Abstract
Background
The aim of this study was to examine the association between serum level of chemerin with AIS and carotid artery atherosclerosis, and to investigate the level of chemerin as a potential novel cerebrovascular risk factor.
Material/Methods
We compared the serum chemerin levels and cerebrovascular parameters between 70 AIS patients and 70 non-AIS subjects in a Chinese population. Enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of serum chemerin. The state of carotid artery plaques in the AIS group was detected by color Doppler ultrasound. We used SPSS software for statistical analysis.
Results
Compared with the non-AIS group, serum level of chemerin in the AIS group increased significantly (p<0.01). Multivariable logistic regression suggested that serum chemerin level, neutrophil count, and BMI were independent risk factors for AIS (p<0.05). Compared with the non-unstable plaque group, there were significant differences from the unstable plaque group in serum chemerin level (p<0.01). Multivariable logistic regression analysis revealed that the LDL-C, FIB, and serum chemerin levels were independent risk factors for carotid artery plaque instability (P<0.05). The levels of serum chemerin in the subjects with no carotid artery plaque were significantly lower than in those with carotid artery plaques of 2 and ≥3 (P=0.013; P=0.01).
Conclusions
The results of this study suggest that the serum chemerin level may be an independent risk factor for AIS and carotid artery plaque instability in Chinese populations.
MeSH Keywords: Adipokines, Carotid Artery Diseases, Stroke
Background
Stroke affects 33 million individuals worldwide each year, and 87% of them are ischemic [1]. In China, ischemic stroke is the leading cause of serious long-term disability and death, and the disease has a tendency to increase year by year [2]. While the main cause of ischemic stroke is atherosclerosis [3], there is a close relationship between acute ischemic stroke (AIS) and atherosclerosis and obesity. Obesity is a pathological state due to the increase in adipose tissue, which can raise the risk of type 2 diabetes, hypertension, coronary heart disease, and a series of other diseases [4]. As is well-known, all of the diseases stated above are established risk factors for AIS. Adipose tissue is no longer considered just an energy storage repository, but is understood to be the largest active endocrine organ releasing a variety of biologically active substances termed adipokines, which exert their biological effects through autocrine, paracrine, and distance secretion mechanisms. In recent years, research on leptin [5], adiponectin [6], visfatin [7], and other adipokines enhance our understanding of the mechanism and development of metabolic syndrome, atherosclerosis, and other diseases. Chemerin is a newly discovered adipokine was first reported in 2007, and it quickly received widespread attention from endocrine and cardiovascular researchers [8].
Chemerin is an immunomodulating factor processed by a variety of proteases linked to inflammation. It activates the G-protein coupled receptor chemerin-like receptor 1 (CMKLR1) and induces chemotaxis in natural killer cells, macrophages, and immature dendritic cells [9]. In 2010, Kaur et al. [10] found that endothelial cells express both chemerin and its receptor, CMKLR1, and it is regulated by tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and other pro-inflammatory cytokines. After being stimulated by inflammatory cytokines, endothelia cells were damaged, triggering macrophages that remain in artery walls by a variety of adhesion and migration activities. Macrophages move lipids into foam cells, promoting the development of atherosclerosis. Chemerin activates extracellular signal-regulated kinase (p38 MAPK and ERK1/2 action), stimulates blood vessels migration, invasion, and formation, and leads to angiogenesis [11]. The current study suggests that platelet granules contains large amounts of pre-chemerin (secreted as a chain containing 163 amino acids with low biological activity), and multiple proteases of the coagulation-fibrinolysis system that can hydrolyze it into active chemerin [12]. Immature blood vessels were easily ruptured, which activates coagulation-fibrinolysis system, producing large amounts of active chemerin. The active chemerin promotes migration of inflammatory cells, including monocytes and macrophages, into atherosclerotic plaques by chemotaxis. The activation and penetration of monocytes and macrophages in atherosclerotic plaques are important factors resulting in plaque instability, which induces the rupture of plaque and eventual thrombus formation [13].
