Abstract
Greatwall (Gwl) functions as an essential mitotic kinase by antagonizing protein phosphatase 2A. In this study we identified Hsp90, Cdc37 and members of the importin α and β families as the major binding partners of Gwl. Both Hsp90/Cdc37 chaperone and importin complexes associated with the N-terminal kinase domain of Gwl, whereas an intact glycine-rich loop at the N-terminus of Gwl was essential for binding of Hsp90/Cdc37 but not importins. We found that Hsp90 inhibition led to destabilization of Gwl, a mechanism that may partially contribute to the emerging role of Hsp90 in cell cycle progression and the anti-proliferative potential of Hsp90 inhibition. Moreover, in agreement with its importin association, Gwl exhibited nuclear localization in interphase Xenopus S3 cells, and dynamic nucleocytoplasmic distribution during mitosis. We identified KR456/457 as the locus of importin binding and the functional NLS of Gwl. Mutation of this site resulted in exclusion of Gwl from the nucleus. Finally, we showed that the Gwl nuclear localization is indispensable for the biochemical function of Gwl in promoting mitotic entry.
Keywords: Greatwall kinase, Hsp90, importin, mitosis, Nuclear localization
Abbreviations
- HSP
heat shock protein
- Gwl
Greatwall
- MPF
maturation-promoting factor
- GVBD
germinal vesicle breakdown
- NLS
nuclear localization signal
- CDK
cyclin-dependent kinase
- IP
immunoprecipitation
Introduction
Entry into mitosis requires activation of maturation-promoting factor (MPF), a complex of cyclin B and Cdk1.1 During mitotic entry, MPF activation is triggered by Cdc25-mediated dephosphorylation of Cdk1. In addition to mechanisms that directly control the activity of Cdc25 and MPF, dynamic and regulated nucleocytoplasmic localization of these factors was also demonstrated to be important. In particular, both cyclin B and Cdc25 need to localize to the nucleus to trigger mitotic entry. It has been shown that phosphorylation of cyclin B by Polo-like kinase 1 (Plk1) promotes its nuclear localization.2,3 Furthermore, checkpoint kinases, Chk1 and Chk2, phosphorylate Cdc25, and thereby creating a binding site for 14-3-3 proteins which sequestrate Cdc25 in cytoplasm and inhibit M-phase entry.4,5
In addition to MPF, another enzyme required for both mitotic entry and maintenance is the Greatwall kinase (Gwl). Recent evidence revealed that Gwl inhibits the activity of protein phosphatase 2A/B55δ, the principal phosphatase acting on Cdk-phosphorylated substrates.6,7 The crucial nature of this inhibition was demonstrated by the failure of M-phase maintenance in egg extracts with full activity of MPF and other mitotic kinases but deficient of Gwl function.6,7 On the other hand, the presence of Gwl greatly reduced the amount of MPF required for mitotic entry.8 The mechanism of PP2A/B55δ inhibition by Gwl has been nicely solved with the recent identification of Endosulfine and its related family member, cAMP-regulated phosphoprotein 19 kDa, as substrates of Gwl. These substrates specifically bind to and inhibit PP2A/B55δ when they are phosphorylated by Gwl.9,10 While compelling evidence obtained in a variety of experimental systems revealed an essential role of Gwl in mitotic regulation, very little has been learned about how Gwl itself is regulated. It has been shown that Gwl is phosphorylated in M-phase, in quantitative correlation with its kinase activity.11,12 Mitotic phosphorylation of Gwl by MPF within its presumptive activation loop is required for Gwl activation, suggesting that MPF acts upstream of Gwl during mitotic entry.11-13 It has also been shown that Gwl can be phosphorylated by Plk1, whereas the specific function and regulation of this modification remains to be further clarified.11,13-15
In the search for new regulators of Gwl, our proteomic analysis revealed 2 groups of proteins as the major binding partners of Gwl which were co-purified with Gwl from G2 and M phase Xenopus oocytes. Specifically, Gwl bound both the chaperone complex Hsp90/Cdc37 and importin α/β through its N-terminus. Hsp90 was required to stabilize Gwl in both interphase and M-phase extracts. Furthermore, we identified the functional NLS in Gwl that mediated its binding to importins. Mutation of amino acid residues KR456/457 within the NLS led to exclusively cytoplasmic localization of the protein in Xenopus S3 cells and interfered its function in promoting mitotic entry in egg extracts.
Results
Identification of novel binding patterns of Gwl
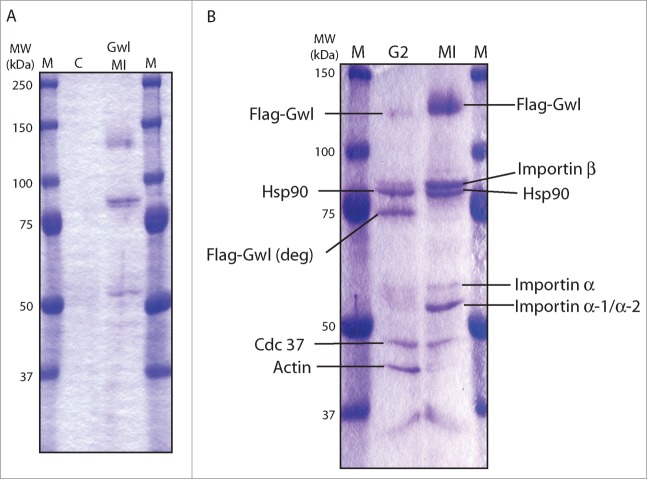
Xenopus oocytes resting at the G2/prophase border can progress to the metaphase of Meiosis I (MI) upon stimulation with progesterone. To identify Gwl binding proteins in both G2 and M phases, mRNA encoding Flag-Gwl was injected into G2 oocytes for protein expression, and after overnight incubation, half of the oocytes were treated with progesterone and collected upon entry into M-phase (M-phase oocytes were morphologically confirmed by germinal vesicle breakdown, GVBD). Flag-Gwl was then immunoprecipitated from oocyte lysates. To exclude proteins that might bind beads non-specifically, Flag-Gwl and co-immunoprecipitated proteins were competitively eluted from the beads by incubation with a soluble Flag peptide as described in Materials and Methods. Whereas no protein was visible in the Flag-eluate of the control oocytes (Fig. 1A), several distinct bands were co-eluted with Flag-Gwl in both G2 and M phase oocytes (Fig. 1B). The bands were excised and subjected to mass spectrometric analysis as described in Materials and Methods. Proteins co-eluted with Gwl in both G2 and M phase were identified as the molecular chaperones Hsp90 and its co-chaperone Cdc37, and members of the importin family, including importin β and importins α1, and α2 (Fig. 1B).
