Abstract
The subventricular zone is one of the 2 germinal niches of the adult brain where neural stem cells (NSC) generate new neurons and glia throughout life. NSC behavior is controlled by the integration of intrinsic signals and extrinsic cues provided by the surrounding microenvironment, or niche. Within the niche, the vasculature has emerged as a critical compartment, to which both neural stem cells and transit-amplifying progenitors are closely associated. A key function of the vasculature is to deliver blood-borne and secreted factors that promote proliferation and lineage progression of committed neural progenitors. We recently found that, in contrast to the established role of soluble cues, juxtacrine signals on vascular endothelial cells maintain neural stem cells in a quiescent and undifferentiated state through direct cell-cell interactions. In this perspective, we discuss how, through these apparently opposing signals, the vascular niche might coordinate stem cell decisions between maintenance and proliferation.
Keywords: adult neurogenesis, cell-cell signaling, neural stem cells, subventricular zone, quiescence, vascular niche
The adult brain contains 2 discrete regions where neural stem cells continuously generate new neurons: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus.1-3 Within the SVZ, neural stem cells, termed type-B cells, are largely quiescent cells with some structural and molecular characteristics of astrocytes.4 Upon activation to a proliferative state, type B stem cells give rise to rapidly proliferating transit amplifying type C progenitors, which first divide symmetrically to expand the progenitor pool and later differentiate into type A neuroblasts. Newborn neuroblasts then exit the SVZ and migrate in chains ensheathed by mature astrocytes along the rostral migratory stream (RMS) to the olfactory bulb where they terminally differentiate into inhibitory interneurons.1,4-7
The cytoarchitecture of the SVZ niche has been well characterized.1,8,9 Type-B, type-C and type-A progenitors are enclosed between 2 main SVZ niche compartments: an apical layer of ependymal cells that line the lateral ventricle, separating the SVZ from the cerebrospinal fluid (CSF), and a basal planar plexus of blood vessels. Type-B cells have a radial morphology and bridge both compartments. Apically, they directly intercalate into the ependymal layer and contact the CSF through a non-motile primary cilium.8,10,11 Basally, they extend long projections, which make direct and stable contact with blood vessels at specialized terminal endfeet.4,8,11,12 Type-C cells are closely apposed to both type-B progenitors and blood vessels, but make smaller and more transient contacts with the vasculature.11,12 Finally, type-A cells predominantly contact the basal processes of type-B cells, which wrap around them.8 Therefore, type-B cells are uniquely poised to receive signals from all SVZ niche compartments, including their immediate progeny, the ependymal/CSF compartment and the vascular/blood compartment. While many of these signals have been identified, how they are integrated to direct neural stem cell behavior, is much less clear.1 Below, we focus on the current knowledge of the vascular regulation of SVZ neural stem cells and their progeny.
Several lines of evidence indicate that soluble signaling cues from the vasculature promote proliferation and differentiation of neural progenitor cells, particularly actively dividing cells, such as activated type-B and type-C cells. First, detailed morphological analysis of the 3-dimensional organization of the niche has revealed that within the SVZ these 2 progenitor subtypes are the cells most closely associated to blood vessels.11,12 Second, the SVZ blood brain barrier (BBB) has been shown to be more permeable than other brain regions due to a relative lack of blood vessel coverage by astrocytic endfeet and pericytes.11,13 Furthermore, activated progenitors often contact the vasculature at these sites, suggesting that they might be directly exposed to blood-borne molecules such as hormones and growth factors, and have immediate access to the vascular basal lamina to which they adhere through α6β1 integrins.11 Finally, vascular endothelial cells themselves produce soluble factors that regulate progenitor cell behavior. This was first demonstrated by Shen and colleagues, who reported that endothelial secreted cues promote progenitor cell self-renewal and prime the cells for neuronal differentiation.14 Consistent with these observations, 2 main studies have since identified specific vascular mitogenic factors, pigment epithelium-derived factor (PEDF) and betacellulin (BTC), which are both secreted by SVZ endothelial cells in vivo. PEDF promotes neural stem cell self-renewal by enhancing Notch signaling in activated type-B cells, which in turn converts their division mode from asymmetric to symmetric.15,16 BTC instead activates EGFR and ErbB4 in type-C and type-A cells, respectively, and increases their proliferation through activation of canonical ERK signaling.17 Furthermore, endothelial cells have been shown to secrete cues that promote neuronal differentiation of type-C cells as well as survival and migration of type-A cells. The effects on type-A cells are of particular note, as blood vessels, which run parallel to the neuroblast chains in the RMS, also serve as scaffolds for guiding their migration to the olfactory bulb. Specifically, a few studies have identified a critical role for endothelial brain-derived neurotrophic factor (BDNF) in both processes.18-21 Similarly, endothelium-secreted SDF1 was also shown to stimulate neuroblast motility and perivascular migration.22
Coupled with the finding that SDF-1 also promotes the homing of transplanted proliferating neural progenitor cells to the vasculature,22,23 this evidence suggests that the SVZ vascular compartment plays an important role in supporting lineage progression of progenitor cells that have already committed to differentiation and are actively cycling.16 However, while the link between the vasculature and proliferation is unquestionable, the discovery that quiescent type-B cells are in intimate physical contact with blood vessels through their basal end feet, suggested additional roles for the vascular niche and raised important questions.8 In particular, do these specialized neurovascular contacts have a functional significance and given the intimate association to the vasculature, what prevents type-B cells from responding to vascular mitogens by undergoing activation and proliferation?
