Abstract
Ethanol has historically been used as an ablative agent for a variety of lesions. One of the more common applications of this technique is celiac plexus neurolysis; however, recent reports have suggested a role for the endoscopic alcohol ablation of a variety of solid and cystic lesions. We report a novel case of endoscopic ethanol ablation of a peripheral nerve sheath tumor presenting as a small bowel obstruction.
Introduction
Ethanol is a widely available and cost-effective agent, which, when used for ablation, induces rapid cell death by means of cell lysis, protein denaturation, and vascular occlusion. The percutaneous approach for alcohol ablation of hepatic, renal, or adrenal lesions is widely reported.1-3 Ethanol is commonly used for endoscopic ultrasound (EUS)-guided celiac plexus neurolysis for the treatment of pain with invasive malignancy or chronic pancreatitis.4,5 Additionally, EUS-guided ethanol ablation has been reported for a variety of cystic and solid malignant and pre-malignant lesions.6
Case Report
A 67-year-old man with a history of morbid obesity presented with symptoms of small bowel obstruction including abdominal pain, nausea, and vomiting. Computed tomography (CT) was concerning for a duodenal mass (Figure 1), which was confirmed by endoscopy and EUS to be a 4-cm hypoechoic mass with cystic spaces (Figure 2). Fine-needle aspiration revealed spindle-shaped cells positive for S100 and negative for CD117 and CD24, consistent with a peripheral nerve sheath tumor.
Figure 1.
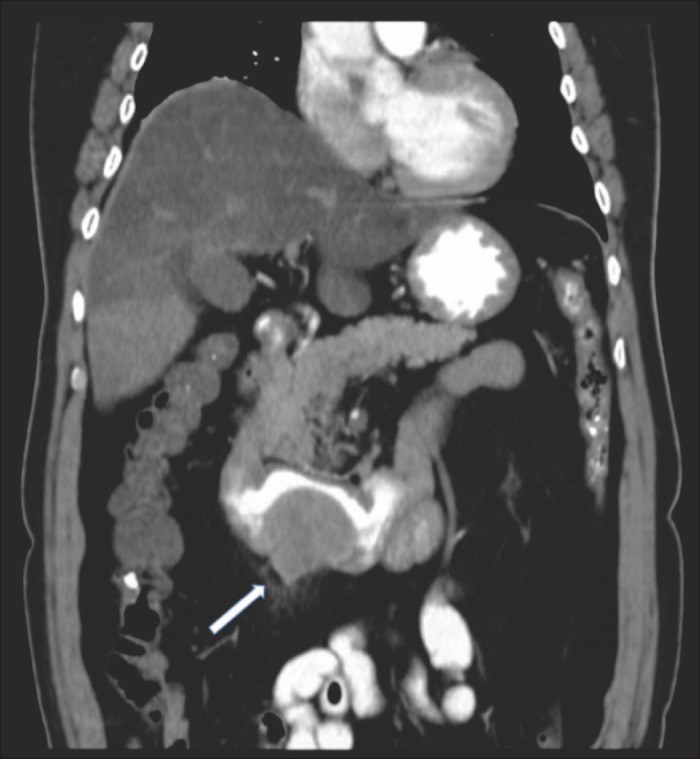
Abdominal CT with oral contrast demonstrating duodenal obstructing mass (arrow).
Figure 2.