Figure 1.
Identification of Gwl binding proteins. (A) Control oocytes or oocytes injected with mRNA encoding Flag-Gwl were incubated overnight, and then treated with progesterone, and monitored for entry into meiosis I (MI). Oocyte extracts were immunoprecipitated with anti-Flag antibody beads, which were then eluted with Flag-peptide as described in Materials and Methods. Eluates were applied to SDS-PAGE gel and stained with Imperial protein stain. Control, uninjected oocytes (C), Flag-Gwl-expressing oocytes at GVBD (Gwl MI), Prestained protein markers (M). (B) Like in panel A, G2 oocytes were injected with mRNA encoding Flag-Gwl and incubated overnight. Some oocytes were then treated with progesterone and monitored for entry in to meiosis I (MI). G2 and MI oocyte extracts were immunoprecipitated with anti-Flag antibody beads, and eluted with the Flag-peptide. Protein bands were excised from the SDS-PAGE gel and analyzed by MALDI-TOF mass spectrometry at the HHMI Mass Spectrometry Resource Laboratory. Identified proteins are shown. The molecular weight of prestained protein markers is indicated on the left.
We confirmed by immunoblotting that both Hsp90 and Cdc37 associated with Gwl (Fig. 2A and B). In particular, the N-terminal segment of Gwl exhibited stronger binding affinity to Hsp90 when compared to the C-terminal segment (Fig. 2A). It has been shown that Hsp90/Cdc37 preferentially binds to the GxFG motif within the glycine-rich loop in client kinases.16 A candidate motif for Hsp90/Cdc37 binding lies in the N-terminal end of Gwl, composed of 41GAG44F residues that are fully conserved from Xenopus to human. As shown in Figure 2B, G41S mutant form of Gwl did not bind either chaperone, confirming the involvement of this motif. Notably, G41S has been previously characterized as a kinase-inactivating mutation.13 By comparison, another kinase-dead form of Gwl with a mutation in the T-loop (T748D Gwl) still bound both chaperones (Fig. 2B and C). Reciprocal co-immunoprecipitation (IP) analysis with Flag and importin antibodies confirmed that both importins coprecipitate with Gwl in G2 and M phase oocytes (Fig. 2D and E). As Flag-Gwl was expressed at several fold over the endogenous level (Fig. 2F), we further confirmed the association between endogenous Gwl and Importin α in the co-IP experiment (Fig. 2G).
Figure 2.
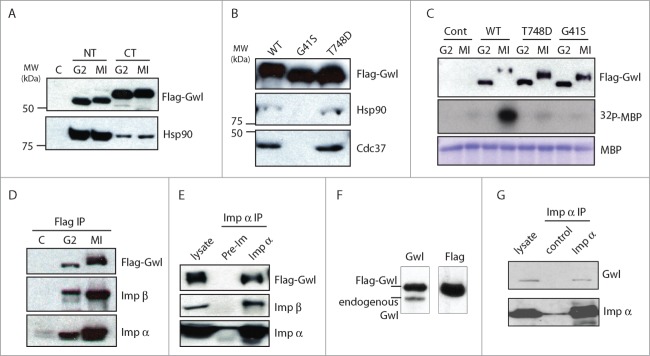
Gwl binds to Hsp90/Cdc37 and importins. (A) Hsp90 binds to the N-terminal half of Gwl kinase. Oocytes were injected with mRNA encoding Flag-tagged N- or C-Terminal Gwl (Fig. 5A) and incubated overnight. Some oocytes were then treated with progesterone and monitored for entry into meiosis I (MI). Oocyte extracts were immunoprecipitated with anti-Flag antibody beads, and the immunoprecipitates were analyzed by immunoblotting for Flag and Hsp90. (B) The GXXGXG motif is essential for binding to Hsp90/Cdc37. Oocytes were injected with mRNA encoding WT, G41S, or T748D Flag-Gwl and incubated overnight. Oocyte extracts were immunoprecipitated with anti-Flag antibody beads and immunoprecipitates were immunoblotted for Flag, Hsp90, and Cdc37. (C) mRNAs encoding WT, G41S, or T748D Flag-Gwl were injected into G2 oocytes followed by overnight incubation. Maturation was then induced in some oocytes by progesterone. Flag-Gwl immunoprecipitates were prepared from G2 and MI oocytes and analyzed for Flag (upper panel) or kinase activity against myelin basic protein (MBP, middle panel, autoradiograph). The level of MBP in the assays is shown as a loading control (lower panel). (D) Gwl binds importins. Immunoprecipitates from oocytes in G2 and MI expressing Flag-Gwl were prepared as in Figure 1, and Flag-Gwl bound proteins were analyzed by immunoblotting using antibodies against Flag, importin α, and importin β, as indicated. (E) Oocytes were injected with mRNA encoding Flag-Gwl and incubated overnight. Oocytes were then treated with progesterone and MI extracts were immunoprecipitated with preimmune or importin α serum, and followed by immunoblotting using Flag, importin β, or importin α antibodies, as indicated. (F) Oocytes were injected with mRNA encoding Flag-Gwl and incubated overnight, and analyzed by immunoblotting using antibodies against Gwl and Flag. (G) Immunoprecipitation of importin α was performed from Xenopus egg extracts, followed by immunoblotting using Gwl and importin α antibodies, as indicated.
Hsp90/Cdc37 is required for the protein stability of Gwl
Binding of Hsp90 chaperone to some of its client proteins has been shown to modulate protein structure, and thereby ensuring the proper protein stability and functionality. We examined a potential role of Hsp90 in regulating the protein stability of Gwl using geldanamycin, a well characterized inhibitor of Hsp90. Interestingly, Hsp90 inhibition in cycling egg extracts led to reduction of Gwl expression within one hour, and these extracts failed to enter mitosis, as judged by the lack of Cdc27 and Cdk-substrate phosphorylation (Fig. 3A). Similar requirement for Hsp90 was observed with exogenously expressed Gwl that was added to egg extracts in either interphase or M-phase (Fig. 3B).
