Abstract
In eukaryotes, the cyclin-dependent kinase Cdk1p (Cdc2p) plays a central role in entry into and progression through nuclear division during mitosis and meiosis. Cdk1p is activated during meiotic nuclear divisions by dephosphorylation of its tyrosine-15 residue. The phosphorylation status of this residue is largely determined by the Wee1p kinase and the Cdc25p phosphatase. In fission yeast, the forkhead-type transcription factor Mei4p is essential for entry into the first meiotic nuclear division. We recently identified cdc25+ as an essential target of Mei4p in the control of entry into meiosis I. Here, we show that wee1+ is another important target of Mei4p in the control of entry into meiosis I. Mei4p bound to the upstream region of wee1+ in vivo and in vitro and inhibited expression of wee1+, whereas Mei4p positively regulated expression of the adjacent pseudogene. Overexpression of Mei4p inhibited expression of wee1+ and induced that of the pseudogene. Conversely, deletion of Mei4p did not decrease expression of wee1+ but inhibited that of the pseudogene. In addition, deletion of Mei4p-binding regions delayed repression of wee1+ expression as well as induction of expression of the pseudogene. These results suggest that repression of wee1+ expression is primarily owing to Mei4p-mediated transcriptional interference.
Keywords: Cdk1, wee1, Mei4, meiosis 1, transcription, pseudogene
Abbreviations
- Cdk1
cyclin-dependent kinase 1
- MI
meiosis 1; Tyr15, tyrosine-15
Introduction
In meiosis, 2 consecutive rounds of chromosome segregation follow a single S phase, producing functional haploid gametes. A high level of homologous recombination occurs between S phase and the first meiotic nuclear division (meiosis I (MI)).1,2 The order of meiosis is regulated by the activities of the cyclin-dependent kinase 1 Cdk1p (also known as Cdc2p)3 and the DNA replication and damage checkpoint proteins.4 Based on the temporal expression of meiosis-specific genes (early, middle, and late), a transcriptional cascade of various meiotic genes regulates sporulation.5,6 In fission yeast, transcription of the middle genes is activated by the meiosis-specific transcription factor Mei4p.6 Mei4p is a member of the group of forkhead transcription factors that bind to the consensus sequence GTAAAYA, also known as FLEX.7,8 In fission yeast, there are 4 forkhead transcription factors, namely, Fkh2p, Fhl1p, Sep1p, and Mei4p.9 These proteins are involved in the cell cycle, mating, septation, and periodic gene expression.10–13 Among them, Mei4p is specifically required during meiosis. Mei4p is essential for recombination, the first meiotic division, and sporulation.8,14 Among the potential Mei4p target genes, spo6+ is required for sporulation8 and mde2+ is required for recombination.7,15
The activity of Cdc2p is not only regulated by the availability of cyclin but by the phosphorylation status of its tyrosine-15 (Tyr15) residue.3,16 During the mitotic cell cycle, the tyrosine kinase Wee1p phosphorylates Cdc2p on Tyr15 and thereby inhibits Cdc2p activity, whereas the tyrosine phosphatase Cdc25p dephosphorylates this residue and thereby activates Cdc2p.3,16 Similar to its regulation during the mitotic cycle, Cdc2p kinase activity gradually increases from meiotic ‘Start’ and peaks around meiotic nuclear division.4,17,18 Consistent with its kinase activity, the Tyr15 residue of Cdc2p is phosphorylated after meiotic ‘Start’ and decreases during meiotic nuclear division.4,17–19 This tyrosine phosphorylation is a rate-limiting step for entry into meiotic division, given that such entry is promoted by forced dephosphorylation of this residue.17
We have previously shown that cdc25+ is an essential target of Mei4p for progression through meiotic nuclear division.20 We also proposed that Mei4p is a rate-limiting factor for entry into MI and coordinates cell cycle progression with other meiotic events such as sporulation and double-strand break formation.20 Here, we show that Mei4p also regulates wee1+ expression, but this negatively affects entry into MI.