In humans, accumulating epidemiological evidence supports that chemerin is related to obesity, metabolic syndrome, type 2 diabetes, and coronary heart disease [14,15]. The associations between chemerin and coronary atherosclerosis have been reported, although with conflicting results [16,17]. Associations between serum levels of chemerin, AIS, and carotid artery atherosclerosis have been less well researched. There is a similar pathogenic link between cardiovascular and cerebrovascular diseases. The aim of this study was to determine whether serum level of chemerin is associated with acute ischemic stroke and carotid artery atherosclerosis, and to investigate the role of chemerin level as a potential novel cerebrovascular risk factor. There is a need to better understand the mechanism of AIS and atherosclerosis to guide clinical treatment and prevention of disease.
Material and Methods
Subject selection
This study was conducted between December 2014 and June 2015, enrolling 70 patients (36 men and 34 women; mean age 64.20±8.14 years) hospitalized at Shengjing Hospital affiliated to China Medical University, Liaoning Province, China. We compared their data to 70 control participants matched for age and sex (36 men and 34 women; mean age 64.14±8.08 years), recruited from our department. The diagnosis of acute ischemic stroke was consistent with the diagnostic criteria established by the Fourth Nationwide Academic Conference on Cerebrovascular Disease of the Chinese Medical Association [18]. We excluded patients with history of cerebral hemorrhage, acute cerebral infarction, coronary heart disease (stable angina pectoris, unstable angina, and myocardial infarction), heart failure, any serious infectious disease, liver or kidney disease, any disease of the immune system, and malignant tumor. We also excluded patients who recently had major surgery. None of the participants were on any anti-inflammatory drugs or steroids. Written informed consent was obtained from all the subjects and this study was approved by the Ethics Committee of Shengjing Hospital.
Clinical and biochemical assessment
A medical history of hypertension, diabetes [19], dyslipidemia [20], smoking, and family history of stroke were recorded. Blood pressure (BP) and heart rate (HR) were measured twice after keeping all participants in a horizontal position for at least 15 minutes. The mean of the 2 measurements was used for analysis. Height, weight, waist circumference (WC), hip circumference (HC), and neck circumference (NC) were measured by the same observer. BMI was calculated as weight (kg) divided by the square of height (m). We defined obesity as BMI ≥25 kg/m2.
Venous blood samples were obtained via sterile venipuncture after participants had fasted overnight. The blood samples were separated by routine procedures and sent to the hospital’s clinical laboratory for measurement of fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), white blood cell count (WBC), neutrophil count, platelet count (PLT), fibrinogen (FIB), and high-sensitivity C-reactive protein (hs-CRP).
To measure chemerin, serum was separated from the blood corpuscles by centrifugation at 3500 r/min for 15 min and kept frozen at −80°C until analysis in the same assay. We quantified serum chemerin using a Human Chemerin enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc, Wuhan, Hubei, China). The inter-assay coefficient of variation was <10% and the intra-assay coefficient of variation was <5%. Measurements of chemerin levels were performed according to the recommendations of the manufacturers.
The stability of carotid artery plaque was determined using standard procedures, with an ALOKA-α 10® Doppler ultrasound system (ALOKA, Japan). Plaques were graded into echolucent or echogenic. This was performed using the classification proposed by Gray-Weale [21]: type 1 has uniformly echolucent lesions; type 2 has echogenic lesions with substantial (>75%) components of echolucency; type 3 has predominately echogenic lesions with small area(s) of echolucency occupying less than a quarter of the plaque; and type 4 has uniformly dense echogenic lesions. Type 1, 2, and 3 plaques composed the unstable plaque group, while type 4 and without plaque composed the non-unstable plaque group.
Statistics and data analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA). The normality of the data distribution was determined using the Kolmogorov-Smirnov test. Continuous data are expressed as means ±SD or median (inter-quartile range) and as percentages for categorical data. Comparisons between groups (patients versus controls) were by Student’s t-test (for data that were normally distribution), Mann-Whitney U-test (for data that were non-normally distributed), and chi-square test (for data that were categorical variables). Correlations between serum chemerin level and continuous variables were determined using Spearman’s correlation. Multiple Logistic regression analysis (stepwise method) was performed to determine whether increased serum chemerin was a risk factor for acute ischemic stroke and atherosclerosis. A p value <0.05 was considered statistically significant.