Figure 3.
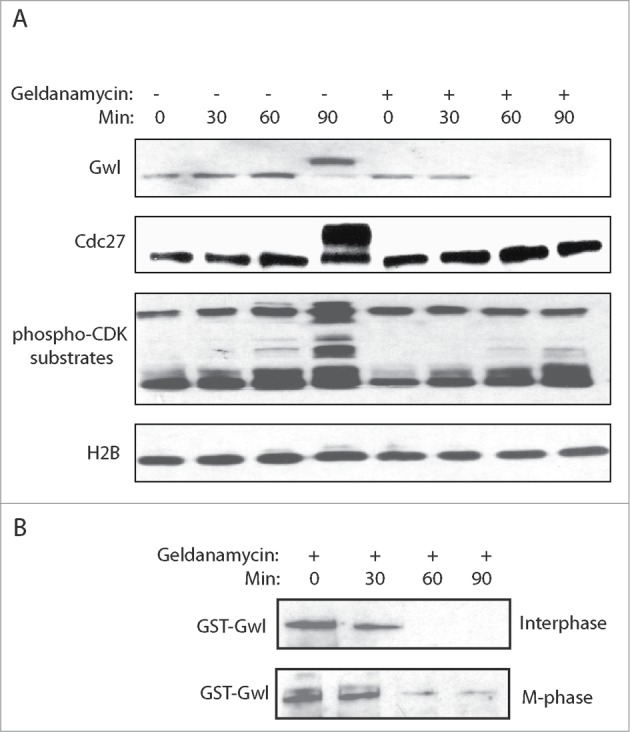
Hsp90 is required for the stabilization of Gwl. (A) Xenopus egg extracts were prepared as described in Materials and Methods, and treated with or without Geldanamycin (2 μM). Extracts at indicated time points were analyzed by immunoblotting for Gwl, Cdc27, phospho-Cdk substrates, and H2B. (B) GST-Gwl was added into Xenopus egg extracts in either interphase or M-phase, and incubated overtime with Geldanamycin. Extract samples were analyzed by immunoblotting for GST.
Dynamic nucleocytoplasmic localization of Gwl during the cell cycle
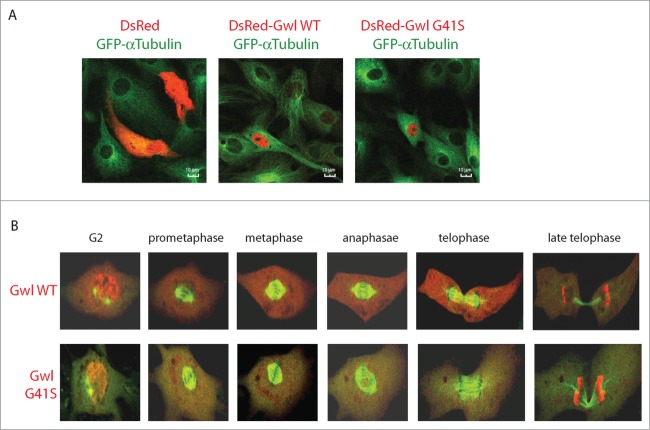
Identification of importins as major binding partners of Gwl prompted us to investigate the nucleocytoplasmic localization of Gwl. It has been reported that both human and Drosophila Gwl localized in the nucleus during interphase,17-19 whereas in Xenopus oocytes Gwl appeared predominantly cytoplasmic.8 To investigate the subcellular localization of Gwl in Xenopus cells, we tagged the WT or G41S Gwl with DsRed, and expressed the recombinant proteins in Xenopus S3 cells harboring stable expression of GFP-α-tubulin. Consistent with previous observations in human and Drosophila cells, Xenopus Gwl was exclusively nuclear in interphase (Fig. 4A). When expressed in Xenopus S3 cells, the G41S Gwl that is kinase-inactive and deficient in chaperone-binding exhibited a similar pattern of localization as the WT (Fig. 4A). Interestingly, live cell imaging revealed dynamic redistribution of Gwl between the nucleus and cytoplasm during mitotic progression. A portion of Gwl began to translocate from the nucleus to cytoplasm at late G2, after 2 centrosomes, as visualized by GFP-α-tubulin, separated from each other. Gwl became fully diffused in cytoplasm before centrosomes reached opposite positions (Fig. 4B; Movies S1 and S2). This finding is in agreement with previous studies in human and Drosophila that reported nuclear export of Gwl before nuclear membrane breakdown.15,20 However, distinct from the previous study that suggested a requirement of Gwl kinase activity for its nuclear export,20 we found a similar pattern of nucleocytoplasmic relocalization for G41S Gwl prior to mitotic entry (Fig. 4B). Gwl was excluded from mitotic chromosomes and diffused in cytoplasm during metaphase and anaphase. Finally, we observed that Gwl was concentrated back in the daughter nuclei in late mitosis, apparently before mitotic exit was completed (Fig. 4B).
Figure 4.
Dynamic localization of Gwl during mitotic progression. (A) Xenopus S3 cells constitutively expressing GFP-α-tubulin were transfected with plasmids encoding DsRed, DsRed-Gwl WT or G41S mutant, and analyzed by confocal microscopy. GFP (green) and DsRed (red) were visualized by direct fluorescence. Bar: 10 μm. (B) As in panel A, GFP-α-tubulin and DsRed-Gwl (WT or G41S) signals were shown in cells progressing through mitosis. Live cell imaging was provided in Movies S1 and S2.