Results and Discussion
wee1+ is a target gene of Mei4p
We previously reported that Mei4p controls phosphorylation of Cdc2p on Tyr15.20 This suggests that wee1+ is also regulated by Mei4p, in addition to cdc25+. We examined the expression of wee1+ mRNA during meiosis by Northern blot analysis (Fig. 1A). To induce meiosis synchronously, we used a temperature-sensitive pat1 strain of Schizosaccharomyces pombe.21 Cells were synchronized in G1 phase by nitrogen deprivation and then shifted to the restrictive temperature to induce meiosis. The amount of wee1+ mRNA decreased around the onset of MI (5 h) in wild-type (WT) cells. By contrast, the level of wee1+ mRNA was almost constant in mei4+-deleted (mei4) cells. Furthermore, the level of wee1+ mRNA was decreased earlier (4 h) in mei4+-overproducing (mei4+-OP) cells. These results suggest that Mei4p negatively regulates wee1+ mRNA.
Figure 1.
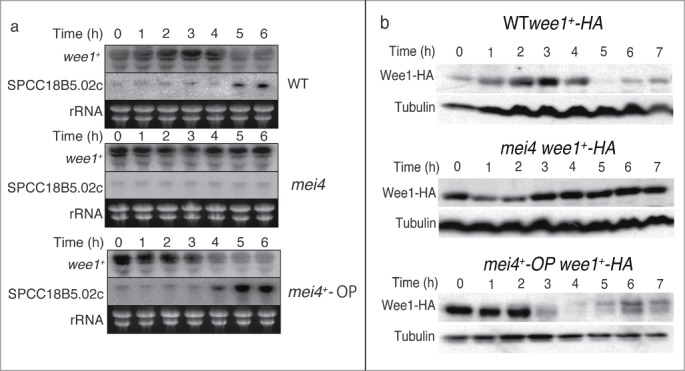
Expression of wee1+ and SPCC18B5.02c during meiosis and the effects of mei4+ deletion or overexpression. Cells of the indicated genotypes (wild-type (WT), HM1307; mei4, HM2163; mei4+-OP, HM4582; WT wee1+-HA, HM4732; wee1+-HA, mei4, HM4833; and mei4+-OP wee1+-HA, HM4735) were subjected to synchronous induction of meiosis as described in the Materials and Methods. Samples were collected at the indicated time points thereafter and subjected to Northern blot analysis of wee1+ and SPCC18B5.02c mRNA (rRNA (rRNA) was stained with ethidium bromide as the loading control) (A) or Western blot analysis with anti-HA and anti-α-tubulin (loading control) antibodies (B).
We next studied the amount of Wee1p protein during meiosis (Fig. 1B). Like the pattern of its mRNA expression, the level of Wee1p protein decreased around the onset of MI (5 h) in WT cells and then slightly increased at later time points. However, the amount of Wee1p was almost constant in mei4 cells. Additionally, the level of Wee1p was decreased earlier in mei4+-OP cells. These data suggest that the Wee1p protein level is negatively controlled by Mei4p, similar to its mRNA expression. The correlation between the expression of wee1+ at the mRNA and protein levels suggested that regulation of the abundance of wee1+ mRNA is important for the physiological function of this gene.
Regulation of the pseudogene adjacent to wee1+ by Mei4p
To address the mechanism by which Mei4p negatively regulates wee1+ mRNA, we measured the abundance of the gene (SPCC18B5.02c) that is located adjacent to wee1+ (Fig. 1A). SPCC18B5.02c is a cinnamoyl-CoA reductase gene (Sanger Center database, http://www.sanger.ac.uk/), although it has frame-shift mutations and is considered to be a pseudogene. The amount of SPCC18B5.02c mRNA increased around MI (5 h) in WT cells, but was not increased in mei4 cells (Fig. 1A). The induction of this mRNA occurred earlier in mei4+-OP cells (Fig. 1A). These data suggest that Mei4p positively regulates this pseudogene.