Results
Baseline characteristics according to AIS
Laboratory findings and anthropometric characteristics of the subjects in AIS (N=70) and non-AIS (N=70) groups are summarized in Table 1. Patients with AIS had higher BMI, TG, FIB, and hypertension history than those of the non-AIS group (P<0.05). Significant differences in FPG, WBC, neutrophil count, and hs-CRP levels were found between the 2 groups (P<0.01). No significant differences were found regarding age, sex, WC, NC, BP, HR, smoking history, diabetes, dyslipidemia, and positive family history of stroke. As expected, there was a significantly difference between two groups in serum chemerin levels (62.96 (58.38–72.15) vs. 85.69 (82.99–91.62) ng/mL, P<0.01, Table 1, Figure 1). We tested for possible associations between AIS and its risk factors using multiple logistic regression analysis, with AIS as the dependent variable. We found that BMI, neutrophil count, and chemerin were the independent risk factors for AIS (P<0.05~0.01, Table 2).
Table 1.
Demographics of the study subjects.
| Parameters | Non-AIS (n=70) | AIS (n=70) | P value |
|---|---|---|---|
| Gender (M/ F) | 36/34 | 36/34 | 1.000 |
| Age (year) | 64.1±8.1 | 64.2±8.1 | 0.967 |
| BMI (kg/m2) | 25.2 (22.9–27.2) | 26.5 (24.0–28.5) | 0.020 |
| WC (cm) | 95.00 (85.00–99.00) | 95.00 (86.00–101.00) | 0.346 |
| HC (cm) | 100.00 (92.00–105.00) | 100.00 (96.00–104.00) | 0.642 |
| NC (cm) | 38.69±3.45 | 39.63±3.77 | 0.127 |
| SBP (mmHg) | 140 (130–170) | 150 (130–175) | 0.104 |
| DBP (mmHg) | 80 (80–90) | 90 (80–100) | 0.101 |
| HR (bmp) | 73 (70–78) | 76 (70–80) | 0.372 |
| FPG (mmol /L) | 5.52 (4.80–6.12) | 6.89 (6.47–7.87) | <0.001 |
| HbA1c (%) | 5.80 (5.50–6.20) | 5.80 (5.50–6.80) | 0.176 |
| TG (mmol/ L) | 5.01 (3.96–5.48) | 4.43 (4.17–5.22) | 0.243 |
| TC (mmol/ L) | 1.27 (0.90–2.07) | 1.58 (1.22–2.16) | 0.011 |
| LDL-C (mmol/ L) | 3.28 (2.51–3.64) | 2.87 (2.59–3.40) | 0.350 |
| HDL-C (mmol/ L) | 0.99 (0.85–1.28) | 1.03 (0.88–1.30) | 0.554 |
| WBC (×109/L) | 6.10 (4.80–7.40) | 7.90 (6.40–9.10) | <0.001 |
| Neutrophil count (×109/L) | 3.36 (2.82–4.14) | 5.04 (3.99–6.95) | <0.001 |
| hs-CRP (mg/L) | 1.17 (0.42–3.57) | 6.55 (2.58–18.10) | <0.001 |
| PLT (×109/L) | 196 (165–228) | 202 (158–257) | 0.529 |
| FIB (mg/mL) | 2.77± 0.51 | 3.06± 0.63 | 0.030 |
| Chemerin (ng/mL) | 62.96 (58.38–72.15) | 85.69 (82.99–91.62) | <0.001 |
| Hypertension (n) | 42 (60%) | 54 (77.1%) | 0.029 |
| Diabetes (n) | 20 (28.6%) | 21 (30%) | 0.853 |
| Dyslipidemia (n) | 60 (85.7%) | 52 (74.3%) | 0.091 |
| Smoking (n) | 16 (22.9%) | 24 (34.3%) | 0.134 |
| Family history of stroke (n) | 8 (11.4%) | 12 (17.1%) | 0.334 |
BMI – indicates body mass index; WC – waist circumferences; HC – hip circumferences; NC – neck circumferences; SBP – systolic blood pressure; DBP – diastolic blood pressure; HR – heart rate; FPG – fasting plasma glucose; HbA1c – glycosylated hemoglobin; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TG – triglycerides; WBC – white blood cell count; hs-CRP – high-sensitivity C-reactive protein; PLT – platelet count; FIB – fibrinoge. Values are median(25 and 75%), except for Age, NC and FIB (mean ±s.d.).
Figure 1.