Identification of the NLS motif of Xenopus Gwl
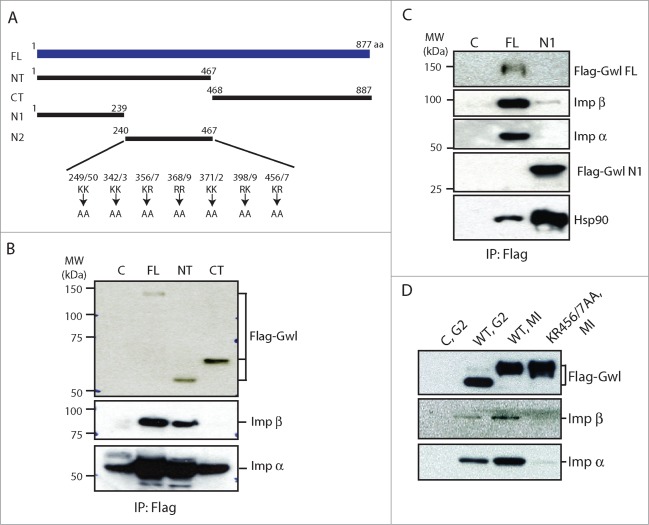
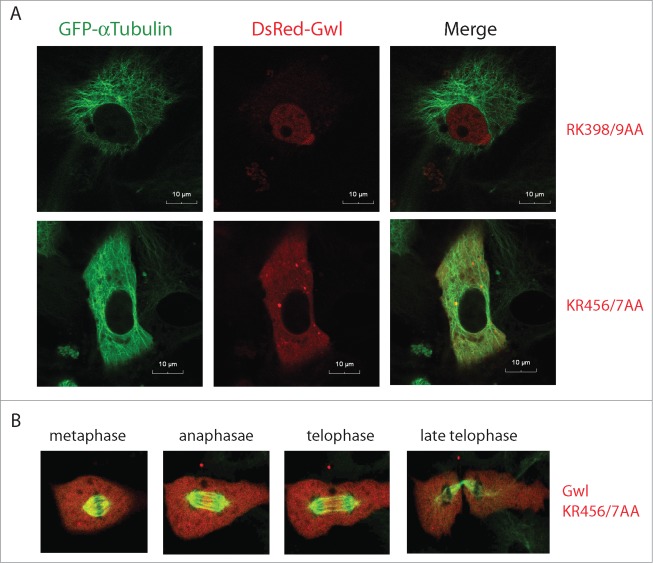
The dynamic and cell cycle-dependent nuclear localization of Gwl may be regulated by the importin complex which has been shown to interact with NLS motifs of client proteins. Like Hsp90 and Cdc37, the importins also bind the NT half of Gwl (Fig. 5A and B). Further analysis of subdomains of the NT half (N1 and N2) indicated that the N1 motif strongly bound Hsp90, but not importins (Fig. 5C). Within the N2 region of Gwl located 7 pairs of consecutive basic residues (Lys and Arg) that potentially function as the NLS of Gwl21 (Fig. 5A). To this end, each of the 7 putative NLS was mutated, and all mutants were analyzed for their subcellular localization and association with importin. Interestingly, the Gwl mutant with KR456/457 changed to 2 alanines did not bind either importin α or β when expressed in and then isolated from MI oocytes (Fig. 5D). The interphase nuclear localization of these mutations was assessed in Xenopus S3 cells by confocal microscopy. KR456/457AA mutation completely abolished the nuclear localization of Gwl (Fig. 6A), whereas mutations of the other 6 K/R motifs had no effect on nuclear localization, as exemplified by RK398/9AA (Fig. 6A). Unlike WT Gwl which exhibited dynamic nucleocytoplasmic redistribution in M-phase, KR456/457AA Gwl stayed diffused in cytoplasm throughout mitotic progression (Fig. 6B; Movie S3). Collectively, these lines of evidence identified KR456/457 as the NLS of Xenopus Gwl which mediates Gwl-importin interaction and the nuclear localization of Gwl.
Figure 5.
KR456/457 of Gwl mediates importin binding. (A) Schematic representation of various Gwl constructs. (B) Oocytes were injected with mRNAs encoding Flag-tagged full length (FL), NT, or CT-Gwl and incubated overnight. Oocytes were then treated with progesterone and collected at MI. Lysates from the MI oocytes were precipitated with anti-Flag antibody beads, and the precipitates were immunoblotted for Flag, importin β, and importin α. Uninjected oocyte lysates treated with Flag antibody beads served as a control (marked as C). (C) The N1 Fragment of Gwl Binds Hsp90 but not importins. Oocytes were injected with mRNA encoding the Flag-tagged full-length and N1 fragment of Gwl and analyzed by immunoblotting using antibodies against Flag, importin β, importin α, and Hsp90. (D) KR456/457 is essential for Gwl binding to importins. Oocytes at G2 phase were injected with mRNA encoding WT or KR456/457AA Flag-Gwl and incubated overnight. Some oocytes were then treated with progesterone and collected at MI. Uninjected G2 oocytes were treated identically as a control (marked as C, G2). Lysates from the G2 and MI oocytes were precipitated with anti-Flag antibody beads, and the precipitates were immunoblotted using Flag, importin β, and importin α antibodies.
Figure 6.
Nuclear exclusion of Gwl KR456/457AA. (A) Xenopus S3 cells expressing GFP-γ-tubulin were transfected with plasmids encoding DsRed-Gwl RK398/9AA or KR456/7AA, as indicated, and analyzed by confocal microscopy. Bar, 10 μm. (B) As in panel A, GFP-α-tubulin and DsRed-Gwl KR456/7AA signals were shown in cells progressing through mitosis. Live cell imaging was provided in Movie S3.
Nuclear localization is required for Gwl function in mitotic entry
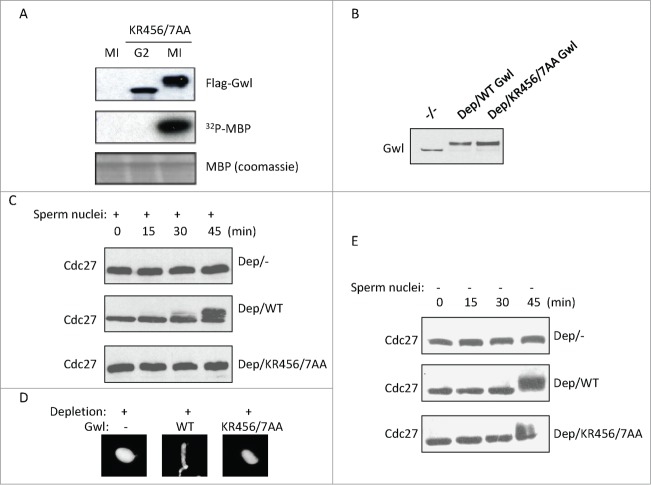
The identification of NLS allowed us to investigate the functional importance of Gwl nuclear localization. Perhaps in line with the fact that the NLS is not in close proximity to the kinase domain of Gwl, NLS-deficient KR456/457AA Gwl exhibited no detectable deficiency in the kinase activity, as demonstrated by both slower mobility of the protein on SDS-PAGE and the in vitro kinase assay (Fig. 7A). We then compared the role of WT and NLS-deficient Gwl in promoting mitotic entry. Cycling extracts were depleted of endogenous Gwl and supplemented with sperm nuclei. As expected, these extracts were unable to enter mitosis (Fig. 7B and C). Interestingly, reconstitution of these extracts with WT, but not KR456/457AA Gwl, restored mitotic entry, as judged by mitotic phosphorylation of Cdc27 (Fig. 7B and C) and the morphology of the sperm nucleus (Fig. 7D). Notably, KR456/457AA Gwl was able to reconstitute mitotic entry in a cycling extract devoid of sperm nuclei (Fig. 7E). Collectively, these results indicated that the NLS-deficient Gwl is enzymatically competent, yet the nuclear localization of Gwl is required for its proper function in mitosis.
