (Figs. 3–6).
The role of CDK and DDK in the initiation of DNA replication has been studied extensively in yeast.14,28 It was shown that Sld2 and Sld3 are the minimal CDK targets required for the initiation of DNA replication,15,16 and the Mcm 2-7 complex was shown to be an important DDK target.17 Though the role of CDK and DDK during the initiation of DNA replication in mammalian cells is poorly understood, phosphorylation of 2 conserved residues in Treslin (Sld3 homolog) by CDK is required for its interaction with TopBP1 (Dpb11 homolog) and DNA replication.20,21 The phosphorylation of these residues was blocked by the activation of Chk1 kinase following replication stress.21 Thus, the Sld3/Treslin-Dpb11/TopBP1 interaction and its control appear to be conserved between yeast and human.
Here, we describe novel interactions among replication initiation proteins that require both CDK and DDK activities. Though the chromatin binding of RecQL4 does not depend on CDK or DDK, its association with Mcm10 on chromatin requires DDK activity, and CDK activity is required for the association of the RecQL4-Mcm10 complex with Ctf4 at replication origins. Although we do not know which proteins are the direct targets of CDK and/or DDK activities, these results suggest that DDK and CDK are required for distinct steps, and DDK acts before CDK in the assembly process. This order of action of these 2 essential kinases is consistent with the previous studies in yeasts and Xenopus.29-31
In budding yeast, replication initiation factors Sld2, Dpb11, GINS and DNA polymerase ε form a labile complex (pre-loading complex), that recruits GINS to origins.7 On the other hand, another initiation factor, Sld3 forms a complex with Cdc45 that binds to replication origins before CDK activation.32 RecQL4, Mcm10, and Ctf4 complex in mammalian cells is comparable to the yeast pre-loading complex in several properties, but also differs in many aspects. One component of the complex, RecQL4 is a homolog of the yeast Sld2, and the formation of both complexes depends on CDK and can occur in the absence of the pre-RC. Therefore, RecQL4, Mcm10, and Ctf4 might play a role as a complex to recruit CMG component proteins to origins. However, RecQL4, Mcm10, and Ctf4 are required for the association of both GINS and Cdc45 with Mcm2-7 complex, and the assembly of RecQL4, Mcm10, and Ctf4 complex occurs only on chromatin and requires both CDK and DDK activities.
The prevention of S phase entry following the exposure of cells to stress is essential for the faithful chromosomal duplication and genome integrity. Since early events in the initiation of DNA replication must be coordinated with the cell cycle, proteins involved in the initiation of DNA replication are plausible targets of checkpoint control. Previously, we showed that the binding of Ctf4 to chromatin and replication origins was prevented by mimosine treatment. Mimosine arrests the cell cycle prior to the onset of DNA replication, and its effect is mediated by the activation of Hif-1α in HeLa cells.33 A decrease in Ctf4 binding to late replication origins by intra-S-phase checkpoint was also observed in hydroxyurea treated cells.34 In this study, we demonstrated that the DNA damage checkpoint pathway affected the origin binding of not only Ctf4, but also RecQL4 and Mcm10 (Fig. 6C). The binding of Ctf4 to chromatin and its interaction with the RecQL4-Mcm10 complex were also prevented in damaged cells (Figs. 6A and B). The addition of caffeine to damaged cells reversed these effects, suggesting that the ATM/ATR-dependent checkpoint pathway is responsible for these responses after DNA damages. Therefore, both the interaction of Treslin with TopBP1 and the interaction and origin binding of RecQL4, Mcm10 and Ctf4 appear to be targets of checkpoint pathways in mammalian cells. While yeast Sld3 is directly phosphorylated by the checkpoint kinase, Rad53, under stress conditions,35,36 direct phosphorylation of Treslin by checkpoint kinase has not been observed after DNA damage in mammalian cells. It remains to be determined whether any of the RecQL4, Mcm10, and Ctf4 proteins are directly phosphorylated and regulated by checkpoint kinases.