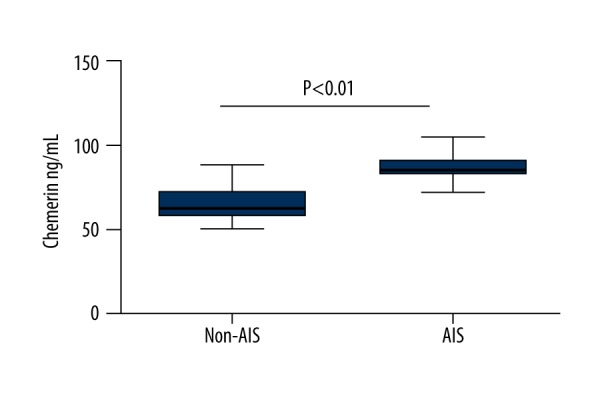
The comparison of serum chemerin levels between AIS and non-AIS groups AIS acute ischemic stroke.
Table 2.
Multiple regression analysis of variables associated with acute ischemic stroke.
| Parameters | Wald | OR | 95% OR | P value |
|---|---|---|---|---|
| BMI | 5.17 | 1.30 | 1.04–1.63 | 0.023 |
| Neutrophil count (×109/L) | 4.41 | 1.69 | 1.02–2.79 | 0.042 |
| Chemerin (ng/mL) | 17.90 | 1.41 | 1.20–1.66 | <0.01 |
BMI – indicates body mass index.
Association of serum chemerin levels with clinical characteristics
Serum chemerin levels were positively correlated with NC, SBP, DBP, smoking, and hypertension history (r=0.206, P<0.05; r=0.214, P<0.05; r=0.185, P<0.05; r=0.170, P<0.05; r=0.293, P<0.01, respectively). Serum chemerin levels were significantly correlated with 2 metabolic factors, FPG and TG (r=0.567, P<0.01 and r=0.342, P<0.01, respectively); 3 inflammatory factors, WBC, neutrophil count, and hs-CRP (r=0.370, P<0.01; r=0.430, P<0.01; and r=0.501, P<0.01, respectively); and 1 coagulation factor, FIB (r=0.315, P<0.01). No significant correlations were found between other parameters (Table 3).
Table 3.
Correlation between serum chemerin levels and AIS risk factors.
| Parameters | r | P value |
|---|---|---|
| Gender (M/ F) | 0.090 | 0.291 |
| Age (year) | 0.050 | 0.557 |
| BMI (kg/m2) | 0.130 | 0.124 |
| SBP (mmHg) | 0.214 | 0.011 |
| DBP (mmHg) | 0.185 | 0.028 |
| FPG (mmol/L) | 0.567 | <0.01 |
| TC (mmol/L) | 0.342 | <0.01 |
| WBC (×109/L) | 0.370 | <0.01 |
| Neutrophil count (×109/L) | 0.430 | <0.01 |
| hs-CRP (mg/L) | 0.501 | <0.01 |
| FIB (mg/mL) | 0.315 | <0.01 |
| Hypertension | 0.293 | <0.01 |
The P values are from Spearman’s correlations. BMI – indicates body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; FPG – fasting plasma glucose; TC – total cholesterol; WBC – white blood cell count; hs-CRP – high-sensitivity C-reactive protein; FIB – fibrinoge.
Baseline characteristics according to the stability of carotid artery atherosclerosis plaques
Table 4 shows the demographic and clinical characteristics of the no plaque group (n=8), stable plaques group (n=8), and unstable plaques group (Group B; n=54). We combined the groups with no plaque and stable plaques as the non-unstable plaques group (Group A; n=16). There was no statistical difference between the groups with stable plaques and non-plaque in serum chemerin levels (83.76 (80.07–88.93) vs. 81.22 (74.00–83.47) ng/mL, P=0.279), while both of them were significantly different from the unstable plaques group in which serum level was 86.86 (83.85–95.22) ng/mL (P<0.01; P=0.05, respectively). Significant differences in sex, SBP, dyslipidemia, NC, hypertension, and smoking history were found between Group A and Group B (P<0.05~0.01). There were significant difference between the 2 groups in terms of FIB and hs-CRP levels (P<0.05); TG, TC, and LDL-C levels (P<0.01). Serum chemerin levels of patients in Group B were 86.86 (83.85–95.22) ng/mL, significantly higher than those in Group A, which were 82.22 (79.36–85.23) ng/mL (P<0.01, Figure 2). We tested for possible associations between the stability of carotid artery atherosclerosis plaques and their risk factors using multiple logistic regression analysis, with the stability of carotid artery atherosclerosis plaques in AIS as the dependent variable. We found that LDL, FIB, and serum chemerin levels were the independent risk factors for the instability of carotid artery atherosclerosis plaques (P<0.05, Table 5).