Figure 7.
The NLS of Gwl is required for its function in mitotic entry. (A) Xenopus oocytes were injected with mRNA encoding Flag-Gwl KR456/7AA, incubated overnight, and followed by Flag-IP. The resulting precipitates were subjected to kinase assay using MBP as a substrate. (B) As described in Methods and Materials, endogenous Gwl was immunodepleted from cycling egg extracts, and Flag-Gwl was expressed and added into extracts. Samples were analyzed by immunoblotting for Gwl. (C) The extracts were prepared as in panel B, supplemented with sperm nuclei, and incubated as indicated. Extract samples were analyzed by immunoblotting for Cdc27. The retarded gel mobility of Cdc27 serves as a mitotic marker. (D) Extracts in panel C (45 min) were stained with DAPI, and analyzed for the morphology of sperm nuclei. (E) Extracts were depleted of Gwl, and supplemented with WT or KR456/457AA Gwl at the same concentrations as in panel C. Extract samples were analyzed by immunoblotting as indicated.
Discussion
Gwl stability is modulated by heat shock protein chaperone
Several heat shock proteins function as molecular chaperones. In particular, Hsp90 has been related to regulators of the cell cycle, including Cdk1, p53, Cdk4, and Plk1.22-25 It has been shown that the evolutionarily conserved protein association between Plk1 and Hsp90 was required for the stability of Plk1, and thereby involved in centrosomal functions and metaphase-to-anaphase transition.22,26 In this study we discovered Hsp90 as a major associated partner for Gwl. Further analysis showed that the N-terminal segment of Gwl mediated its association with Hsp90. Interestingly, a glycine-rich loop in the N-terminal Gwl was required for Hsp90 association, and a G41S mutant form of Gwl was deficient in Hsp90 association. Our proteomic identification of Gwl-associated proteins also revealed its association with Cdc37, the co-chaperone of Hsp90 involved in recognition of some substrates. Initially identified in budding yeast as a cell division cycle regulator, Cdc37 has been shown to interact with Cdk4, Cdk5, and several other cell cycle factors.25,27 Consistently, we found that G41S Gwl lost the association with Cdc37, confirming the involvement of the glycine-rich loop. Importantly, inhibition of Hsp90 reduced the protein stability of Gwl and suppressed M-phase entry, suggesting that complexing to the Hsp90/Cdc37 chaperone is essential for Gwl function. The study therefore revealed Gwl as a key client protein through which Hsp90/Cdc37 chaperone regulates cell division. This new discovery contributes to better understanding of cell cycle regulation, and yields potential implication to cancer therapy. In fact, Hsp90 is frequently overexpressed in cancer cells, whereas its inhibition exhibits anti-proliferative potentials and is being explored in multiple clinical trials for treatment of various types of cancer.28,29
Gwl association with importin mediates its nuclear localization
The proper function of cellular enzymes often involves sophisticated regulation of subcellular localization, in addition to enzymatic activity and protein stability. For instance, cyclin B/Cdk1 is imported into the nucleus before nuclear membrane breaks down to promote mitotic entry. Similarly, dynamic subcellular localization of Plk1 and Aurora A has been shown to regulate their functions during mitotic progression.30-33 One mechanism to regulate the nuclear localization of a protein is through association with the importin family members. Importin is typically composed of 2 subunits, importin α and importin β. For nuclear transport, importin β either directly binds and transports cargo proteins, or is adapted to the cargo proteins through importin α. In this study, we report that Gwl associates with both importin α and Importin β, suggesting that the nuclear localization of Gwl is mediated by importin. In cultured Xenopus cells, we showed that Gwl localized to the nucleus in interphase, and was diffused into the cytoplasm in mitosis. Our detailed analysis identified a functional NLS around KR456/457. Mutation of the NLS abolished the association of Gwl with importin α and Importin β, and led to Gwl nuclear exclusion and cytoplasmic sequestration.
Independent studies in Drosophila and mammalian cells also characterized the nuclear localization of Gwl.15,20 The Drosophila homolog of Gwl contains 2 essential NLS motifs within the central region, mutation of which disrupted its nuclear location.15 However, neither of these NLS motifs in Drosophila is conserved in vertebrates. The NLS of human Gwl was found around KR444/445,20 the corresponding residues of KR456/457 in Xenopus Gwl. Thus, the NLS of Xenopus Gwl, as identified in this study, represents a well-conserved mechanism in vertebrate animals.
Dynamic nucleocytoplasmic localization of Gwl during the cell cycle
Studies in Drosophila and human cells suggested a pre-mitotic transport of Gwl from nucleus to cytoplasm.15,20 We also observed in Xenopus cells that nuclear Gwl started to diffuse into cytoplasm before centrosomes reached opposite positions. While it remained unclear how this nucleocytoplasmic translocation of Gwl may contribute to the regulation of mitotic entry, previous studies indicated that the translocation was dependent on Gwl phosphorylation by CDK1, and possibly also Plk1.15,20 It was also suggested that the kinase activity of Gwl was required for its nuclear export as a kinase-dead form of Gwl harboring a mutation at the ATP-binding site was deficient in nuclear export.20 However, a different form of kinase-dead Gwl used in our study exhibited a similar pattern of nucleocytoplasmic translocation as the WT. Notably, this mutant form of Gwl was efficiently phosphorylated in mitosis, as judged by the retarded gel migration. Furthermore, we observed that Gwl was reconcentrated into the daughter nuclei in telophase, seemingly before mitotic chromosomes are fully decondensed. By comparison, the NLS-deficient form of Gwl remained diffused in the cytoplasm during mitotic exit.