To begin to address these questions and identify potential regulatory mechanisms, we initially took a reductionist approach and investigated the effects of direct co-culture of SVZ neural precursor cells (NPC) with brain microvascular endothelial cells in vitro.24 Remarkably, we found that direct cell-cell contact, independent of soluble endothelial-secreted factors, arrested the stem cells in the G0/G1 phase of the cell cycle and induced expression of type-B marker genes. Furthermore, we showed that under culture conditions that promote NPC differentiation, contact with endothelial cells fully suppressed differentiation into both glial and neuronal lineages while maintaining the NPC as type-B marker positive, quiescent cells. Overall, these findings indicated that juxtacrine (i.e. cell-cell contact-dependent) signaling cues on endothelial cells override intrinsic differentiation programs and extrinsic mitogenic cues, to maintain stem cells as quiescent, type-B like cells and inhibit their differentiation.
Using loss and gain-of-function approaches in co-culture we further identified the membrane-bound ligands Ephrin-B2 and Jagged-1 on endothelial cells as the main mediators of these effects. Specifically, we found that endothelial Ephrin-B2 mainly suppresses NPC cell-cycle entry by activating Eph signaling, which in turn quenches MAPK signaling downstream of extracellular mitogens resulting in the downregulation of Cyclin-D1. However, our results indicated that Eph signaling did not affect cell fate. By contrast, we showed that Jagged-1 mostly maintained type-B cell identity through activation of canonical Notch signaling and downstream target gene expression, in the absence of a robust effect on proliferation. Therefore, these 2 pathways appear to function cooperatively, but independently.
Together, our results in vitro pointed to a role for vascular Ephrin-B2 and Jagged-1 at the basal neurovascular contacts between type-B end feet and endothelial cells in the SVZ. If these signals were indeed important for the maintenance of quiescent type-B cells in vivo, we would expect that in their absence, type-B cell behavior would be deregulated in favor of lineage progression. Indeed, we found this to be the case: upon acute conditional genetic deletion of either Ephrin-B2 or Jagged-1 from endothelial cells in the adult brain in vivo, we observed increased activation of quiescent type-B cells in the absence of changes in other progenitor subpopulations. This aberrant activation increased neuron production in the olfactory bulb initially, but later depleted the stem cell pool resulting in an overall loss of neurogenesis.
Interestingly, while in vitro Ephrin-B2 and Jagged-1 affected different cellular processes, namely quiescence vs. fate, we found a very similar phenotype upon deletion of either ligand in vivo. A likely explanation for this apparent paradox is that both signals are required to enforce neural stem cell maintenance, but do so by controlling different downstream mechanisms. Indeed, maintenance is a complex and actively regulated process, by which the integration of a multitude of intrinsic and extrinsic cues keeps stem cells in a quiescent and undifferentiated state to protect them from damage and prevent depletion.13,25 Thus, although Jagged-1 does not directly control the cell-cycle of type-B cells, its deletion results in aberrant activation of the cells to a state that is intrinsically more proliferative. Conversely, while Ephrin-B2 does not regulate fate per se, its loss, by de-repressing quiescence signals, favors the acquisition of an activated type-B fate. Our results in double Ephrin-B2/Jagged1 endothelial knock-out mice support this idea, as concomitant deletion of both ligands further exacerbated the activation phenotype, confirming that the pathways are independent also in vivo and function combinatorially to suppress the transition of type-B stem cells from quiescence to activation.24
Our data therefore suggested that in addition to proliferation, the vasculature is also a niche for stem cell maintenance. This idea has been further supported by recent work from Delgado et al., which showed that endothelial- and choroid plexus-derived NT3 suppresses type-B cell proliferation and is required for their long-term maintenance through a mechanism dependent on nitric oxide synthase.26 Thus, a picture is beginning to emerge of a dual control of neural progenitor behavior by the vascular niche. In this view, blood vessels both maintain quiescent type-B stem cells through a combination of secreted and cell contact-dependent signals and, concomitantly, promote proliferation and differentiation of activated type-B cells and transit-amplifying progenitors through soluble cues.