Mei4p binds to FLEX elements adjacent to wee1+
A 27-bp oligonucleotide harboring a FLEX heptameric core (GTAAAYA) is required for binding to Mei4p in vitro.8 We searched for core FLEX sequences in the genomic regions adjacent to wee1+. Five FLEX sequences (FLEX1, FLEX2, FLEX3, FLEX4, and FLEX5) were found within a 3-kb region upstream of the start codon of wee1+ (Fig. 2A).
Figure 2.
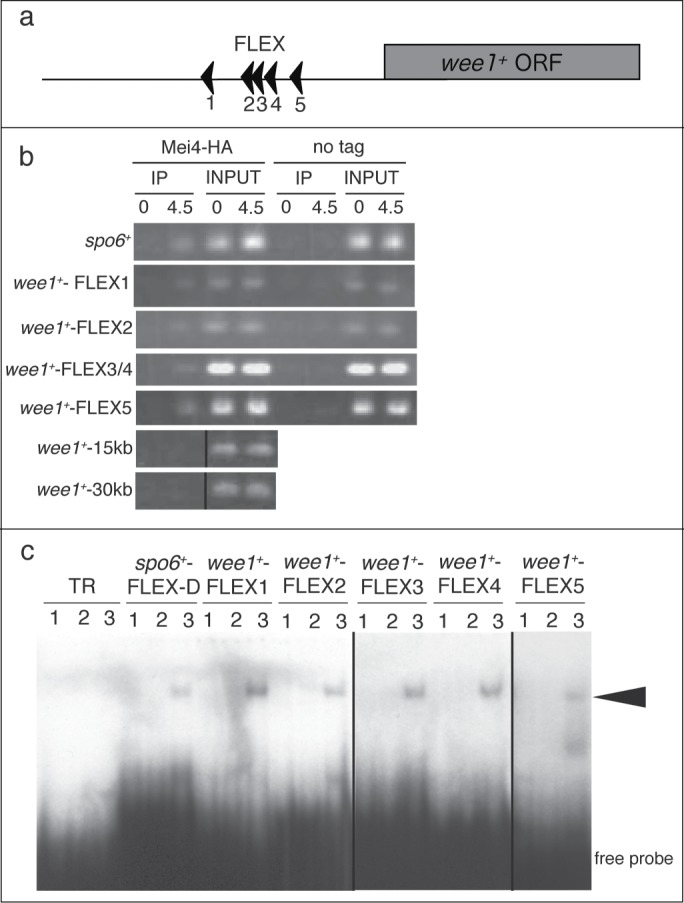
Mei4p binds to the FLEX sequences upstream of wee1+ both in vivo and in vitro. (A) Schematic representation of the positions of the FLEX sequences associated with wee1+. The arrowheads indicate the direction of the FLEX sequence from 5′ to 3′. ORF, open reading frame; (B) A chromatin immunoprecipitation assay of FLEX sites associated with wee1+ was performed with antibodies against HA or control IgG at the indicated times after induction of meiosis in cells expressing (Mei4-HA) or not expressing (no tag) mei4+-HA. wee1+-15kb and wee1+-30kb were about 15 kb and 30 kb downstream, respectively, of the start codon of wee1+ and were used as negative controls. TheFLEX-D site of spo6 was used as a positive control. The images of the immunoprecipitates of wee1+-15kb and wee1+-30kb are different from those of the inputs, but the experimental conditions were the same; (C) Electrophoretic mobility shift assay analysis of wee1+ FLEX sites with a recombinant glutathione S-transferase (GST)-Mei4p fusion protein. Buffer only (lane 1), GST (lane 2), or purified recombinant GST-Mei4p (71–182) (lane 3) were incubated with the indicated labeled oligonucleotide probe. The TR probe is unrelated to the FLEX sequence and was used as a negative control. The arrowhead indicates shifted bands. The image of wee1+−FLEX3 and wee1+−FLEX4 is different from the rest of the image, but the experimental conditions were the same.