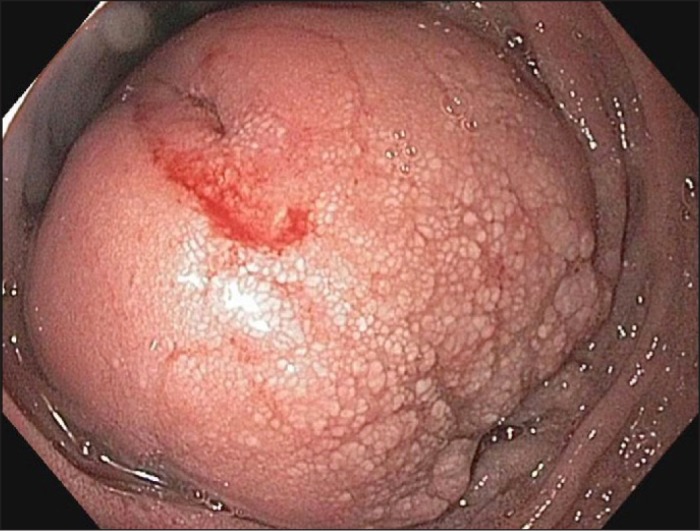
Obstructive lesion shown on EGD.
Laparoscopic examination demonstrated no evidence of metastatic disease; however, intraoperatively, it became apparent that the patient's body habitus precluded the possibility of a safe surgical resection. In the postoperative setting, the patient continued to suffer from obstructive symptoms and the decision was made to pursue ethanol ablation of his lesion. The patient underwent 4 sessions of endoscopically administered ethanol ablation. On his first session, a total of 10 mL of 98% ethanol was injected in divided doses, immediately resulting in white, necrotic-appearing mucosa (Figure 3). The clinical response was dramatic; 2 weeks after initial intervention, the patient had advanced his diet from liquids to solids. At week 6, there was an evident reduction of tumor burden with a shallow ulcer (Figure 3). Small foci of residual tumor were treated with serial ethanol injections approximately 6 weeks apart. On final endoscopy, 25 weeks after initial intervention, no residual tumor was noted (Figure 3).
Figure 3.
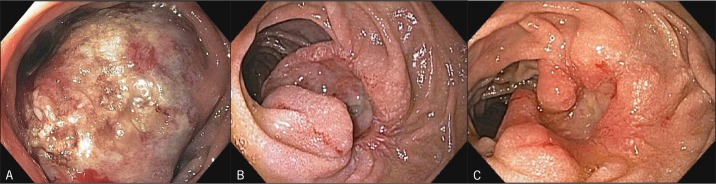
Obstructive lesion (A) after initial ethanol injection, (B) 6 weeks after initial injection, and (C) 25 weeks after the initial injection, during which time 3 additional injections were administered.
Discussion
We demonstrate the feasibility and safety of an endoscopic approach to the ethanol ablation of a peripheral nerve sheath tumor. An endoscopic approach circumvents technical constraints of both percutaneous and surgical treatment. While there is little data to demonstrate the long-term efficacy of such an approach, our case illustrates that endoscopic ethanol ablation can significantly palliate symptoms when surgery is not an option. Endoscopic alcohol ablation therapy should be considered in a multidisciplinary approach to select patients.
Disclosures
Author contributions: M. Chin wrote the article and is the article guarantor. C-L Chen, K. Chang, and J. Lee provided editorial input. J. Samarasena edited the final manuscript and supervised manuscript preparation.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Bean WJ. Renal cysts: Treatment with alcohol. Radiology. 1981;138(2):329–331. [DOI] [PubMed] [Google Scholar]
- 2.Livraghi T, Bolondi L, Lazzaroni S, et al. . Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: A study on 207 patients. Cancer. 1992;69(4):925–929. [DOI] [PubMed] [Google Scholar]
- 3.Xiao YY, Tian JL, Li JK, et al. . CT-guided percutaneous chemical ablation of adrenal neoplasms. Am J Roentgenol. 2008;190(1):105–110. [DOI] [PubMed] [Google Scholar]
- 4.Puli SR, Reddy JB, Bechtold ML, et al. . EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: A meta-analysis and systematic review. Dig Dis Sci. 2009;54(11):2330–7. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman M, Singh G, Das S, et al. . Efficacy of endoscopic ultrasoundguided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44(2):127–34. [DOI] [PubMed] [Google Scholar]
- 6.Zhang WY, Li ZS, Jin ZD. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol. 2013;19(22):3397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]