How then can the same niche compartment achieve such differential control of its resident neural progenitors? While additional work will be required to fully answer this question, from current evidence it is tempting to speculate that the interplay of cell intrinsic differences and mode of vascular interaction among progenitor cells might be responsible. It is well established that the complement of cell surface receptors expressed by neural stem cells changes during lineage progression, as exemplified by EGFR, which is undetectable in quiescent type-B cells, upregulated upon their activation, highly expressed in type-C progenitors and downregulated in neuroblasts.27-29 Thus, quiescent type-B cells might not respond to vascular proliferating cues simply because they do not express the molecular machinery required to sense them. However, a recent characterization of the gene expression profiles of quiescent and activated type-B cells has revealed that quiescent stem cells are enriched for genes involved in cell communication and response to extrinsic cues, suggesting that these cells are primed for activation, yet other prevailing signals might actively maintain their quiescence.29 These signals are likely to be a combination of cell intrinsic and non-cell autonomous mechanisms. Indeed, a very recent study by Crouch et al, demonstrated that quiescent type-B cells are intrinsically resistant to the mitogenic activity of vascular secreted factors even when they express the relevant receptors, as shown for placental growth factor 2 (PIGF2) by the authors.30 Our work indicates that direct cell-cell interactions with the vascular niche are also critical in the active maintenance of neural stem cell quiescence, in that juxtacrine signals presented at the site of neurovascular contact render the stem cells refractory to mitogenic cues and differentiation programs.24 Additionally, as the specialized end feet through which these cues are presented are unique to type-B cells, this mechanism enables the selective inhibition of stem cell proliferation, while permitting the unimpeded proliferation of their more differentiated progeny in response to vascular mitogens.8
Great progress has been made over the past decade in elucidating the regulation of adult neurogenesis by the vascular niche.31,32 Together, these studies have revealed a perhaps unanticipated level of complexity in the system and the presence of multiple co-existing regulatory mechanisms that jointly orchestrate maintenance, proliferation and differentiation of most, if not all, progenitor subpopulations. Many questions however still remain unanswered. For example, what other vascular cues are involved and do these signals change in response to physiological and pathological stimuli to modulate neurogenesis accordingly. Similarly, how is type-B activation induced within a quiescence-promoting environment? Addressing these questions will be an exciting challenge for the future and holds great promise for the identification of strategies aimed at harnessing neurogenesis for brain repair and the treatment of neurodegenerative disease.
Figure 1.
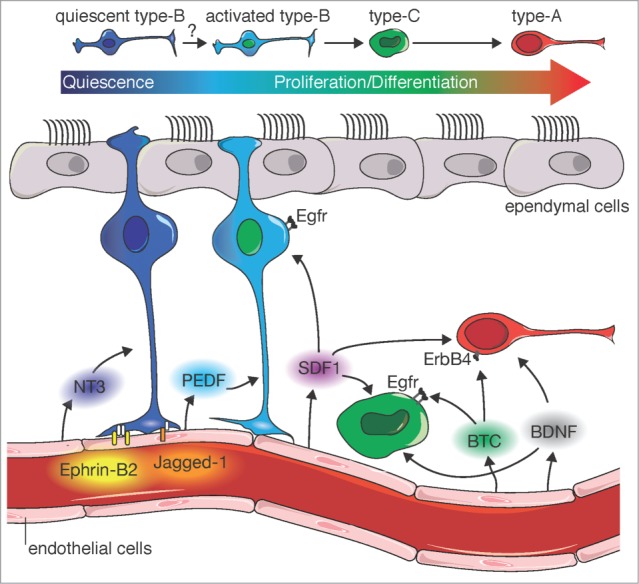
Model for vascular regulation of SVZ neurogenesis. Juxtacrine signals and paracrine factors from vascular endothelial cells (Ephrin-B2, Jagged-1, NT-3) cooperate with cell-intrinsic programs to maintain type-B cells in a quiescent and undifferentiated state in which they are refractory to proliferative cues. In contrast, activated type-B cells and their type-C progeny sense and respond to paracrine vascular cues, which promote their proliferation and neuronal differentiation into type-A cells (PEDF, BTC, BDNF). Paracrine factors such as BDNF and SDF1 induce Type-A neuroblast migration along blood vessels to the olfactory bulb.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank B. Krusche for help with artwork.