We examined whether Mei4p can bind to the identified FLEX sequences adjacent to wee1+ by performing a chromatin immunoprecipitation (ChIP) assay. ChIP analysis showed that Mei4p associated with the FLEX1, FLEX2, FLEX3/4, and FLEX5 sites of wee1+, as well as with the FLEX-D site of spo6+, which was used as a positive control (Fig. 2B). This binding was greatly reduced at the 0 h time point, when the level of Mei4p is low.20 Furthermore, the association of Mei4p with a genomic region located ∼15 kb or 30 kb downstream of the start codon of wee1+ was minimal. These results showed that Mei4p binds to the FLEX sites adjacent to wee1+ in vivo when the abundance of wee1+ mRNA is low.
To examine whether Mei4p can bind directly to the FLEX sites adjacent to wee1+ in vitro, we performed an electrophoretic mobility shift assay (EMSA) with a fusion protein composed of glutathione S-transferase (GST) and the forkhead domain of Mei4p (amino acids 71–182) as described previously,20 together with radioactive oligonucleotides containing FLEX1, FLEX2, FLEX3, FLEX4, or FLEX5 of wee1+ as probes. A band shift with each of the 5 wee1+ FLEX probes was observed only in the presence of the fusion protein, not in its absence or in the presence of GST alone (Fig. 2C). These were similar to the results obtained with a spo6+ FLEX-D probe, which was used as a positive control.8 A shifted band was not detected with the GST-Mei4 fusion protein and a negative control (TR) oligonucleotide (Fig. 2C). These data suggested that Mei4p binds directly in vitro to all 5 FLEX elements located close to wee1+.
Role of the FLEX sites in the control of wee1+ mRNA expression
We next examined whether the FLEX sequences associated with wee1+ function as transcriptional cis elements. We deleted the chromosomal region containing FLEX1, FLEX2, FLEX3, and FLEX4 of wee1+ (Fig. 3A). In the resulting cells, wee1+ mRNA was downregulated 1 h later than in WT cells following the induction of meiosis, and induction of the adjacent pseudogene was also delayed (Fig. 3B). This result shows that 4 of the FLEX elements located close to wee1+ are required for efficient down-regulation of wee1+ mRNA and up-regulation of the adjacent pseudogene mRNA. This suggests that these sites serve as cis-acting elements for Mei4p.
Figure 3.
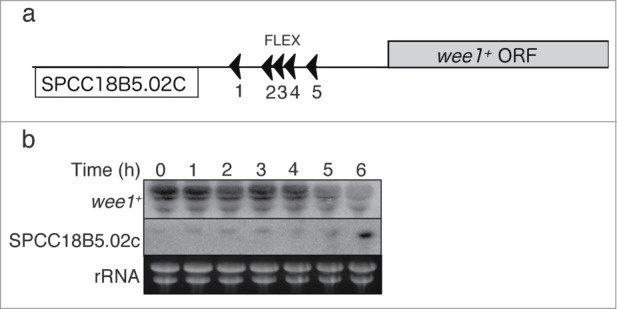
Effects of deletion of the FLEX sites near to wee1+ on wee1+ gene expression. (A) Schematic representation of the wee1+ and SPCC18B5.02c loci according to the Sanger Center database. ORF, open reading frame; (B) The region of the wee1+ gene containing FLEX1, FLEX2, FLEX3, and FLEX4 was deleted (HM5678). Meiosis was induced in the resulting cells as described in Fig. 1. Samples were collected at the indicated time points after meiosis induction and subjected to Northern blot analysis of wee1+ and SPCC18B5.02c mRNA. Ribosomal RNA (rRNA) was stained with ethidium bromide as the loading control.
Negative regulation of wee1+ expression by Mei4p
This study of the role of Mei4p in the control of meiotic nuclear division in fission yeast revealed that: (1) the major target of Mei4p in this context is wee1+, in addition to cdc25+; (2) Mei4p down-regulates wee1+ mRNA, whereas it up-regulates the adjacent pseudogene mRNA; and (3) FLEX sequences are located close to wee1+ and function as cis-regulatory elements for Mei4p.