Functional importance of Gwl nuclear localization
An important lesson learned from previous studies on Cdk1/cyclin B, Plk1, Aurora kinases, and other mitotic regulators was that their subcellular localization often constitutes an essential mechanism to ensure the proper function of these factors. Regulation of Gwl nuclear localization is functionally important as the NLS-deficient Gwl was unable to fully rescue the mitotic defects caused by Gwl-depletion in both Drosophila and mammalian cells.15,20 To shed new light on the role of Gwl nuclear localization, we investigated if the NLS is required for the biochemical function of Gwl. It has been shown that Gwl promotes mitotic entry through phosphorylation of Ensa/Arpp-19, which subsequently inhibit PP2A/B55δ.9,10 Interestingly, our results showed that the NLS-deficient Gwl failed to promote mitotic entry in extracts supplemented with sperm nuclei, despite that this mutant possesses intact and functional kinase domains and promoted mitotic entry in extracts devoid of nucleus formation. Notably, a number of previous studies also characterized the nuclear import of cyclin B1 and Cdc25C in Xenopus egg extracts.34-38 These studies and our current finding argue that even though Xenopus egg extracts can biochemically undergo cell cycle progression in the absence of nuclei, nucleocytoplasmic regulation of the cell cycle is recapitulated in this system when sperm nuclei are reconstituted. As another example, although Xenopus egg extracts support DNA replication without nuclei, it has been shown that the NLS of cyclin E is required for DNA replication in Xenopus egg extracts with the supplementation of sperm nuclei.39 Moreover, the nuclear import of cyclin B1 is required for mitotic entry in Xenopus oocytes,40 despite that enucleated Xenopus oocytes still exhibit mitotic activation of Cdk1.41 While future analyses are required to reveal how the nuclear localization of Gwl contributes to its biochemical function, a number of possibilities are proposed. First, Gwl may phosphorylate its substrates in the nucleus to promote mitotic entry. Alternatively, Gwl may be initially activated in the nucleus prior to its nuclear export during the early stage of mitotic entry. The latter possibility may be attributed to the nuclear localization of its activating signal, such as MPF activity, or a lower level of inhibitory activity in the nucleus, such as phosphatases that target Gwl. Finally, Gwl negatively regulates the DNA damage checkpoint in Xenopus egg extracts,42 it is thus possible that the nuclear localization of Gwl contributes to mitotic entry by suppressing an inhibitory signal, such as the cell cycle checkpoint governing DNA damage or incomplete DNA replication.
Materials and Methods
Xenopus techniques and cloning procedures
Xenopus oocyte arrested in G2 were manually dissected from ovarian fragments, and Flag tagged Gwl expression was achieved by microinjecting oocytes with 40 nl of mRNA (0.25 mg/ml) encoding various Gwl constructs. After overnight incubation at 18°C, progesterone (10 μg/ml) was added to some oocytes to induce maturation, and MI (GVBD) oocytes were collected when a well-defined white spot appeared at the animal pole. Full length Xenopus Gwl cDNA was purchased from Open Biosystems (MXL1736–9507526, Clone ID: 6637446, Accession number: BC068968). Full length, NT (1–467 a.a.), or CT (468–887 a.a.) Gwl was cloned into a pCS-Flag vector using LIC cloning (Novagen). Mutants of all NLS candidate sites, T748D Gwl, and G41S Gwl were generated using a Quick Change Site-Directed mutagenesis kit (Stratagene), and mRNA was produced using a mMessage mMachine SP6 kit (Ambion). For transfection of Xenopus S3 cells, full length Gwl was cloned into a DsRed C1 vector (Clontech), and DsRed-Gwl was transfected into Xenopus S3 cells using Lipofectamine LTX (Invitrogen). Cytostatic factor (CSF) extracts were freshly prepared as previously described.14 Eggs were dejellied with 2% cysteine in 1× XB (1 M KCl, 10 mM MgCl2, 100 mM HEPES pH 7.7, and 500 mM sucrose), washed 4 times with 1× XB, and then once with 1× MEB (1 M KCl, 11 mM MgCl2, 100 mM HEPES pH 7.7, 500 mM sucrose, and 5 mM EGTA, pH 7.7). Eggs were packed in centrifuge tubes with low speed centrifugation then crushed by centrifugation at 10,000 g at 4°C for 10 min. The cytoplasmic layer was further separated by centrifugation at 10,000 g for 15 min at 4°C. Interphase extracts were obtained by incubation of CSF extracts with CaCl2 (40 nM). For cycling extracts, eggs were rinsed with distilled water then soaked in water for 10 min before being dejellied with 2% cysteine in 1× XB. They were washed 5 times in 0.2× MMR buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EDTA, 10 mM HEPES, and KOH to pH 7.8). The Ca2+ ionophore, A23187, was added to 10 ng/ml until the animal poles rotated. The eggs were then washed with 0.2× MMR 10 times and 1× XB 4 times. Eggs were packed by low speed centrifugation. The eggs were crushed 35 mins after addition of A23187 by centrifugation at 10,000 g at 4°C. The cytoplasmic layer was transferred to new tubes and energy mix (7.5 mM creatine phosphate, 1 mM ATP, 1 MgCl2) was added. The cytoplasmic layer was further separated by centrifugation at 10,000 g for 15 min at 4°C. Demembraned Xenopus sperm nuclei were added into egg extracts at 1000 nuclei/μl. WT and mutant forms of Flag-Gwl added to egg extracts was expressed using TNT SP6 High-Yield Wheat Germ Protein Expression System (Promega).
