Abstract
TOR is involved in aging in a wide range of species from yeast to mammals. Here we show that, after overnight fasting, mTOR activity is higher in the livers of 28 months old female mice compared with middle-aged mice. Taken together with previous reports, our data predict that the life-extending effect of calorie restriction (CR) may be diminished, if CR is started in very old age. In contrast, rapamycin is known to be effective, even when started late in life.
Keywords: aging, mTOR, rapalogs, rapamycin, senescence
mTOR and Aging
The nutrient-sensing TOR pathway stimulates protein synthesis, cellular growth and functions.1-4 mTOR drives geroconversion from cellular quiescence to senescence5 and is involved in organismal aging and age-related diseases.6-10 In agreement, rapamycin extends life span in mice.11-21 However, this does not mean that the activity of mTOR is necessarily increased in old mice compared with young mice. In fact, even unchanged mTOR activity would be excessive in old animals compared with young, growing animals. In theory the activity may even be decreased but still be inappropriately high for an aging organism.22 In analogy, consider a car moving at 65 mph on highway. When a car exits highway to a small street, then even 50 mph will be too fast. As a more relevant example, protein synthesis is decreased (but not enough) in old yeast, worm and fly. Still, inhibition of protein synthesis further prolongs life span.23-26
Thus on pure theoretical grounds, the effect of age on mTOR activity is difficult to predict. First, we will discuss mTOR activity in cellular senescence.
mTOR in Cellular Aging
In the absence of mitogenic stimulation, normal cells become quiescent. In culture, cellular quiescence is usually caused by withdrawal of growth factors (serum) and/or nutrients. In quiescent cells, mTOR is not activated. The condition is reversible: growth stimulation causes cell proliferation. In proliferating cells, mTOR is active, which is manifested by phosphorylated S6 (p-S6). When the cell cycle is forcefully arrested, by DNA damaging agents and CDK inhibitors, then still active mTOR converts cycle arrest to senescence.27-38 Any conditions that inhibit mTOR suppress geroconversion.33,34,39,40 During geroconversion, levels of p-S6 are the same as its levels in proliferating cells. So both proliferating and senescent cells are characterized by activated mTOR. It is important to emphasize that chronically active mTOR renders cells resistant to acute mTOR activation.41 For example, chronic activation of mTOR by glucose decreases responsiveness to insulin.41 The response is diminished upstream of mTOR and absent downstream of mTOR (post-mTOR resistance). In the presence of active mTOR, insulin did not increase translation of proteins such as HIF-1a., Thus, basal activation of mTOR is a characteristic of senescent cells.
In summary, in vitro model of cellular senescence suggests that in the organism:
mTOR activity should be measured in the organs with minimal number of proliferating cells. In fact, proliferating cells are expected to have high levels of p-S6, mimicking senescent cells;
mTOR activity should be measured at “basal” levels. Food is a physiological activator of mTOR. Therefore, we choose to measure mTOR activity after fasting.
Fasting Levels of Hepatic pS6
We measured p-S6 and p-Akt in the liver in mice fasting overnight, comparing mice at the age of 28 months (old) and 9 months (adult, middle-aged). As shown in Figure 1, fasting p-S6 levels were higher in old mice than in middle-aged mice. Noteworthy, fasting levels of p-S6 were lower compared with non-fasting levels in middle-aged mice (Fig. 1). However, we did not have sufficient material to compare fasting and non-fasting levels in old mice. Careful analysis of the literature allowed us to find missing data. In the paper by Sengupta et al,42 Figure 4 C shows that fasting strongly decreased levels of p-S6 in young mice, while only marginally affected levels of p-S6 in the old mice. This publication and our data complement each other. Levels of p-Akt-S473 were not changed in old mice (Fig. 1). Noteworthy, total body weight and insulin levels were slightly yet statistically significantly elevated in old mice (Fig. 2).
Figure 1.
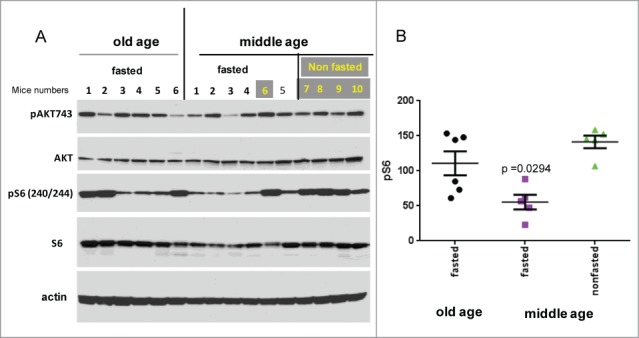
Hepatic p-S6 and p-Akt in old and adult (middle-aged) mice. (A) Immunoblot analysis. Protein lysates from the liver of 28 and 10 month old (old vs middle aged) female C57BL/6NCr mice were prepared as described.56,57 Fasted: mice were fasted overnight. Non-fasted: regular conditions. Numbers indicate individual mice. (B) Quantitative analysis of p-S6 signal shown in panel A. Data presented as mean ± SE.