We propose the following model (Fig. 4). Before MI, wee1+ is transcribed by an unknown transcription factor, whereas the adjacent pseudogene is not expressed. When Mei4p is produced, Mei4p binds to the FLEX regions close to wee1+ and displaces the unknown transcription factor at the onset of MI. The affinity of Mei4p for the FLEX regions is likely stronger than that of the unknown transcription factor. Thereafter, the level of wee1+ mRNA decreases, as well as the level of Wee1p protein. Conversely, the level of the pseudogene mRNA increases. Due to the downregulation of Wee1p, phosphorylation of the Tyr15 residue of Cdc2p decreases, Cdc2p is activated, and MI begins. In this way, Mei4p negatively regulates wee1+, whereas it positively regulates transcription of the adjacent psudogene. In the process of transcriptional interference, a transcriptional event affects another transcriptional process.22 Therefore, inhibition of wee1+ expression is likely primarily owing to Mei4p-mediated transcriptional interference.
Figure 4.
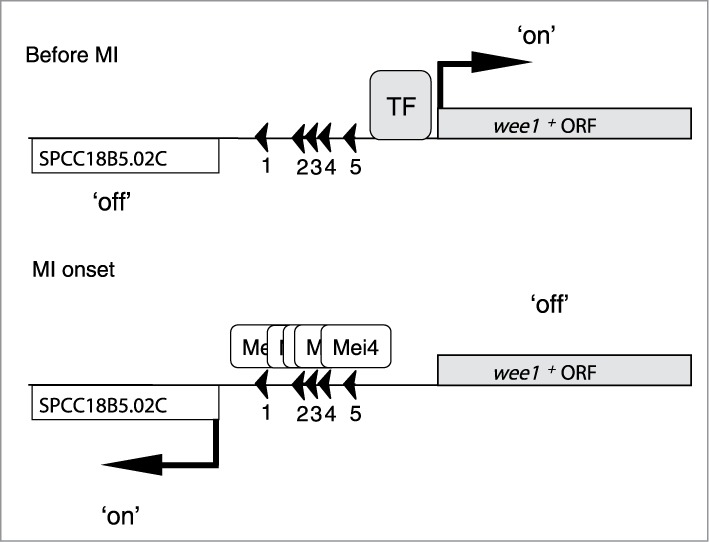
Model of wee1+ regulation during meiosis. See the text for details. MI, meiosis I; ORF, open reading frame; TF, transcription factor.
Positive and negative regulation of genes by the same transcription factor has been described previously. In budding yeast, Fkh1p and Fkh2p both activate and inhibit transcription.23 The serine-dependent factor Cha4p also serves as both an activator and repressor of transcription simultaneously.24 In mammals, the tumor suppressor p53 up-regulates the transcription of genes involved in several essential processes including cell cycle control and apoptosis.25 It also acts as a transcriptional repressor by competing with more efficient activators for DNA binding or by blocking the functions of DNA-bound activators.26–28 Similar to p53, it is possible that Mei4p negatively regulates its target genes by competing with an activator for binding to overlapping sites in the gene promoter. The promoter context, such as the relative positions of the Mei4p-binding sites and other factors, may influence whether Mei4p activates or represses transcription.
Control of wee1+ during mitosis versus meiosis
During the mitotic cell cycle, the level of wee1+ mRNA does not fluctuate markedly, although the Wee1p protein level moderately fluctuates in fission yeast.29 Recently, it has been proposed that Wee1p is localized at the center of the cell and its activity is regulated by the medially-located sensor Cdr2 in actively proliferating cells.30,31 In this way, a geometry-sensing mechanism is used to coordinate cell length and division. However, during meiosis, cells do not grow and cell length is relatively constant. Therefore, it would be difficult to use a geometry-sensing mechanism to regulate Wee1p. It seems likely that Mei4p evolved later to transcriptionally regulate wee1+ mRNA during meiosis.