Immunoprecipitation, Immunoblotting and Immunodepletion
For immunoprecipitation of Flag Gwl, 20 oocytes treated as indicated in the figures were homogenized in 100 μl of EB (80 mM β-glycerophosphate, 20 mM EGTA, 5 mM MgCl2, 20 mM Hepes, pH 7.5), and the oocyte cytosol was collected by centrifugation at 10,000 × g for 1 min at 4°C. 5 μl of cytosol was mixed with 5 μl of 2× Laemmli sample buffer (Bio-Rad) and used as the input fraction for Western blotting. The remaining cytosol was added to 20 μl of anti-Flag agarose beads (Sigma, M2 Flag agarose) pre-equilibrated in EB, and the mixture was incubated for 60 min at 4°C. The beads were then washed 3 times in EB. For mass spectrometry, Flag-Gwl beads were then eluted with 10 μg of Flag peptides (Sigma) in 20 μl of EB for 2 hours on ice and eluates were suspended in 20 μl of 2× Laemmli sample buffer. SDS-PAGE was performed using a 10% Criterion gel (Bio-Rad) that was then stained with Imperial Coomassie stain (Thermo Scientific), after which bands were cut out individually for analysis by the HHMI Mass Spectrometry Resource Laboratory (Berkeley). For immunoblotting analysis, bead eluates or beads were resuspended in 20 μl of 2× Laemmli sample buffer. SDS-PAGE was performed using either a 7.5% or 10% Criterion gel (Bio-Rad) The gels were transferred to nitrocellulose membranes (Whatman) using a semi dry Western blotting apparatus (Pharmacia), and blocked with 5% milk before incubation with the indicated primary antibodies. Antibodies against Flag were obtained from Sigma. Antibodies to Hsp90, Cdc37, and H2B were from Cell Signaling, antibodies to importin β and PCNA were from Abcam, and serum recognizing importin α was a kind gift from Dr. Manfred Lohka (University of Calgary).The kinase activity of immunoprecipitated Gwl was measured as previously described.42 Gwl depletion from Xenopus egg extracts was performed as previously described.43
Cell culture, transfection, and microscopy analysis
Xenopus S3 cell culture, transfection of DsRed-Gwl, and cell imaging by confocal microscopy were performed as previously described.44 Xenopus S3 GFP-α-tubulin-expressing cells were a kind gift from Dr. Gary Gorbsky (Oklahoma Medical Research Foundation). In general, cells were transfected in suspension immediately after trypsinization using Lipofectamine LTX in combination with PLUS reagent (Invitrogen) according to the manufacturer's instructions. Subsequently, cells underwent double thymidine treatment (2 mM thymidine for 14–16 h), then released for 8 h, followed by a second thymidine treatment for 14–16 h to synchronize cells at the G1/S boundary. Detection of GFP and DsRed by direct fluorescence using confocal microscopy began 5 h after release from the second thymidine block. For live cell imaging, XS3 cells were seeded onto 0.17 mm glass-bottom wells (WillCo Wells), and viewed at room temperature using a Nikon Eclipse TE 300, PCM 2000 inverted microscope.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Manfred Lohka (University of Calgary) and Dr. Gary Gorbsky (Oklahoma Medical Research Foundation) for reagents; Dr. James Maller (University of Colorado Denver) for the initial development of this project, and Ms. Andrea Lewellyn (University of Colorado Denver) for technical support.
Funding
This work was supported by National Institutes of Health grant 1R01CA172574 to A.P.
Supplemental Material
Supplemental data for this article can be found on the publisher's website.
References
- 1. Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nature reviews. Mol Cell Biol 2001; 2:21-32; PMID:11413462, http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 2. Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 2001; 410:215-20; PMID:11242082; http://dx.doi.org/ 10.1038/35065617 [DOI] [PubMed] [Google Scholar]
- 3. Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene 2002; 21:8282-92; PMID:12447691; http://dx.doi.org/ 10.1038/sj.onc.1206011 [DOI] [PubMed] [Google Scholar]
- 4. Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Ann Rev Biochem 2004; 73:39-85; PMID:15189136; http://dx.doi.org/ 10.1146/annurev.biochem.73.011303.073723 [DOI] [PubMed] [Google Scholar]
- 5. Aressy B, Ducommun B. Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med Chem 2008; 8:818-24; PMID:19075563; http://dx.doi.org/ 10.2174/187152008786847756 [DOI] [PubMed] [Google Scholar]
- 6. Vigneron S, Brioudes E, Burgess A, Labbé JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. Embo J 2009; 28:2786-93; PMID:19680222; http://dx.doi.org/ 10.1038/emboj.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase greatwall (Gwl) promotes inactivation of PP2A/B55 delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 2009; 20:4777-89; PMID:19793917; http://dx.doi.org/ 10.1091/mbc.E09-07-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hara M, Abe Y, Tanaka T, Yamamoto T,Okumura E, Kishimoto T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun 2012; 3:1059; PMID:22968705; http://dx.doi.org/ 10.1038/ncomms2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gharbi-Ayachi A, Labbé JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010; 330:1673-7; PMID:21164014; http://dx.doi.org/ 10.1126/science.1197048 [DOI] [PubMed] [Google Scholar]
- 10. Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010; 330:1670-3; PMID:21164013; http://dx.doi.org/ 10.1126/science.1195689 [DOI] [PubMed] [Google Scholar]
- 11. Vigneron S., Gharbi-Ayachi A, Raymond AA, Burgess A, Labbé JC, Labesse G, Monsarrat B, Lorca T, Castro A. Characterization of the mechanisms controlling greatwall activity. Mol Cell Biol 2011; 31:2262-75; PMID:21444715; http://dx.doi.org/ 10.1128/MCB.00753-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blake-Hodek KA, Williams BC, Zhao Y, Castilho PV, Chen W, Mao Y, Yamamoto TM, Goldberg ML. Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol Cell Biol 2012; 32:1337-53, PMID:22354989; http://dx.doi.org/ 10.1128/MCB.06525-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu JT, Zhao Y, Li ZX, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 2006; 22:83-91; PMID:16600872; http://dx.doi.org/ 10.1016/j.molcel.2006.02.022 [DOI] [PubMed] [Google Scholar]