Materials and Methods
Cell culture and reagents
HeLa, human cervical adenocarcinoma cells and 293T cells stably expressing FLAG-RecQL4 or FLAG-Ctf4 were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and antibiotics, and maintained at 37°C in a humidified incubator containing 5% CO2. For the synchronization of the cell cycle at G2/M, cells were treated with 100 ng/ml of nocodazole for 24 hr. For thymidine double block, cells were treated with 2.5 mM thymidine for 12 hr, incubated in fresh medium without thymidine for 14 hr, and then incubated for an additional 20 hr in the medium containing 2.5 mM thymidine. All siRNAs were chemically synthesized by ST Pharm (Daejon, Korea). Transfections of siRNAs (120 nM) were performed with oligofectamine (Invitrogen) following the manufacturer's instruction; when co-transfection of siRNA and plasmid DNAs was carried out, lipofectamine-PLUS (Invitrogen) was used. The sense strand sequences of siRNAs used in this study were as followings: GL2, 5′-AACGUACGCGGAAUACUUCGA-3′; Cdc6, 5′-AGAUCGACUUAAUCAGGUA-3′; Cdc7, 5′-AAGCUCAGCAGGAAAGGUG-3′; Ctf4, 5′-GAUCAGACAUGUGCUAUUA-3′; Mcm7, 5′-CUCGGGAAGAAGCAGUUCA-3′; Mcm10, 5′- AAAGAGUUGCAAGAGGAAUUA -3′; Orc2, 5′-AAGAAGGAGCGAGCGCAGCUU-3′; RecQL4, 5′-AACUUCUGAUCCCUGGUGAGU-3′.
Cell fractionation and immunoprecipitation
Chromatin fraction preparations were carried out by lysing cells in a buffer containing 50 mM Hepes-NaOH, pH 7.5, 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.3% NP-40, 1 mM ATP, 10 mM NaF, 0.1 mM sodium vanadate and protease inhibitors (5 μg/ml leupeptin, 5 μg/ml pepstatin A, 1mM benzamidine and 1mM PMSF). After incubation at 4°C for 3 min, the chromatin fraction was separated from the soluble fraction by centrifugation at 13,000 rpm for 10 min. To prepare whole cell extracts for immunoblotting, cells were re-suspended in cell lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM sodium vanadate and protease inhibitors. The cells were disrupted by sonication and the amount of protein measured using the Bradford assay. Approximately 30 μg of protein was subjected to SDS-polyacrylamide gel electrophoresis. For immunoprecipitation, total cell extracts were prepared with immunoprecipitation buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1% NP-40, 0.5 mM EDTA, 0.5 mM EGTA, 1 mM ATP, 5 mM MgCl2, 10 mM NaF, 0.1 mM sodium vanadate, and protease inhibitors). After adding benzonase (Sigma-Aldrich, USA) to a final concentration of 150 U/ml, 300∼500 μg aliquots of cell extracts were used for immunoprecipitation with appropriate antibodies. Antibodies against Mcm2, Cdc45, and Actin were purchased from Santa Cruz Biotechnology (USA). Antibodies against Cdc7 and Mcm10 were from Abcam (UK). Antibodies against Sld5, RecQL4, Ctf4, and Orc2 were from Strategic Diagnostics Inc. (USA), Cell signaling (USA), Biolegend (USA), and BD Biosciences (USA), respectively.
Immunofluorescence
For immunofluorescence assays, HeLa cells were grown on a coverslip and fixed with 3.7% paraformaldehyde. Fixed cells were treated with blocking buffer containing 3% BSA and 0.1% Triton X-100 in PBS for 30 min at room temperature. Indicated proteins were labeled with the primary antibodies and Alexa 488 or 555-conjugated secondary antibodies (Invitrogen) in the same blocking buffer for 1 h at room temperature. For microscopic analysis, the nuclei of cells were stained with DAPI and the stained cells imaged by Zeiss AXIO observer A-1 equipped with Zeiss EC Plan-Apochromax 63X/1.4 oil and Zeiss AXIOCAM MRC. Acquired images were analyzed using Axiovision software. Antibodies against Mcm10 and Ctf4 were kindly provided from Dr. Anindya Dutta (University of Virginia) and Cyclin E antibody was purchased from Santa Cruz Biotechnology (USA).