Simultaneous transcriptional regulation of wee1+ and cdc25+
We have shown that Mei4p regulates wee1+ (this study) and cdc25+20 simultaneously. This simultaneous regulation is related to 2 observations. First, overexpression of mei4+ induces earlier meiotic nuclear division and Mei4p is a rate-limiting factor for entry into MI.20 Simultaneous regulation of wee1+ and cdc25+ by Mei4p decreases wee1+ mRNA expression and increases cdc25+ mRNA expression. Consequently, the protein levels of Wee1p and Cdc25p change according to their mRNA levels. Therefore, phosphorylation of the Tyr15 residue of Cdc2p decreases rapidly and its kinase activity increases quickly. Second, when mei4+ is disrupted, MI does not occur.8,20 Simultaneous regulation of wee1+ and cdc25+ likely contributes to this tight control. We conclude that Mei4p is a critical regulator of MI by activating cdc25+ transcription and inhibiting wee1+ transcription.
Experimental Procedures
Fission yeast strains
The strains used in the present study were constructed according to standard procedures32 and are listed in Table S1. Synchronous meiosis was induced in liquid culture with temperature-sensitive pat1 mutants as described previously.2
Primers and probes
The primers and double-stranded oligonucleotides used in the present study are listed in Table S2.
Isolation of RNA and Northern blot analysis
Total RNA was isolated from synchronized pat1 cells as described previously.32 Aliquots (5–6 μg) of the isolated RNA were subjected to Northern blot analysis. For probes, fragments of open reading frames were amplified from genomic DNA by PCR with the following sets of primers: 477 and 478 for wee1+ and 1040 and 1041 for SPCC18B5.02c.
Immunoblot analysis
Whole cell extracts (20 μg of protein) were prepared by the boiling method17 and analyzed as described previously.17,33
ChIP
ChIP and quantitative real-time PCR analyses were performed as described previously.20 Aliquots of precipitated DNA as well as DNA fragments isolated from 0.4 mg of whole cell extract were subjected to semi-quantitative PCR analysis with the following primers: wee1+−FLEX1, 915 and 916; wee1+−FLEX2, 866 and 867; wee1+−FLEX3/4, 868 and 869; wee1+−FLEX5, 913 and 914; wee1+−15K, 870 and 871; and wee1+−30K, 872 and 873.
EMSA analysis
EMSA analysis was performed as described previously.20 The double-stranded oligonucleotides used as probes were as follows: TR, 742 and 743; spo6+−FLEX, 740 and 741; wee1+−FLEX1, 907 and 908; wee1+−FLEX2, 909 and 910; wee1+−FLEX3, 860 and 861; wee1+−FLEX4, 862 and 863; and wee1+−FLEX5, 911 and 912.
Deletion of wee1+ FLEX elements from the chromosome
The chromosomal region containing FLEX1, FLEX2, FLEX3, and FLEX4 of wee1+ was replaced with a PCR-generated DNA fragment containing the corresponding region interrupted by kanr.