- 14. Peng A, Wang L, Fisher LA. Greatwall and Polo-like kinase 1 coordinate to promote checkpoint recovery. J Biol Chem 2011; 286:28996-9004; PMID:21708943; http://dx.doi.org/ 10.1074/jbc.M111.257121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang P, Galan JA, Normandin K, Bonneil É, Hickson GR, Roux PP, Thibault P, Archambault V. Cell cycle regulation of Greatwall kinase nuclear localization facilitates mitotic progression. J Cell Biol 2013; 202:277-93; PMID:23857770; http://dx.doi.org/ 10.1083/jcb.201211141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terasawa K, Yoshimatsu K, Iemura S, Natsume T, Tanaka K, Minami Y. Cdc37 interacts with the glycine-rich loop of Hsp90 client kinases. Mol Cell Biol 2006; 26:3378-89; PMID:16611982; http://dx.doi.org/ 10.1128/MCB.26.9.3378-3389.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu JT, Fleming SL, Williams B, Williams EV, Li Z, Somma P, Rieder CL, Goldberg ML. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 2004; 164:487-92; PMID:14970188; http://dx.doi.org/ 10.1083/jcb.200310059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voets E, Wolthuis RM. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 2010; 9:3591-601; PMID:20818157 [DOI] [PubMed] [Google Scholar]
- 19. Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010; 107:12564-569; PMID:20538976; http://dx.doi.org/ 10.1073/pnas.0914191107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez-Fernandez M, Sánchez-Martínez R, Sanz-Castillo B, Gan PP, Sanz-Flores M, Trakala M, Ruiz-Torres M, Lorca T, Castro A, Malumbres M. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc Natl Acad Sci U S A 2013; 110:17374-9; PMID:24101512; http://dx.doi.org/ 10.1073/pnas.1310745110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dingwall C, Laskey RA. Nuclear import: a tale of two sites. Curr Biol 1998; 8, R922-4; PMID:9889096; http://dx.doi.org/ 10.1016/S0960-9822(98)00010-4 [DOI] [PubMed] [Google Scholar]
- 22. de Carcer G. Heat shock protein 90 regulates the metaphase-anaphase transition in a polo-like kinase-dependent manner. Cancer Res 2004; 64:5106-12; PMID:15289312; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-2214 [DOI] [PubMed] [Google Scholar]
- 23. Munoz MJ, Jimenez J. Genetic interactions between Hsp90 and the Cdc2 mitotic machinery in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 1999; 261:242-50; PMID:10102358; http://dx.doi.org/ 10.1007/s004380050963 [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Chen J. Phosphorylation and hsp90 binding mediate heat shock stabilization of p53. J Biol Chem 2003; 278:2066-71; PMID:12427754; http://dx.doi.org/ 10.1074/jbc.M206697200 [DOI] [PubMed] [Google Scholar]
- 25. Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev 1996; 10:1491-502; PMID:8666233; http://dx.doi.org/ 10.1101/gad.10.12.1491 [DOI] [PubMed] [Google Scholar]
- 26. de Carcer G, do Carmo Avides M, Lallena MJ, Glover DM, Gonzalez C. Requirement of Hsp90 for centrosomal function reflects its regulation of Polo kinase stability. EMBO J 2001; 20:2878-84; PMID:11387220; http://dx.doi.org/ 10.1093/emboj/20.11.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta GF, Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene 1997; 14:1999-2004; PMID:9150368; http://dx.doi.org/ 10.1038/sj.onc.1201036 [DOI] [PubMed] [Google Scholar]
- 28. Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. J Breast Cancer 2011; 14:167-74; PMID:22031796; http://dx.doi.org/ 10.4048/jbc.2011.14.3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Curr Top Med Chem 2006; 6:1205-14; PMID:16842157; http://dx.doi.org/ 10.2174/156802606777812068 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez JA, Lens SM, Span SW, Vader G, Medema RH, Kruyt FA, Giaccone G. Subcellular localization and nucleocytoplasmic transport of the chromosomal passenger proteins before nuclear envelope breakdown. Oncogene 2006; 25:4867-79; PMID:16547492; http://dx.doi.org/ 10.1038/sj.onc.1209499 [DOI] [PubMed] [Google Scholar]
- 31. Taniguchi E, Toyoshima-Morimoto F, Nishida E. Nuclear translocation of plk1 mediated by its bipartite nuclear localization signal. J Biol Chem 2002; 277:48884-8; PMID:12364337; http://dx.doi.org/ 10.1074/jbc.M206307200 [DOI] [PubMed] [Google Scholar]
- 32. Golsteyn RM, Mundt KE, Fry AM, Nigg EA. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol 1995; 129:1617-28; PMID:7790358; http://dx.doi.org/ 10.1083/jcb.129.6.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee MS, Huang YH, Huang SP, Lin RI, Wu SF, Li C. Identification of a nuclear localization signal in the polo box domain of Plk1. Biochim Biophys Acta 2009; 1793:1571-78; PMID:19631697; http://dx.doi.org/ 10.1016/j.bbamcr.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Bardes ES, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev 1998; 12:2131-43; PMID:9679058; http://dx.doi.org/ 10.1101/gad.12.14.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumagai A, Dunphy WG. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev 1999; 13:1067-72; PMID:10323858; http://dx.doi.org/ 10.1101/gad.13.9.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell 1998; 9:345-54; PMID:9450960; http://dx.doi.org/ 10.1091/mbc.9.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walsh S, Margolis SS, Kornbluth S. Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and Plx. Mol Cancer Res 2003; 1:280-89; PMID:12612056 [PubMed] [Google Scholar]
- 38. Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol 2000; 12:658-65; PMID:11063929; http://dx.doi.org/ 10.1016/S0955-0674(00)00149-6 [DOI] [PubMed] [Google Scholar]
- 39. Moore JD, Kornbluth S, Hunt T. Identification of the nuclear localization signal in Xenopus cyclin E and analysis of its role in replication and mitosis. Mol Biol Cell 2002; 13:4388-400; PMID:12475960; http://dx.doi.org/ 10.1091/mbc.E02-07-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Meyer AN, Donoghue DJ. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc Natl Acad Sci U S A 1997; 94:502-7; PMID:9012813; http://dx.doi.org/ 10.1073/pnas.94.2.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wasserman WJ, Smith LD. The cyclic behavior of a cytoplasmic factor controlling nuclear membrane breakdown. J Cell Biol 1978; 78, R15-22; PMID:307553; http://dx.doi.org/ 10.1083/jcb.78.1.R15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng AM, Yamamoto TM, Goldberg ML, Maller JL. A novel role for greatwall kinase in recovery from DNA damage. Cell Cycle 2010; 9:4364-9; PMID:20980823; http://dx.doi.org/ 10.4161/cc.9.21.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang L, Fisher LA, Wahl JK, 3rd, Peng A. Monoclonal antibodies against Xenopus greatwall kinase. Hybridoma (Larchmt) 2011; 30:469-74; PMID:22008075; http://dx.doi.org/ 10.1089/hyb.2011.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eckerdt F, Eyers PA, Lewellyn AL, Prigent C, Maller JL. Spindle pole regulation by a discrete Eg5-interacting domain in TPX2. Curr Biol 2008; 18:519-25; PMID:18372177; http://dx.doi.org/ 10.1016/j.cub.2008.02.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.