Bimolecular fluorescence complementation (BiFC) assay
The BiFC assay was performed as described.18 Briefly, various BiFC constructs containing the complete open reading frame of RECQL4, MCM10 or CTF4 fused with the N- or C-terminal half of the fluorescence protein were transfected into HeLa cells. After incubation for 24 hr, fluorescence signals reflecting protein-protein interactions were determined by fluorescent microscopy (NIKON, TE2000).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed as described previously.37 PCR reaction was carried out in a single tube using 2 primer sets for the origin in the Mcm4 upstream promoter region (M4Ori) and the 4 kb distal region of M4Ori (M4Distal) or the origin in the β-globin locus (β-globin) and the 9 kb distal region of β-globin (β-globin Distal). Sequences of the primer sets used in this study were; M4Ori-F, 5′-AAACCAGAAGTAGGCCTCGCTCGG-3′; M4Ori-R, 5′-GTCTGACCTGCGGAGGTAGTTTGG-3′; M4Distal-F, 5′-TAATCCGTCACCTTGACTACCACC-3′; M4Distal-R, 5′-ACAGCACGTGCATGATTCTGTAGG-3′; β-globin-F, 5′-GTCTCCCGGAACTATCACTCT-3′; β-globin-R, 5′-GCACCATAAGGGACATGATAAG-3′; β-globin Distal-F, 5′-AGTTCATGTCCTTTGTAGGGAC-3′; β-globin Distal-R, 5′-GCTCTACGGATGTGTGAGATC-3′.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Education, Science and Technology (no. 2011-0015907 and no. 2009-0072305).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Monnat RJ, Jr. Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin Cancer Biol 2010; 20:329-39; PMID:20934517; http://dx.doi.org/ 10.1016/j.semcancer.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh DK, Ghosh AK, Croteau DL, Bohr VA. RecQ helicases in DNA double strand break repair and telomere maintenance. Mutat Res 2012; 736:15-24; PMID:21689668; http://dx.doi.org/ 10.1016/j.mrfmmm.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu X, Liu Y. Dual DNA unwinding activities of the Rothmund-Thomson syndrome protein, RECQ4. EMBO J 2009; 28:568-77; PMID:19177149; http://dx.doi.org/ 10.1038/emboj.2009.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larizza L, Magnani I, Roversi G. Rothmund-Thomson syndrome and RECQL4 defect: splitting and lumping. Cancer Lett 2006; 232:107-20; PMID:16271439; http://dx.doi.org/ 10.1016/j.canlet.2005.07.042 [DOI] [PubMed] [Google Scholar]
- 5. Dietschy T, Shevelev I, Stagljar I. The molecular role of the Rothmund-Thomson-, RAPADILINO- and Baller-Gerold-gene product, RECQL4: recent progress. Cell Mol Life Sci 2007; 64:796-802; PMID:17364146; http://dx.doi.org/ 10.1007/s00018-007-6468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh AK, Rossi ML, Singh DK, Dunn C, Ramamoorthy M, Croteau DL, Liu Y, Bohr VA. RECQL4, the protein mutated in Rothmund-Thomson syndrome, functions in telomere maintenance. J Biol Chem 2012; 287:196-209; PMID:22039056; http://dx.doi.org/ 10.1074/jbc.M111.295063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muramatsu S, Hirai K, Tak YS, Kamimura Y, Araki H. CDK-dependent complex formation between replication proteins Dpb11, Sld2, Pol (epsilon}, and GINS in budding yeast. Genes Dev 2010; 24:602-12; PMID:20231317; http://dx.doi.org/ 10.1101/gad.1883410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sangrithi MN, Bernal JA, Madine M, Philpott A, Lee J, Dunphy WG, Venkitaraman AR. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell 2005; 121:887-98; PMID:15960976; http://dx.doi.org/ 10.1016/j.cell.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 9. Abe T, Yoshimura A, Hosono Y, Tada S, Seki M, Enomoto T. The N-terminal region of RECQL4 lacking the helicase domain is both essential and sufficient for the viability of vertebrate cells. Role of the N-terminal region of RECQL4 in cells. Biochim Biophys Acta 2011; 1813:473-9; PMID:21256165; http://dx.doi.org/ 10.1016/j.bbamcr.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 10. Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem 2010; 79:89-130; PMID:20373915; http://dx.doi.org/ 10.1146/annurev.biochem.052308.103205 [DOI] [PubMed] [Google Scholar]
- 11. Labib K, Tercero JA, Diffley JF. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 2000; 288:1643-7; PMID:10834843; http://dx.doi.org/ 10.1126/science.288.5471.1643 [DOI] [PubMed] [Google Scholar]
- 12. Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol 2006; 8:358-66; PMID:16531994; http://dx.doi.org/ 10.1038/ncb1382 [DOI] [PubMed] [Google Scholar]
- 13. Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A 2006; 103:10236-41; PMID:16798881; http://dx.doi.org/ 10.1073/pnas.0602400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev 2010; 24:1208-19; PMID:20551170; http://dx.doi.org/ 10.1101/gad.1933010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 2007; 445:328-32; PMID:17167415; http://dx.doi.org/ 10.1038/nature05465 [DOI] [PubMed] [Google Scholar]
- 16. Zegerman P, Diffley JF. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 2007; 445:281-5; PMID:17167417; http://dx.doi.org/ 10.1038/nature05432 [DOI] [PubMed] [Google Scholar]