Table 4.
Demographic characteristic of the non-unstable plaques and unstable plaques groups.
| Parameters | Group A (n=16) | Group B (n=54) | P value |
|---|---|---|---|
| Gender (M/F) | 4/12 | 32/22 | 0.016 |
| NC (cm) | 36.50 (34.00–39.75) | 41.00 (37.00–44.00) | 0.001 |
| SBP (mmHg) | 140 (123–150) | 150 (130–180) | 0.026 |
| TG (mmol/L) | 4.17 (3.66–4.72) | 4.75 (4.27–5.29) | 0.002 |
| TC (mmol/L) | 1.07 (0.93–1.89) | 1.68 (1.33–2.32) | 0.009 |
| LDL-C (mmol/L) | 2.62 (2.23–2.96) | 2.94 (2.63–3.51) | 0.002 |
| hs-CRP (mg/L) | 2.40 (0.49–12.28) | 6.86 (2.93–19.90) | 0.024 |
| FIB (mg/mL) | 2.80 (2.40–3.13) | 3.20 (2.70–3.40) | 0.035 |
| Chemerin (ng/mL) | 82.22 (79.36–85.23) | 86.86 (83.85–95.22) | <0.001 |
| Hypertension (n) | 8 (50.0%) | 46 (85.2%) | 0.009 |
| Dyslipidemia (n) | 8 (50.0%) | 44 (81.5%) | 0.027 |
| Smoking (n) | 0 (0.0) | 24 (44.4%) | 0.001 |
Group A – patients with stable plaques and non-plaque group; Group B – patients with unstable plaques group; NC – neck circumferences; SBP – systolic blood pressure; TC – total cholesterol; LDL-C – low-density lipoprotein cholesterol; TG – triglycerides; hs-CRP – high-sensitivity C-reactive protein; FIB – fibrinoge. Values are median (25 and 75%), except for age and WC (mean ±s.d.).
Figure 2.
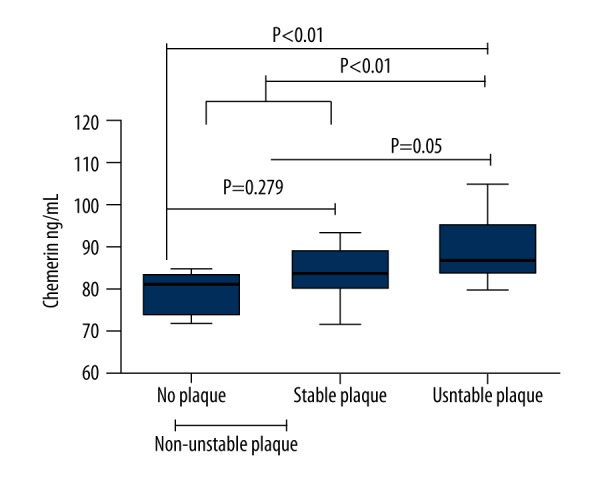
The comparison of serum chemerin levels and carotid atherosclerotic plaque stability.
Table 5.
Multiple regression analysis of variables associated with unstable plaques groups.
| Parameters | Wald | OR | 95% OR | P value |
|---|---|---|---|---|
| TC (mmol/ L) | 5.11 | 0.21 | 0.001–0.60 | 0.024 |
| LDL-C (mmol/ L) | 4.02 | 1.98 | 1.02–3.87 | 0.045 |
| FIB (mg/mL) | 5.43 | 35.74 | 1.77–722.69 | 0.020 |
| Chemerin (ng/mL) | 4.23 | 1.27 | 1.01–1.60 | 0.040 |
TC – total cholesterol; LDL-C – low-density lipoprotein cholesterol; FIB – fibrinoge.