Funding Statement
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to H M and Y M-T).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank C Shimoda and P Nurse for providing yeast strains, and A Tochigi for technical help.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Roeder GS. Meiotic chromosomes: it takes two to tango. Genes Dev 1997; 11:2600-21; PMID:9334324; http://dx.doi.org/ 10.1101/gad.11.20.2600 [DOI] [PubMed] [Google Scholar]
- 2. Tonami Y, Murakami H, Shirahige K, Nakanishi M. A checkpoint control linking meiotic S phase and recombination initiation in fission yeast. Proc Natl Acad Sci USA 2005; 102:5797-801; PMID: 15805194; http://dx.doi.org/ 10.1073/pnas.0407236102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacNeill SA, Nurse P. Cell cycle control in fission yeast, schizosaccharomyces pombe. In: Pringle JR, Broach JR, Jones EW. (Eds), The Molecular and Cellular Biology of the Yeast Saccharomyces: Life Cycle and Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1997, pp 697-763 [Google Scholar]
- 4. Murakami H, Nurse P. DNA replication and damage checkpoints and meiotic cell cycle controls in the fission and budding yeasts. Biochem J 2000; 349:1-12; PMID:10861204; http://dx.doi.org/ 10.1042/0264-6021:3490001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu S, Herskowitz I. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell 1998; 1:685-96; PMID:9660952; http://dx.doi.org/ 10.1016/S1097-2765(00)80068-4 [DOI] [PubMed] [Google Scholar]
- 6. Mata J, Lyne R, Burns G, Bahler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet 2002; 32:143-7; PMID:12161753; http://dx.doi.org/ 10.1038/ng951 [DOI] [PubMed] [Google Scholar]
- 7. Abe H, Shimoda C. Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics 2000; 154:1497-508; PMID:10747048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horie S, Watanabe Y, Tanaka K, Nishiwaki S, Fujioka H, Abe H, Yamamoto M, Shimoda C. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol Cell Biol 1998; 18:2118-29; PMID:9528784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murakami H, Aiba H, Nakanishi M, Murakami-Tonami Y. Regulation of yeast forkhead transcription factors and FoxM1 by cyclin-dependent and polo-like kinases. Cell Cycle 2010; 9:3233-42; PMID:20716958; http://dx.doi.org/ 10.4161/cc.9.16.12599 [DOI] [PubMed] [Google Scholar]
- 10. Shimada M, Yamada-Namikawa C, Murakami-Tonami Y, Yoshida T, Nakanishi M, Urano T, Murakami H. Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast. EMBO J 2008; 27:132-42; PMID:18059475; http://dx.doi.org/ 10.1038/sj.emboj.7601949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bulmer R, Pic-Taylor A, Whitehall SK, Martin KA, Millar JB, Quinn J, Morgan BA. The forkhead transcription factor Fkh2 regulates the cell division cycle of Schizosaccharomyces pombe. Eukaryotic cell 2004; 3:944-54; PMID:15302827; http://dx.doi.org/ 10.1128/EC.3.4.944-954.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szilagyi Z, Batta G, Enczi K, Sipiczki M. Characterisation of two novel fork-head gene homologues of Schizosaccharomyces pombe: their involvement in cell cycle and sexual differentiation. Gene 2005; 348:101-9; PMID:15777722; http://dx.doi.org/ 10.1016/j.gene.2004.12.043 [DOI] [PubMed] [Google Scholar]
- 13. Buck V, Ng SS, Ruiz-Garcia AB, Papadopoulou K, Bhatti S, Samuel JM, Anderson M, Millar JB, McInerny CJ. Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast. J Cell Sci 2004; 117:5623-32; PMID:15509866; http://dx.doi.org/ 10.1242/jcs.01473 [DOI] [PubMed] [Google Scholar]
- 14. Bresch C, Muller G, Egel R. Genes involved in meiosis and sporulation of a yeast. Mol Gen Genet 1968; 102:301-6; PMID:5743433; http://dx.doi.org/ 10.1007/BF00433721 [DOI] [PubMed] [Google Scholar]
- 15. Gregan J, Rabitsch PK, Sakem B, Csutak O, Latypov V, Lehmann E, Kohli J, Nasmyth K. Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr Biol 2005; 15:1663-9; PMID: 16169489; http://dx.doi.org/ 10.1016/j.cub.2005.07.059 [DOI] [PubMed] [Google Scholar]