- 17. Sheu YJ, Stillman B. The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 2010; 463:113-7; PMID:20054399; http://dx.doi.org/ 10.1038/nature08647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Im JS, Ki SH, Farina A, Jung DS, Hurwitz J, Lee JK. Assembly of the Cdc45-Mcm2-7-GINS complex in human cells requires the Ctf4/And-1, RecQL4, and Mcm10 proteins. Proc Natl Acad Sci U S A 2009; 106:15628-32; PMID:19805216; http://dx.doi.org/ 10.1073/pnas.0908039106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumagai A, Shevchenko A, Dunphy WG. Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell 2010; 140:349-59; PMID:20116089; http://dx.doi.org/ 10.1016/j.cell.2009.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumagai A, Shevchenko A, Dunphy WG. Direct regulation of Treslin by cyclin-dependent kinase is essential for the onset of DNA replication. J Cell Biol 2011; 193:995-1007; PMID:21646402; http://dx.doi.org/ 10.1083/jcb.201102003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boos D, Sanchez-Pulido L, Rappas M, Pearl LH, Oliver AW, Ponting CP, Diffley JF. Regulation of DNA replication through Sld3-Dpb11 interaction is conserved from yeast to humans. Curr Biol 2011; 21:1152-7; PMID:21700459; http://dx.doi.org/ 10.1016/j.cub.2011.05.057 [DOI] [PubMed] [Google Scholar]
- 22. Zhu W, Ukomadu C, Jha S, Senga T, Dhar SK, Wohlschlegel JA, Nutt LK, Kornbluth S, Dutta A. Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev 2007; 21:2288-99; PMID:17761813; http://dx.doi.org/ 10.1101/gad.1585607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Rochette PJ, Feyissa EA, Su TV, Liu Y. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J 2009; 28:3005-14; PMID:19696745; http://dx.doi.org/ 10.1038/emboj.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeon Y, Lee KY, Ko MJ, Lee YS, Kang S, Hwang DS. Human TopBP1 participates in cyclin E/CDK2 activation and preinitiation complex assembly during G1/S transition. J Biol Chem 2007; 282:14882-90; PMID:17293600; http://dx.doi.org/ 10.1074/jbc.M609116200 [DOI] [PubMed] [Google Scholar]
- 25. Hanna JS, Kroll ES, Lundblad V, Spencer FA. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol Cell Biol 2001; 21:3144-58; PMID:11287619; http://dx.doi.org/ 10.1128/MCB.21.9.3144-3158.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shyu YJ, Liu H, Deng X, Hu CD. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques 2006; 40:61-6; PMID:16454041; http://dx.doi.org/ 10.2144/000112036 [DOI] [PubMed] [Google Scholar]
- 27. Chattopadhyay S, Bielinsky AK. Human Mcm10 regulates the catalytic subunit of DNA polymerase-alpha and prevents DNA damage during replication. Mol Biol Cell 2007; 18:4085-95; PMID:17699597; http://dx.doi.org/ 10.1091/mbc.E06-12-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanaka S, Araki H. Regulation of the initiation step of DNA replication by cyclin-dependent kinases. Chromosoma 2010; 119:565-74; PMID:20686781; http://dx.doi.org/ 10.1007/s00412-010-0291-8 [DOI] [PubMed] [Google Scholar]
- 29. Walter JC. Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J Biol Chem 2000; 275:39773-8; PMID:11005825; http://dx.doi.org/ 10.1074/jbc.M008107200 [DOI] [PubMed] [Google Scholar]
- 30. Yabuuchi H, Yamada Y, Uchida T, Sunathvanichkul T, Nakagawa T, Masukata H. Ordered assembly of Sld3, GINS and Cdc45 is distinctly regulated by DDK and CDK for activation of replication origins. EMBO J 2006; 25:4663-74; PMID:16990792; http://dx.doi.org/ 10.1038/sj.emboj.7601347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heller RC, Kang S, Lam WM, Chen S, Chan CS, Bell SP. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell 2011; 146:80-91; PMID:21729781; http://dx.doi.org/ 10.1016/j.cell.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamimura Y, Tak YS, Sugino A, Araki H. Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J 2001; 20:2097-107; PMID:11296242; http://dx.doi.org/ 10.1093/emboj/20.8.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park SY, Im JS, Park SR, Kim SE, Wang HJ, Lee JK. Mimosine arrests the cell cycle prior to the onset of DNA replication by preventing the binding of human Ctf4/And-1 to chromatin via Hif-1alpha activation in HeLa cells. Cell Cycle 2012; 11:761-6; PMID:22374673; http://dx.doi.org/ 10.4161/cc.11.4.19209 [DOI] [PubMed] [Google Scholar]
- 34. Karnani N, Dutta A. The effect of the intra-S-phase checkpoint on origins of replication in human cells. Genes Dev 2011; 25:621-33; PMID:21406556; http://dx.doi.org/ 10.1101/gad.2029711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature 2010; 467:474-8; PMID:20835227; http://dx.doi.org/ 10.1038/nature09373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature 2010; 467:479-83; PMID:20865002; http://dx.doi.org/ 10.1038/nature09377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell 1998; 92:463-73; PMID:9491888; http://dx.doi.org/ 10.1016/S0092-8674(00)80940-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