Funding
This work was supported by the Medical Research Council.
References
- 1.Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 2011; 70:674-86; PMID:21609824; http://dx.doi.org/ 10.1016/j.neuron.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132:645-60; PMID:18295581; http://dx.doi.org/ 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- 3.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005; 28:223-50; http://dx.doi.org/ 10.1146/annurev.neuro.28.051804.101459; PMID:16022595 [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97:703-16; PMID:10380923; http://dx.doi.org/ 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Verdugo JM, Doetsch F, Wichterle H., Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol 1998; 36:234-48; PMID:9712307 [DOI] [PubMed] [Google Scholar]
- 6.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 2003; 6:507-8; PMID:12704391; http://dx.doi.org/ 10.1038/nn1048 [DOI] [PubMed] [Google Scholar]
- 7.Doetsch F., Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci: Off J Soc Neurosci 1997; 17:5046-61; PMID:9185542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzadeh Z, Merkle FT., Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008; 3:265-78; PMID:18786414; http://dx.doi.org/ 10.1016/j.stem.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell 2009; 4:507-10; PMID:19497279; http://dx.doi.org/ 10.1016/j.stem.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A 1999; 96:11619-24; PMID:10500226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008; 3:289-300; PMID:18786416; http://dx.doi.org/ 10.1016/j.stem.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008; 3:279-88; PMID:18786415; http://dx.doi.org/ 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev. Mol Cell Biol 2013; 14:329-40; PMID:23698583; http://dx.doi.org/ 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004; 304:1338-40; PMID:15060285; http://dx.doi.org/ 10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, Mira H, Escribano J, Fariñas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci 2006; 9:331-339; PMID:16491078; http://dx.doi.org/ 10.1038/nn1657 [DOI] [PubMed] [Google Scholar]
- 16.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci 2009; 12:1514-23; PMID:19898467; http://dx.doi.org/ 10.1038/nn.2437 [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Gaviro MV, Scott CE, Sesay AK, Matheu A, Booth S, Galichet C, Lovell-Badge R. Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc Natl Acad Sci U S A 2012; 109:1317-22; PMID:22232668; http://dx.doi.org/ 10.1073/pnas.1016199109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leventhal C, Rafii S, Rafii D, Shahar A Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 1999; 13:450-64, http://dx.doi.org/ 10.1006/mcne.1999.0762; PMID:10383830 [DOI] [PubMed] [Google Scholar]
- 19.Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Götz M, Barker PA, et al.. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci: Off J Soc Neurosci 2009; 29:4172-88, http://dx.doi.org/ 10.1523/JNEUROSCI.4956-08.2009; PMID:19339612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci U S A 1995; 92:210-214; PMID:7816819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol 2009; 516:94-104; http://dx.doi.org/ 10.1002/cne.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 2010; 7:163-73; PMID:20682445; http://dx.doi.org/ 10.1016/j.stem.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornsson CS, Apostolopoulou M, Tian Y, Temple S. It Takes a village: constructing the neurogenic niche. Dev Cell 2015; 32:435-46; PMID:25710530; http://dx.doi.org/ 10.1016/j.devcel.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 2014; 16:1045-56; PMID:25283993; http://dx.doi.org/ 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132:598-611; PMID:18295578; http://dx.doi.org/ 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado AC, Ferrón SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocón P, Fariñas I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 2014; 83:572-85; PMID:25043422; http://dx.doi.org/ 10.1016/j.neuron.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 27.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A 2009; 106:6387-92, http://dx.doi.org/ 10.1073/pnas.0810407106; PMID:19332781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 2002; 36:1021-34; PMID:12495619; http://dx.doi.org/ 10.1016/S0896-6273(02)01133-9 [DOI] [PubMed] [Google Scholar]
- 29.Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014; 82:545-59; PMID:24811379; http://dx.doi.org/ 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crouch EE, Liu C, Silva-Vargas V, Doetsch F. Regional and stage-specific effects of prospectively purified vascular Cells on the Adult V-SVZ neural stem cell lineage. J Neurosci: Off J Soc Neurosci 2015; 35:4528-39; PMID:25788671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med 2009; 4:879-97; PMID:19903006; http://dx.doi.org/ 10.2217/rme.09.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci 2011; 14:1382-9; PMID:22030549; http://dx.doi.org/ 10.1038/nn.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]