Figure 2.
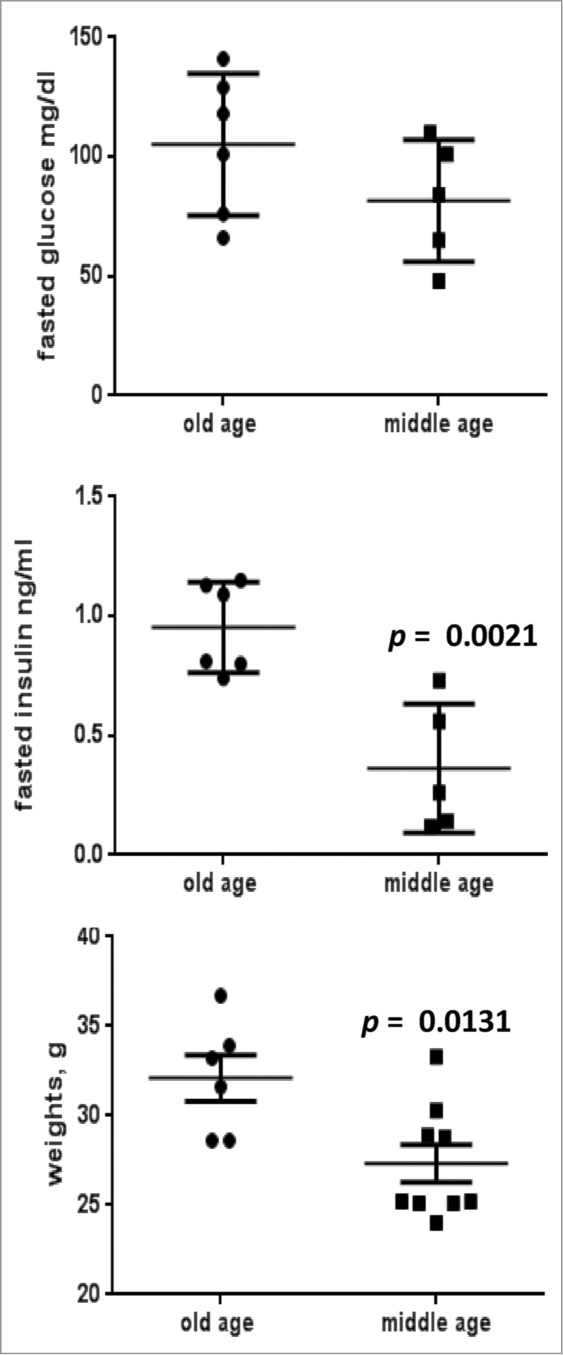
Comparison of weight and metabolic parameters in old (28 months old) and middle aged (10 months old) female mice of C57BL/6NCr strain. Blood was collected after overnight fasting and concentrations of insulin and triglycerides were determined in blood sera using, using Insulin (Mouse) Ultrasensitive ELISA kit (Alpco Diagnostics, Salem, NH) and Triglyceride Colorimetric Assay kit (Cayman Chemical Company, Ann Arbor, MI), respectively. Glucose was measured directly in blood using Accu-Chek Aviva strips (MaKesson, Atlanta, GA). Data presented as mean ± SE.
Implications for Health- and Life-Extension
In rodents, life-long CR prolongs life span by 40% in some strains.43 The life extending effect of CR can be explained by inhibition of mTOR.44-49 Fasting only minimally downregulated p-S6 in old mice, compared with young animals.42 As we showed here, fasting levels of p-S6 were higher in old mice compared with middle-aged animals. This predicts that fasting is less effective in old animals than in young/middle-aged animals. Therefore, late-life CR should be less effective than life-long CR. Indeed, the later in life the onset of CR, the less extension of life span was observed.50,43 Furthermore, neither CR restriction nor amino-acid restriction had any longevity benefits in very old humans.43,51 In contrast, rapamycin extends life span of mice, when started late in life.11,52 Rapamycin also extends mouse lifespan when given as a brief (6 week) treatment to aged C57BL/6 mice.52 Thus in old humans, rapamycin rather than CR should be considered to extend health- and life span.