Serum chemerin levels and the number of carotid artery atherosclerosis plaques
When all subjects in the AIS group were further divided into 4 groups according to the number of carotid artery atherosclerosis plaques (n=0, 1, 2, ≥3), the serum chemerin levels were 80.82 (72.21–84.05), 83.08 (81.53–90.25), 87.95 (81.76–93.57, and 86.86 (84.24–94.84) ng/mL for the plaques numbers of 0, 1, 2, and ≥3, respectively. The levels of serum chemerin in the subjects with no carotid artery plaque were significantly lower than those with carotid artery plaques of 2 and ≥3 (P=0.013; P=0.01), but there was no significant difference in plaque number of 1 (p=0.284). The serum chemerin levels were not significantly different among groups in plaques numbers ≥1 (P>0.05, Figure 3).
Figure 3.
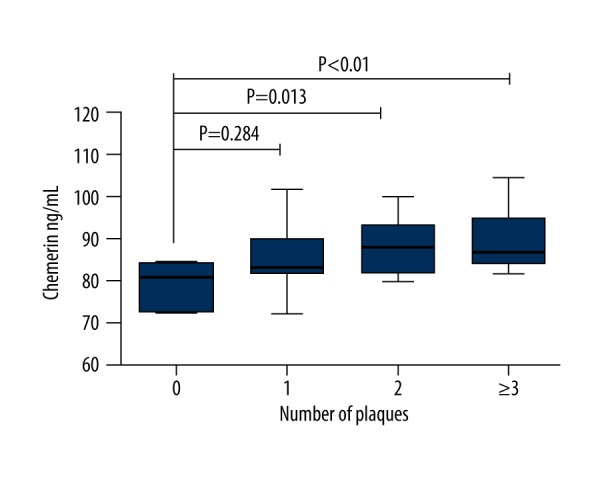
The comparison of serum chemerin levels according to the number of carotid atherosclerotic plaque in acute ischemic stroke group.
Association of serum chemerin levels with diabetes and obesity
All subjects were divided into a diabetic and a non-diabetic group, as well as an obese and a non-obese group, according to diabetes and obesity. We found no significant difference in serum chemerin levels in those groups (P>0.05, Table 6).
Table 6.
Relationship between chemerin, hs-CRP, diabetes and obesity [median (25 and 75%)].
| Parameters | n | Chemerin (ng/ mL) | P value | hs-CRP (mg/ L) | P value |
|---|---|---|---|---|---|
| Diabetes | 41 | 80.13 (67.21–87.07) | 0.434 | 2.52 (0.87–8.90) | 0.757 |
| Non-diabetes | 99 | 77.40 (62.24–85.89) | 3.12 (0.68–9.96) | ||
| Obesity | 84 | 73.64 (60.79–83.98) | 0.091 | 2.97 (0.88–8.83) | 0.508 |
| Non-obesity | 56 | 81.37 (64.12–86.87) | 2.06 (0.67–11.72) |
We define obesity as BMI ≥25kg/m2.
Discussion
The present study shows for the first time that patients with AIS had a higher serum chemerin level than those without AIS. Increasing circulating level of chemerin was also found to be associated with the instability of carotid artery atherosclerosis plaques.
We found a significant difference between the AIS group and the non-AIS group in BMI, FPG, WBC, neutrophil counts, hs-CRP, TC, and FIB. This is consistent with previous reports that the occurrence of AIS is closely related with obesity, diabetes, acute inflammatory response, and hypercoagulable state. Serum chemerin level was significantly increased in patients with AIS compared to those with non-AIS, and was positively correlated with SBP, FPG, TC, WBC, neutrophil count, hs-CRP, FIB, and NC. Chemerin binds with chemerin receptors on the cell membrane, leading to inhibition of adenylate cyclase activity and cyclic adenosine monophosphate aggregation. This process induces the release of calcium ions, increases the concentration of intracellular calcium, and causes hypertension-related vasoconstriction [22]. There is broad consensus about the close relationship between chemerin, glucose, and TC in several studies of endocrine and metabolic syndrome [14]. Chemerin plays a role in chemotaxis and its level was positively correlated with inflammation status. Chemerin might affect the macrophages and dendritic cells to migrate to inflammatory peripheral regions and release a variety of inflammatory mediators. The inflammatory mediators could further promote the release of chemerin to expand the inflammatory response by a positive feedback mechanism [23]. We found a lack of association between serum chemerin level, BMI, HC, and WC, which does not agree with previously reported studies. We believe this difference is associated with ethnic diversity, which is supported by a number of studies in Asian populations [15].