- 16. Nurse P. Universal control mechanism regulating onset of M-phase. Nature 1990; 344:503-8; PMID:2138713; http://dx.doi.org/ 10.1038/344503a0 [DOI] [PubMed] [Google Scholar]
- 17. Murakami H, Nurse P. Meiotic DNA replication checkpoint control in fission yeast. Genes Dev 1999; 13:2581-93; PMID:10521402; http://dx.doi.org/ 10.1101/gad.13.19.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borgne A, Murakami H, Ayte J, Nurse P. The G1/S cyclin Cig2p during meiosis in fission yeast. Mol Biol Cell 2002; 13:2080-90; PMID:12058071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daya Makin M, Szankasi P, Tang L, MacRae D, Pelech SL. Regulation of p105wee1 and p34cdc2 during meiosis in Schizosaccharomyces pombe. Biochem Cell Biol 1992; 70:1088-96; PMID:1297333; http://dx.doi.org/ 10.1139/o92-154 [DOI] [PubMed] [Google Scholar]
- 20. Murakami-Tonami Y, Yamada-Namikawa C, Tochigi A, Hasegawa N, Kojima H, Kunimatsu M, Nakanishi M, Murakami H. Mei4p coordinates the onset of meiosis I by regulating cdc25+ in fission yeast. Proc Natl Acad Sci U S A 2007; 104:14688-93; PMID:17804800; http://dx.doi.org/ 10.1073/pnas.0702906104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto M, Imai Y, Watanabe Y. Mating and Sporulation in Scizosaccharomyces pombe. In: Pringle JR, Broach JR, Jones EW. (Eds), The Molecular and Cellular Biology of the Yeast Saccharomyces: Life Cycle and Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1997, pp 1037-106 [Google Scholar]
- 22. Shearwin KE, Callen BP, Egan JB. Transcriptional interference–a crash course. Trends Genet 2005; 21:339-45; PMID:15922833; http://dx.doi.org/ 10.1016/j.tig.2005.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu G, Spellman PT, Volpe T, Brown PO, Botstein D, Davis TN, Futcher B. Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature 2000; 406:90-4; PMID:10894548; http://dx.doi.org/ 10.1038/35021046 [DOI] [PubMed] [Google Scholar]
- 24. Martens JA, Wu PY, Winston F. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev 2005; 19:2695-704; PMID:16291644; http://dx.doi.org/ 10.1101/gad.1367605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 2002; 2:594-604; PMID:12154352; http://dx.doi.org/ 10.1038/nrc864 [DOI] [PubMed] [Google Scholar]
- 26. Lee KC, Crowe AJ, Barton MC. p53-mediated repression of α-fetoprotein gene expression by specific DNA binding. Mol Cell Biol 1999; 19:1279-88; PMID:9891062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson RA, Ince TA, Scotto KW. Transcriptional repression by p53 through direct binding to a novel DNA element. J Biol Chem 2001; 276:27716-20; PMID:11350951; http://dx.doi.org/ 10.1074/jbc.C100121200 [DOI] [PubMed] [Google Scholar]
- 28. Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem 2002; 277:3247-57; PMID:11714700; http://dx.doi.org/ 10.1074/jbc.M106643200 [DOI] [PubMed] [Google Scholar]
- 29. Aligue R, Wu L, Russell P. Regulation of schizosaccharomyces pombe wee1 tyrosine kinase. J Biol Chem 1997; 272:13320-5; PMID:9148953; http://dx.doi.org/ 10.1074/jbc.272.20.13320 [DOI] [PubMed] [Google Scholar]
- 30. Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 2009; 459:852-6; PMID:19474792; http://dx.doi.org/ 10.1038/nature08054 [DOI] [PubMed] [Google Scholar]
- 31. Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 2009; 459:857-60; PMID:19474789; http://dx.doi.org/ 10.1038/nature08074 [DOI] [PubMed] [Google Scholar]
- 32. Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast schizosaccharomyces pombe. Methods Enzymol 1991; 194:795-823; PMID:2005825; http://dx.doi.org/ 10.1016/0076-6879(91)94059-L [DOI] [PubMed] [Google Scholar]
- 33. Sugimoto I, Murakami H, Tonami Y, Moriyama A, Nakanishi M. DNA replication checkpoint control mediated by the spindle checkpoint protein Mad2p in fission yeast. J Biol Chem 2004; 279:47372-8; PMID:15347659; http://dx.doi.org/ 10.1074/jbc.M403231200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