Noteworthy, physical exercise and IGF-I/nutrients cause mTOR-dependent increase of protein synthesis and muscle hypertrophy. In the aging muscle, this response is diminished.53,54 This may be caused by basal mTOR activity in aged mice,55 which precludes appropriate response to stimulation.53 Therefore, in order to prevent sarcopenia, one can consider an intermittent rapamycin treatment. This may decrease basal levels of mTOR activity (during rapamycin treatment), while allowing physical exercise and other physiological stimuli to induce mTOR (during rapamycin withdrawal). This prediction needs to be tested.
References
- 1. Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002; 10:457-68; PMID:12408816 [DOI] [PubMed] [Google Scholar]
- 2. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004; 18:1926-45; PMID:15314020 [DOI] [PubMed] [Google Scholar]
- 3. Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 2011; 189:1177-1201; PMID:22174183; http://dx.doi.org/ 10.1534/genetics.111.133363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010;40:310-322; PMID:20965424; http://dx.doi.org/ 10.1016/j.molcel.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle 2009;8:1883-1887; PMID:19448395 [DOI] [PubMed] [Google Scholar]
- 6. Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging 2009; 1:357-362; PMID:20157523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol 2011; 23(6):744-55; PMID:21963299; http://dx.doi.org/ 10.1016/j.ceb.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 8. Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol 2012;181: 1142-1146; PMID:22841821; http://dx.doi.org/ 10.1016/j.ajpath.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 9. Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013; 5:5; PMID:23513177; http://dx.doi.org/ 10.12703/P5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013;493:338-345; PMID:23325216; http://dx.doi.org/ 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature 2009;460:392-396; PMID:19587680; http://dx.doi.org/ 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011;66:191-201; PMID:20974732; http://dx.doi.org/ 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011;10:4230-4236; PMID:22107964; http://dx.doi.org/ 10.4161/cc.10.24.18486 [DOI] [PubMed] [Google Scholar]
- 14. Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, et al. Rapamycin reverses elevated mTORC1 signaling in lamin AC-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 2012;4:144ra103; PMID:22837538; http://dx.doi.org/ 10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH, III, Zhang Y, Becker KG, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One 2013;9:e83988; PMID:24409289; http://dx.doi.org/ 10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell 2012;11:675-82; PMID:22587563; http://dx.doi.org/ 10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle 2012;11. 845; PMID:22356747; http://dx.doi.org/ 10.4161/cc.11.5.19607 [DOI] [PubMed] [Google Scholar]
- 18. Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, et al. Rapamycin-mediated lifespan increase in mice is dose and sex-dependent and appears metabolically distinct from dietary restriction. Aging Cell 2013; 13(3):468-77; PMID:24341993; http://dx.doi.org/ 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selman C, Partridge L. A double whammy for aging? Rapamycin extends lifespan and inhibits cancer in inbred female mice. Cell Cycle 2012;11:17-18; PMID:22186783; http://dx.doi.org/ 10.4161/cc.11.1.18736 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin extends life and health in C57BL6 mice. J Gerontol A Biol Sci Med Sci 2014;69:119-130; PMID:23682161; http://dx.doi.org/ 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popovich IG, Anisimov VN, Zabezhinski MA, Semenchenko AV, Tyndyk ML, Yurova MN, Blagosklonny MV. Lifespan extension and cancer prevention in HER-2neu transgenic mice treated with low intermittent doses of rapamycin. Cancer Biol Ther 2014;15:586-592; PMID:24556924; http://dx.doi.org/ 10.4161/cbt.28164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blagosklonny MV. mTOR-driven aging: speeding car without brakes. Cell Cycle 2009;8:4055-4059; PMID:19923900 [DOI] [PubMed] [Google Scholar]
- 23. Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007;6:95-110; PMID:17266679 [DOI] [PubMed] [Google Scholar]
- 24. Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 2007;6:111-119; PMID:17266680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell 2011;10:185-90; PMID:21176090; http://dx.doi.org/ 10.1111/j.1474-9726.2010.00665.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta R, Chandler-Brown D, Ramos FJ, Shamieh LS, Kaeberlein M. Regulation of mRNA translation as a conserved mechanism of longevity control. Adv Exp Med Biol 2011;694:14-29; PMID:20886753 [DOI] [PubMed] [Google Scholar]