NC is a new index of anthropometry; it is simple to use and better shows upper body subcutaneous fat deposition. It has gradually drawn wide attention from endocrinology and metabolism researchers. Recent publications reported that this index is correlated with sleep apnea syndrome, metabolic syndrome, and coronary heart disease, perhaps due to sharing common risk factors: hypertension, dyslipidemia, and hyperglycemia [24]. Previous studies found that chemerin level generally along with all of these aforementioned risk factors [14]. In conclusion, we can now explain the correlation between NC and chemerin, as found in this study. Fitch et al. [25] found a significant relationship between NC and carotid intima thickness, and it was independent of the traditional cardiovascular disease risk factors such as hypertension, dyslipidemia, hyperglycemia, and smoking. A 2014 study in a large, community-based population in Chinese in confirmed that NC is a good indicator of early-stage atherosclerosis, independent of other metabolic factors [26]. But, until now, few studies have reported on the relationship between NC and stability of carotid plaques. Findings of the present study suggest that NC is related to stability of carotid plaques, but is not an independent risk factor.
Compared with the no plaque group, the stable plaque group had no significant difference in serum chemerin level, but both of them were obviously different from the unstable plaque group (Group B). Even when these 2 groups were merged into a non-unstable plaque group (Group A), a significant difference between Group A and Group B was still detected. Stepwise multiple regression analysis suggests that the occurrence of atherosclerosis increased along with increased serum chemerin level, but as the number of plaques increased, there was no apparent rising trend in serum chemerin level. In 2014, after the analysis of the serum chemerin levels in 220 Chinese subjects, researchers found that an elevated level of chemerin was an independent risk factor for acute coronary syndrome (unstable angina and CHD), but not for stable angina pectoris. This suggests that serum chemerin level is associated with instability of arterial plaques [27], perhaps because the inflammatory reaction plays a crucial role in determining the stability of plaques [28]. Similarly, Yan Q et al. [15] found that after dividing patients with coronary heart disease according to the number of lesion vessels, compared to patients without obvious stenosis, serum level of chemerin in patients with vascular lesions significantly increased, but the number of lesions and serum chemerin level were not related. Although previous studies found chemerin level generally was higher in diabetic and obese patients, our study found no differences in serum chemerin levels between diabetic and non-diabetic groups, or between BMI <25 kg/m2 and ≥25 kg/m2 groups. The level of chemerin and hs-CRP were significantly positively correlated in the AIS group, but diabetes and obesity had no obvious correlations with the latter. Serum chemerin level probably primarily reflected the activation state of the body inflammatory response. Inflammation in patients with AIS was stronger than patients with diabetes and obesity, which diluted the serum levels of chemerin in the diabetes and obesity groups. In 2013, after testing serum chemerin level in obese patients with diabetes, Weigert et al. [29] found that the circulating serum chemerin level was mainly related to inflammation, which supports our speculation.
Our study is a cross-sectional survey with a small sample size, which only uncovered a relevant relationship but cannot identify the causal connection between AIS, atherosclerosis, and chemerin. A larger prospective study might be able to observe the changes in chemerin at 24 hours, 48 hours, 72 hours, 7 days, and 1 month after AIS, to elucidate the role of chemerin during the occurrence, development, and recovery of AIS. Some investigators pointed out that in local atherosclerotic plaques, chemerin level can better reflect their relationship with atherosclerosis [15,30]. Our measurement of circulating chemerin might not provide information on the local tissue activity of the peptides. In addition, our study, by design, failed to clarify the effects of distinct pharmaceutical agents on serum levels of chemerin, which might influence the results.
Conclusions
Our findings suggest that serum chemerin level is an independent risk factor for AIS and carotid artery plaque instability, which might be associated with chemerin involvement in inflammatory reactions. Further studies are necessary to investigate the biological function of chemerin and its role in the occurrence and development of AIS. These results may play a guiding role in diagnosis and prognosis of cerebral infarction and atherosclerosis, providing new targets for the treatment of such diseases.
Acknowledgments
We would like to thank all the subjects and all the members of the Department of Neurology of Shengjing Hospital for their participation in this study.
Footnotes
Source of support: Departmental sources
References
- 1.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–332. [Google Scholar]
- 2.Neurology branch of Chinese Medical Association. China ischemic stroke and transient ischemic attack secondary prevention guidelines. Chinese Journal For Clinicians. 2011;39:68–74. [Google Scholar]
- 3.Nisa NM, Gudzune K, Hutfless S, et al. Avoiding weight gain in cardiometabolic disease: a systematic review. J Obes. 2014:358919. doi: 10.1155/2014/358919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131–45. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Balsan GA, Vieira JL, Oliveira AM, Portal VL. Relationship between adiponectin, obesity and insulin resistance. Rev Assoc Med Bras. 2015;61:72–80. doi: 10.1590/1806-9282.61.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Romacho T, Sanchez-Ferrer CF, Peiro C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013:946427. doi: 10.1155/2013/946427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 8.Teiger E, Castaigne A. Description and mechanisms of ischemia in atherosclerosis. Rev Prat. 1999;49:2110–16. [PubMed] [Google Scholar]
- 9.Mattern A, Zellmann T, Beck-Sickinger AG. Processing, signaling and physiological function of chemerin. IUBMB Life. 2014;66:19–26. doi: 10.1002/iub.1242. [DOI] [PubMed] [Google Scholar]
- 10.Kaur J, Adya R, Tan BK, et al. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem Biophys Res Comnmn. 2010;391:1762–68. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 11.Bozaoglu K, Curran JE, Stocker CJ, et al. Chemerin a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–85. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du XY, Zabel BA, Myles T, et al. Regulation of Chemerin bioaetivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor) and platelets. J Biol Chem. 2009;284:751–58. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan XX, Tao H. The relationship between chemerin and atherosclerosis. Int J Endocrinol Metab. 2013;33:195–98. [Google Scholar]
- 14.Li Y, Shi B, Li S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: a meta analysis. PloS One. 2014;9:e113915. doi: 10.1371/journal.pone.0113915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Q, Zhang Y, Hong J, et al. The association of serum Chemerin level with risk of coronary artery disease in Chinese adults. Endocrine. 2012;41:281–88. doi: 10.1007/s12020-011-9550-6. [DOI] [PubMed] [Google Scholar]
- 16.Lehrke M, Becker A, Greif M, et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161:339–44. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 17.Spiroglou SG, Kostopoulos CG, Varakis JN, Papdaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;26:115–30. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 18.Li W. The fourth cerebrovascular diseases academic meeting of the Chinese Medical Association: Diagnostic points of various cerebrovascular diseases. Chinese J Neurol. 1996;29:379–80. [Google Scholar]
- 19.American Diabetes Association. Standards of Medcial Care in Diabetes. Diabetes Care. 2014;37:S16–77. [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Gray-Weale AC, Graham JC, Burnett JR, et al. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. 1988;29:676–81. [PubMed] [Google Scholar]
- 22.Wang D, Yuan GY, Wang XZ, et al. Plasma chemerin level in metabolic syndrome. Genet Mol Res. 2013;12:5986–91. doi: 10.4238/2013.November.26.8. [DOI] [PubMed] [Google Scholar]
- 23.Wittamer V, Bondue B, Guillabert A, et al. Neutrophil-mediated maturation of chermerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–93. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Hong J. Neck circumference-a new indicator of cadiovascular disease risk factors. J Intern Med Concepts Pract. 2011;6:448–50. [Google Scholar]
- 25.Fitch KV, Stanley TL, Looby SE, et al. Relationship between neck circumference and cardiometabolic parameters in HIV-infected and non-HIV-infected adults. Diabetes Care. 2011;34:1026–31. doi: 10.2337/dc10-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang J, Wang Y, Li H, et al. Neck circumference and early stage atherosclerosis: the cardiometabolic risk in Chinese (CRC) study. Cardiovasc Diabetol. 2014;13:107–13. doi: 10.1186/s12933-014-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Q, Lin Y, Liang Z, et al. Chemerin is a novel biomarker of acute coronary syndrome but not of stable angina pectoris. Cardiovasc Diabetol. 2014;13:145–53. doi: 10.1186/s12933-014-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruganandan S, Roman AA, Sinal CJ. Role of Chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrowstem cells. J Bone Miner Res. 2010;25:222–34. doi: 10.1359/jbmr.091106. [DOI] [PubMed] [Google Scholar]
- 29.Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrino. 2010;72:342–48. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 30.Gao X, Mi S, Zhang F, et al. Association of chemerin mRNA expression in human epicadial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2010;10:87–95. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]