- 27. Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle 2009;8:4112-8; PMID:19946210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Diff. 2013;20:1241-9; PMID:23852369; http://dx.doi.org/ 10.1038/cdd.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leontieva OV, Blagosklonny MV. CDK46-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell Cycle 2013;12:3063-9; PMID:23974099; http://dx.doi.org/ 10.4161/cc.26130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leontieva OV, Blagosklonny MV. M(o)TOR of pseudo-hypoxic state in aging: rapamycin to the rescue. Cell Cycle 2014;13:(4):509-15; PMID:24496328; http://dx.doi.org/ 10.4161/cc.27973 [DOI] [PubMed] [Google Scholar]
- 31. Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159-65; PMID:22394614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho S, Hwang ES. Status of mTOR activity may phenotypically differentiate senescence and quiescence. Mol Cells 2012;33:597-604; PMID:22570149; http://dx.doi.org/ 10.1007/s10059-012-0042-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A 2010;107:9660-4; PMID:20457898; http://dx.doi.org/ 10.1073/pnas.1002298107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A 2012;109:13314-8; PMID:22847439; http://dx.doi.org/ 10.1073/pnas.1205690109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012;11:2391-401; PMID:22627671; http://dx.doi.org/ 10.4161/cc.20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serrano M. Dissecting the role of mTOR complexes in cellular senescence. Cell Cycle 2012;11:2231-2; PMID:22714590; http://dx.doi.org/ 10.4161/cc.21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 2012;11:401-14; PMID:22958932; http://dx.doi.org/ 10.1016/j.stem.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dulic V. Senescence regulation by mTOR. Methods Mol Biol 2013;965:15-35; PMID:23296649; http://dx.doi.org/ 10.1007/978-1-62703-239-1_2 [DOI] [PubMed] [Google Scholar]
- 39. Leontieva OV, Demidenko ZN, Blagosklonny MV. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc Natl Acad Sci U S A 2014; 111:8832-7; PMID:24889617; http://dx.doi.org/ 10.1073/pnas.1405723111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol 2012;47:958-65; PMID:22981852; http://dx.doi.org/ 10.1016/j.exger.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leontieva OV, Demidenko ZN, Blagosklonny MV. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis 2014;5:e1214; PMID:24810050; http://dx.doi.org/ 10.1038/cddis.2014.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010;468:1100-4; PMID:21179166; http://dx.doi.org/ 10.1038/nature09584 [DOI] [PubMed] [Google Scholar]
- 43. Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr 2007;137:1078-86; PMID:17374682 [DOI] [PubMed] [Google Scholar]
- 44. Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta 2009;1790:1067-1074; PMID:19539012; http://dx.doi.org/ 10.1016/j.bbagen.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle 2010;9:683-8; PMID:20139716 [DOI] [PubMed] [Google Scholar]
- 46. Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 2012;11:390-8; PMID:22210414; http://dx.doi.org/ 10.1016/j.arr.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaeberlein M, Kennedy BK. Ageing: a midlife longevity drug? Nature. 2009;460:331-2; PMID:19606132; http://dx.doi.org/ 10.1038/460331a [DOI] [PubMed] [Google Scholar]
- 48. Cava E, Fontana L. Will calorie restriction work in humans? Aging (Albany NY) 2013; 5:507-14; PMID:23924667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Emran S, Yang M, He X, Zandveld J, Piper MD. Target of rapamycin signaling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging (Albany NY) 2014; 6:390-8; PMID:24861087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 1982;215:1415-8; PMID:7063854 [DOI] [PubMed] [Google Scholar]
- 51. Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19:407-17; PMID:24606898; http://dx.doi.org/ 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2009;2:ra75; PMID:19934433; http://dx.doi.org/ 10.1126/scisignal.2000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11; PMID:21798089; http://dx.doi.org/ 10.1186/2044-5040-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. D’Antona G, Nisoli E. mTOR signaling as a target of amino acid treatment of the age-related sarcopenia. Interdiscip Top Gerontol 2010;37:115-41; PMID:20703059; http://dx.doi.org/ 10.1159/000319998 [DOI] [PubMed] [Google Scholar]
- 55. Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2014;14:303-23; PMID:23686362; http://dx.doi.org/ 10.1007/s10522-013-9432-9 [DOI] [PubMed] [Google Scholar]
- 56. Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012;4:899-916; PMID:23443503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 2014. Mar 22; 13(4):616-22; PMID:24655348; http://dx.doi.org/ 10.1111/acel.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]


